Dementia Associated With Vitamin B12 Deficiency
Abstract
Vitamin B12 deficiency has long been associated with a wide variety of hematological, neurological, and psychiatric disorders. The role of vitamin B12 deficiency as one of the few treatable causes of dementia, however, is still controversial. The authors report on 2 elderly patients suffering from cognitive impairment and psychotic symptomatology probably related to cobalamin deficiency, who showed improvement after parenteral vitamin B12 substitution. The literature concerning the pathophysiology and the diagnostic and therapeutic aspects of cobalamin deficiency is reviewed.
Dementia is a frequently occurring syndrome, especially in the elderly population. The differential diagnosis of the dementia syndromes includes a large number of disorders, from Alzheimer's disease and alcoholic dementia to posttraumatic and vascular dementia. Hypovitaminosis is one of the few disorders causing dementia that are potentially curable today.
The following two case reports illustrate the importance of vitamin B12 deficiency as a possible cause for the development of dementia. They also underline that vitamin B12 deficiency is a frequent condition in the elderly population that is often overlooked in clinical practice. Whereas common routine parameters like the serum level of vitamin B12 or the Schilling test may not be sufficient to diagnose borderline cobalamin deficiency, newer functional assays may help to exclude vitamin B12 deficiency as a cofactor for dementia in the future.
CASE REPORTS
Patient 1. Patient O.F., male, was referred to a hospital at the age of 64 years because of confusion and collapse. According to relatives and colleagues from work, O.F. had shown a slowly progressive change of behavior and deterioration of working efficiency as an administrative employee over the previous 5 years. Recently he had been able to manage only the workload of a part-time employee, although he voluntarily worked additional hours on Saturdays. Several times he was sent home from work because of confused speech, walking impediment, and inability to complete his work correctly. Additionally, he showed an increasing withdrawal from social activities and a loss of interest in previous hobbies.
On admission to the hospital he was awake and cooperative, but disoriented. He showed serious impairment of cognitive functions and short-term and long-term memory. Formal thinking was slowed. Drive, facial expression, spontaneous movements, and abstract thinking ability were reduced. He frequently complained of memory deficits, and he had a lack of appetite resulting in a weight loss of 11 kg over a period of 6 weeks.
Physical examination revealed a pale and dry skin and emphysematic lungs. The neurological examination showed diminished tendon reflexes and a reduced sense of posture and vibration of the legs. Primitive reflexes (palmomental and snout reflexes) were positive. CT scan and MRI of the brain showed a generalized cerebral atrophy. EEG, cerebrospinal fluid, Doppler sonography of the cerebral arteries, and xenon SPECT for the measurement of cerebral blood flow did not show any abnormalities. The serum vitamin B12 level was in the lower normal range (307 pg/ml). The red blood count was normal. The Schilling test was pathological (9% absorption of cobalamin), but normalized after substitution of intrinsic factor (18% absorption), indicating a lack of intrinsic factor. Gastroscopy revealed atrophic gastritis, which was confirmed by biopsy. Serum titers of antibodies specific for parietal cells were not elevated, however.
Detailed psychological testing (complete Wechsler Adult Intelligence Scale–Revised, Benton Visual Retention Test, Trail Making Test, and Mini-Mental State Examination [MMSE]) reflected an average overall IQ (102). Concentration and the ability to solve complex cognitive tasks were reduced, however. At the retest approximately 5 weeks later (under substitution of vitamin B12 and additional therapy with doxepin and lorazepam), the patient showed a significantly better performance IQ (112) and improved cognitive speed.
Patient 2. Patient A.H., male, was admitted to our psychiatric clinic at the age of 77 years. For the previous 13 years he had had the delusional fear of impoverishment. However, when he encountered special offers, he bought unnecessary amounts of cheap and often useless items, which he then collected in his apartment. For the past 11 years he had been known to suffer from pernicious anemia, yet he had only rarely accepted parenteral vitamin B12 substitutions.
On admission he was disoriented to time and situation, was afraid of impoverishment, and had a depressed mood. Neurological examination revealed bilaterally reduced visual acuity, reduced ankle jerks, and a markedly diminished sense of posture and vibration. CT scan of the brain showed generalized atrophy. Doppler/duplex sonography of the cerebral arteries did not indicate significant stenosis. Visual evoked potentials were normal; somatosensory evoked potentials were pathologically delayed. Nerve conduction studies measured delayed and reduced action potentials, indicating polyneuropathy.
The red blood count revealed a macrocytosis with elevated mean corpuscular hemoglobin concentration (36.1 g/dl) and volume (102 fl μm3). The vitamin B12 level was borderline low (203 pg/ml), with a borderline pathological Schilling test (9.85% absorption; after substitution of intrinsic factor, 12.38% absorption). Antibodies specific for thyroglobulin, parietal cells, and microsomes were not detectable. Stool tests for parasites were negative. A gastroscopy was refused by the patient. The CSF had a normal cell count but an elevated protein content (59 mg/100ml), indicating a disturbed blood–CSF barrier.
Psychological testing was difficult to perform and revealed an average overall IQ (104). Short-term memory was severely impaired and cognitive flexibility was reduced. The patient was treated with vitamin B12 in combination with an antidepressant (clomipramine) and initial anxiolytic medication (lorazepam). A second psychological testing was refused by the patient. On dismissal from the clinic, in contrast to the time of admission, he was fully oriented and his mood was balanced.
VITAMIN B12 DEFICIENCY: LITERATURE REVIEW
Clinical Features
Apart from the well-characterized hematological changes of vitamin B12 deficiency, a variety of neurological impairments have been described. Typically, signs of peripheral neuropathy and myelopathy can be observed: distal paresthesias, impairment of vibratory and position sense, reduced ankle jerks, Lhermitte's sign.1 In more severe cases symmetrical limb weakness and other pyramidal deficits can occur2–4 (Table 1). Paresthesia is the most common initial complaint, affecting more than 70% of the patients with neurological symptoms.4 Electrophysiological signs of demyelination can be detected in tests of nerve conduction velocity and somatosensory evoked potentials.5
Associated Mental Changes
Possible links between vitamin B12 deficiency and cerebral symptoms were first observed in patients who suffered from funicular myelosis or pernicious anemia.6,7 Today there is an increasing evidence that hematological and neuropsychiatric effects due to cobalamin deficiency do not necessarily occur simultaneously.3,8–11The true incidence of cerebral symptoms in vitamin B12 deficiency is unknown; reports vary between 4% and more than 50%,2,10,12,13 depending on the population studied and the definition of vitamin B12 deficiency used. The mental effects described in the context of cobalamin deficiency cover a scope from mood changes (agitation, depression, mania) to psychotic episodes (paranoia, auditory and visual hallucinations, delusions) to cognitive impairment (slow mentation, memory deficits, confusion, dementia)2,4,10,11,13 (Table 1). Mental or psychological changes may precede hematological signs by months or years—they can in fact be the initial or only symptoms.3,9,14 In a study with patients suffering from vitamin B12 deficiency after gastric resections, 50% displayed intellectual impairment, whereas only 14% had megaloblastic bone marrow.12 However, most or all patients with central nervous system involvement also show some signs of peripheral neuropathy.15
Cobalamin deficiency has been shown to be the most frequent associated physical disease in patients with dementia.16 The incidence of low vitamin B12 levels among dementia patients has been found to range between 29%8 and 47%.17 Even in healthy elderly patients, a correlation between serum cobalamin level and cognitive function (as tested in IQ, MMSE, verbal, and memory tests) has been observed.18,19 Other studies, on the contrary, have questioned the correlation between dementia and serum cobalamin levels and the reversibility of dementia under cobalamin substitution (see review20). Furthermore, low serum cobalamin levels have also been detected in healthy control subjects21 and nondemented patients with other neurological diseases (our own observation).
Pathophysiology
Historical reports describe perivascular degeneration and foci of myelin degeneration in the brains of patients with pernicious anemia.6,22 MRI images of patients with vitamin B12 deficiency sometimes show signs of disseminated demyelination similar to those found in multiple sclerosis.23 A number of animal models provide additional evidence for the detrimental effect of vitamin B12 deficiency on the integrity of myelin,24,25 which is aggravated under the oxidizing influence of N2O.26,27 The exact mechanism of the myelin damage following cobalamin deficiency, however, is still unknown.28,29
Several important functions have been attributed to vitamin B12 as a coenzyme.10,29,30 Both cobalamin and folate are needed for the methylation of homocysteine to methionine and in the synthesis of S- adenosylmethionine, a major methyl donor in the CNS. S-adenosylmethionine participates in various methylation steps involving proteins, phospholipids, DNA, and neurotransmitter metabolism. A defect in methylation processes is thought to play a central role in the biochemical basis of the neuropsychiatry of these vitamin deficiencies.30
Additionally, hyperhomocysteinemia, which is a metabolic consequence of vitamin B12 deficiency, is an independent vascular risk factor associated with cerebral microvascular disease.31 Cerebral microangiopathy is considered to be the basis for vascular dementia. High homocysteine (HY) levels may also lead to an excessive production of homocysteic acid and cysteine sulphinic acid, which act as endogenous agonists of N-methyl-d- aspartatate receptors and may impair cognitive functions by excitotoxic mechanisms (see review32).
Causes
Vitamin B12 is contained in a wide variety of animal proteins. After intestinal absorption it enters enterohepatic circulation. The reported body half-life exceeds 1,300 days. With normal absorption, the recommended daily intake ranges from 2 μg to 5 μg.10,33 Malnutrition as a sole cause is rare and occurs in cases of chronic alcoholism, extreme vegetarianism, and other forms of insufficient dietary supply.33 Intestinal absorption of vitamin B12 depends on a series of steps including the liberation of protein-bound vitamin B12 from proteins by gastric acid34 and its transfer to intrinsic factor produced by gastric parietal cells.
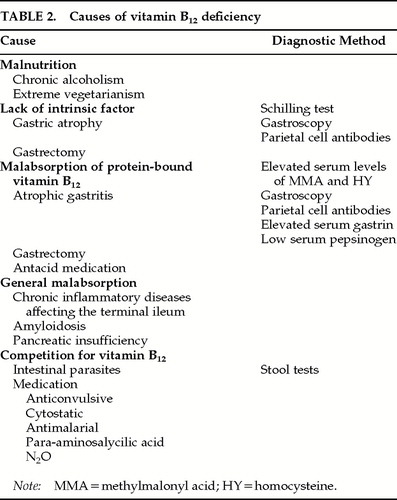
The most common cause of impaired vitamin B12 absorption is the lack of intrinsic factor, which can be measured by the Schilling test.11 A cause of vitamin B12 malabsorption in spite of a normal Schilling test, which is frequently encountered in the elderly population, is an insufficient amount of gastric acid to proteolyze vitamin B12 from other nutritional proteins.35,36 This hypo- or achlorhydria is often due to the reduction of fundus glands and parietal cell mass found in atrophic gastritis, which is reported to be present in more than 30% of patients above 60 years of age.37 Other rare causes of cobalamin deficiency33 are summarized in Table 2.
Diagnostic Aspects
Numerous studies report an age-related decline in serum vitamin B12 levels. Depending on the population studied and the criteria and methods used, the prevalence of vitamin B12 deficiency varies from 3% to 40% in elderly patients.38–40 The minimum serum level of vitamin B12 is usually recommended to be above 200 pg/ml.33 However, metabolic evidence of cobalamin deficiency has been observed in patients with serum levels between 200 and 300 pg/ml with a frequency similar to that in patients with levels below 200 pg/ml.39 Van Goor et al.11 therefore suggest the diagnostic measurement of serum levels of methylmalonyl acid (MMA) and homocysteine (HY). Elevated serum levels of these metabolites have been shown to be more reliable and sensitive indicators of cobalamin deficiency than serum cobalamin levels alone.3,29,41
With this procedure, a functional lack of vitamin B12 was demonstrated in 5% to 9% of symptomatic elderly patients with normal to only borderline subnormal vitamin B12 levels,39,41and a positive correlation between serum MMA and HY and the degree of neurological deficit has been observed.42,43 Serum HY levels were also found to be significantly higher, and serum folate and vitamin B12 levels lower, in patients with senile dementia of Alzheimer type than in control subjects.44,45 Elevated serum levels of MMA and HY have been shown to fall to normal when vitamin B12 is substituted,3,39,43,46 whereas MMA rises under the substitution of folic acid.21
One possible explanation for vitamin B12 deficiency with only borderline subnormal vitamin B12 serum levels is the presence of cobalamin analogues that cause high vitamin B12 values in presently used assays with R-binders. Patients with neurological deficits have been shown to have higher analogue levels than patients with hematological disorders.47
Falsely high serum levels of MMA and HY can occur in chronic renal failure or intravascular volume depletion.48 Certain anaerobic intestinal flora can cause MMA elevation due to the production of propionic acid, a precursor of MMA. Folic acid deficiency, on the other hand, can result in elevated HY levels.49 Van Goor et al.11 therefore suggest the measurement of both MMA and HY. Normal levels of MMA and HY seem to rule out clinically significant vitamin B12 deficiency.41
Treatment
Only one-third of patients with low vitamin B12 levels receive adequate therapy.50 Parenteral substitution remains the surest form of vitamin B12 replacement. The recommended dosage is 1,000 μg cobalamin im daily for 1 week, weekly for 1 month, then monthly.10,33 Occasionally deaths have been reported in early phases of replacement due to the fall of potassium in patients with megaloblastosis. For patients with this condition, monitoring of potassium levels and a lower starting dose of vitamin B12 is recommended (5 μg/day in the first days).10 Folate administration in patients with vitamin B12 deficiency can partially correct megaloblastosis but may aggravate encephalopathy; therefore both values should be monitored together.10 Frequently iron deficiency occurs together with vitamin B12 deficiency and requires replacement.51
Prognosis
Whereas hematological abnormalities normalize within 2 months of vitamin B12 substitution,11 the reversal of neurological symptoms depends on their severity and duration.3,4 Mental symptoms have also been described to be partially or completely reversible after cobalamin substitution.4,14,52,53 Chatterjee et al.54 even reported the partial reversal of associated white matter lesions. Interestingly, in a recent case report cognitive improvement under cobalamin substitution was paralleled by a significant improvement of the P300 latency.55 P300 has previously been suggested as a valuable parameter in the assessment of dementia56,57 and has been used in the follow-up of treatment studies.58
The improvement of mental impairment seems to be possible only in early stages, however; after longer duration there may be structural changes without the possibility of neuronal repair. Martin et al.59 found improvement of cognitive dysfunction in 11 of 18 patients with low serum cobalamin only if symptoms persisted for less than 1 year. Similar observations were reported by other authors.53,60 Chronic dementia seems to respond poorly but should, nevertheless, be treated if there is metabolic evidence for vitamin B12 deficiency.61
DISCUSSION
In the two case reports we presented, cobalamin deficiency is a likely contributor to the neuropsychiatric malfunctioning. In Patient 1, a borderline low vitamin B12 serum level, pathological Schilling test, and histologically proven atrophic gastritis imply cobalamin deficiency. Patient 2 had long been known to suffer from pernicious anemia but had refused regular vitamin B12 therapy. Cobalamin deficiency was confirmed by a low vitamin B12 serum titer, macrocytosis, and a pathological Schilling test. Substitution of vitamin B12 together with additional treatment resulted in improved cognitive function, which was documented by psychological retesting (Patient 1) or was indicated clinically by restored orientation (Patient 2).
Although in our patients, as well as in previously reported cases, the effects of vitamin B12 substitution cannot be positively distinguished from the effects of co- medication, supporting therapeutic measures, and retest improvement, there is substantial evidence supporting the crucial involvement of vitamin B12 in several pathophysiological conditions affecting the CNS, reaching from myelination to transmitter function. Even though the causal relationship between cobalamin deficiency and dementia in individual patients is hard to prove and may often remain circumstantial, subclinical vitamin B12 deficiency, which today can be unambiguously identified, is a common condition in the elderly population. Considering the devastating impact of dementia on the quality of life of the individual and also the vast costs this often incurable condition causes, the proper diagnosis and inexpensive treatment of cobalamin deficiency should not be missed, especially in the early phases of cognitive decline.
Modern diagnostic tools like the measurement of HY and MMA, as well as longitudinal testing of cognitive function and neurophysiological parameters, will help to further define the role of vitamin B12 deficiency as a cofactor in the development of dementia and to elucidate why not all cobalamin-deficient patients develop mental symptoms.
 |
 |
1 Butler WM, Taylor HG, Diehl LF: Lhermitte's sign in cobalamin (vitamin B12) deficiency. JAMA 1981; 245:1059Crossref, Medline, Google Scholar
2 Spatz R: Perniziöse Anämie–Funikuläre Spinalerkrankung–Perniciosa-Psychose [Pernicious anemia, funicular myelopathy, psychosis]. Nervenarzt 1969; 40:475–481Medline, Google Scholar
3 Lindenbaum J, Healton EB, Savage DG, et al: Neuropsychiatric disorders caused by cobalamin deficiency in the absence of anemia or macrocytosis. N Engl J Med 1988; 318:1720–1728Google Scholar
4 Healton EB, Savage DG, Brust JC, et al: Neurologic aspects of cobalamin deficiency. Medicine 1991; 70:229–245Crossref, Medline, Google Scholar
5 Karnaze DS, Carmel R: Neurologic and evoked potential abnormalities in subtle cobalamin deficiency states, including deficiency without anemia and with normal absorption of free cobalamin. Arch Neurol 1990; 47:1008–1012Google Scholar
6 Woltman HW: Brain changes associated with pernicious anemia. Arch Intern Med 1918; 21:791–843Crossref, Google Scholar
7 Woltman HW: The mental changes associated with pernicious anemia. Am J Psychiatry 1924; 80:435–449Crossref, Google Scholar
8 Karnaze DS, Carmel R: Low serum cobalamin levels in primary degenerative dementia: do some patients harbor atypical cobalamin deficiency states? Arch Intern Med 1987; 147:429–431Google Scholar
9 Carmel R: Pernicious anemia: the expected findings of very low serum cobalamin levels, anemia, and macrocytosis are often lacking. Arch Intern Med 1988; 148:1712–1714Google Scholar
10 Martin DC: B12 and folate deficiency dementia. Clin Geriatr Med 1988; 4:841–852Crossref, Medline, Google Scholar
11 van Goor L, Woiski MD, Lagaay AM, et al: Review: cobalamin deficiency and mental impairment in elderly people. Age Aging 1995; 24:536–542Crossref, Medline, Google Scholar
12 Roos D, Willanger R: Various degrees of dementia in a selected group of gastrectomized patients with low serum B12. Acta Neurol Scand 1977; 55:363–376Crossref, Medline, Google Scholar
13 Stabler SP, Allen RH, Savage DG, et al: Clinical spectrum and diagnosis of cobalamin deficiency. Blood 1990; 76:871–881Crossref, Medline, Google Scholar
14 Evans DL, Edelsohn GA, Golden RN: Organic psychosis without anemia or spinal cord symptoms in patients with vitamin B12 deficiency. Am J Psychiatry 1983; 140:218–221Crossref, Medline, Google Scholar
15 Shorvon SD, Carney MW, Chanarin I, et al: The neuropsychiatry of megaloblastic anaemia. British Medical Journal 1980; 281:1036–1038Google Scholar
16 Nagga AK, Marcusson J: Associated physical disease in a demented population. Aging (Milano) 1998; 10:440–444Medline, Google Scholar
17 Cole MG, Prehal JF: Low serum vitamin B12 in Alzheimer-type dementia. Age Aging 1984; 13:101–105Crossref, Medline, Google Scholar
18 Goodwin JS, Goodwin JM, Garry PJ: Association between nutritional status and cognitive functioning in a healthy elderly population. JAMA 1983; 249:2917–2921Google Scholar
19 Bell IR, Edman JS, Marby DW, et al: Vitamin B12 and folate status in acute geropsychiatric inpatients: affective and cognitive characteristics of a vitamin nondeficient population. Biol Psychiatry 1990; 27:125–137Crossref, Medline, Google Scholar
20 Hutto BR: Folate and cobalamin in psychiatric illness. Compr Psychiatry 1997; 38:305–314Crossref, Medline, Google Scholar
21 Allen RH, Stabler SP, Savage DG, et al. Diagnosis of cobalamin deficiency, I: usefulness of serum methylmalonic acid and total homocysteine concentrations. Am J Hematol 1990; 34:90–98Crossref, Medline, Google Scholar
22 Adams RD, Kubik CS: Subacute degeneration of the brain in pernicious anemia. N Engl J Med 1944; 231:1–9Crossref, Google Scholar
23 Haan J, Haupts M, Uhlenbrock D: Magnetic resonance imaging (MRI), cranial computerized tomography (CCT), evoked potentials and cerebrospinal fluid (CSF) analysis in five patients with funicular myelosis. Neurosurg Rev 1987; 10:209–211Crossref, Medline, Google Scholar
24 Agamanolis DP, Victor M, Harris JW, et al: An ultrastructural study of subacute combined degeneration of the spinal cord in vitamin B12-deficient rhesus monkeys. J Neuropathol Exp Neurol 1978; 37:273–299Crossref, Medline, Google Scholar
25 Scalabrino G, Monzio Compagnoni B, Ferioli ME, et al: Subacute combined degeneration and induction of ornithine decarboxylase in spinal cords of totally gastrectomized rats. Lab Invest 1990; 62:297–304Medline, Google Scholar
26 Duffield MS, Phillips JI, Vieira Makings E, et al: Demyelinisation in the spinal cord of vitamin B12 deficient fruit bats. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol 1990; 96:291– 297Crossref, Google Scholar
27 Flippo TS, Holder WDJ: Neurologic degeneration associated with nitrous oxide anesthesia in patients with vitamin B12 deficiency. Arch Surg 1993; 128:1391–1395Google Scholar
28 Metz J: Cobalamin deficiency and the pathogenesis of nervous system disease. Annu Rev Nutr 1992; 12:59–79Crossref, Medline, Google Scholar
29 Allen RH, Stabler SP, Savage DG, et al: Metabolic abnormalities in cobalamin (vitamin B12) and folate deficiency. FASEB J 1993; 7:1344–1353Google Scholar
30 Bottiglieri T: Folate, vitamin B12, and neuropsychiatric disorders. Nutr Rev 1996; 54:382–390Crossref, Medline, Google Scholar
31 Fassbender K, Mielke O, Bertsch T, et al: Homocysteine in cerebral macroangiography and microangiopathy (letter). Lancet 1999; 353:1586–1587Google Scholar
32 Parnetti L, Bottiglieri T, Lowenthal D: Role of homocysteine in age-related vascular and non-vascular diseases. Aging (Milano) 1997; 9:241–257Medline, Google Scholar
33 Dieterich M: Vitaminstoffwechselstörungen [Disturbances of vitamin metabolism], in Therapie und Verlauf neurologischer Krankheiten, 3rd edition, edited by Brandt T, Dichgans J, Diener HC. Stuttgart, Kohlhammer, 1998, pp 830–844Google Scholar
34 Lindenbaum J: Aspects of vitamin B12 and folate metabolism in malabsorption syndromes. Am J Med 1979; 67:1037–1045Google Scholar
35 Jones BP, Broomhead AF, Kwan YL, et al: Incidence and clinical significance of protein-bound vitamin B12 malabsorption. Eur J Haematol 1987; 38:131–136Crossref, Medline, Google Scholar
36 Scarlett JD, Read H, O'Dea K: Protein-bound cobalamin absorption declines in the elderly. Am J Hematol 1992; 39: 79–83Google Scholar
37 Krasinski SD, Russell RM, Samloff IM, et al: Fundic atrophic gastritis in an elderly population: effect on hemoglobin and several serum nutritional indicators. J Am Geriatr Soc 1986; 34:800– 806Crossref, Medline, Google Scholar
38 Nilsson Ehle H, Jagenburg R, Landahl S, et al: Serum cobalamins in the elderly: a longitudinal study of a representative population sample from age 70 to 81. Eur J Haematol 1991; 47:10–16Crossref, Medline, Google Scholar
39 Pennypacker LC, Allen RH, Kelly JP, et al: High prevalence of cobalamin deficiency in elderly outpatients. J Am Geriatr Soc 1992; 40:1197–1204Google Scholar
40 Lindenbaum J, Rosenberg IH, Wilson PW, et al: Prevalence of cobalamin deficiency in the Framingham elderly population. Am J Clin Nutr 1994; 60:2–11Crossref, Medline, Google Scholar
41 Savage DG, Lindenbaum J, Stabler SP, et al: Sensitivity of serum methylmalonic acid and total homocysteine determinations for diagnosing cobalamin and folate deficiencies. Am J Med 1994; 96:239–246Crossref, Medline, Google Scholar
42 Stabler SP, Marcell PD, Podell ER, et al: Assay of methylmalonic acid in the serum of patients with cobalamin deficiency using capillary gas chromatography-mass spectrometry. J Clin Invest 1986; 77:1606–1612Google Scholar
43 Stabler SP, Marcell PD, Podell ER, et al: Elevation of total homocysteine in the serum of patients with cobalamin or folate deficiency detected by capillary gas chromatography-mass spectrometry. J Clin Invest 1988; 81:466–474Crossref, Medline, Google Scholar
44 Clarke R, Smith AD, Jobst KA, et al: Folate, vitamin B12, and serum total homocysteine levels in confirmed Alzheimer disease. Arch Neurol 1998; 55:1449–1455Google Scholar
45 McCaddon A, Davies G, Hudson P, et al: Total serum homocysteine in senile dementia of Alzheimer type. Int J Geriatr Psychiatry 1998; 13:235–239Crossref, Medline, Google Scholar
46 Naurath HJ, Joosten E, Riezler R, et al: Effects of vitamin B12, folate, and vitamin B6 supplements in elderly people with normal serum vitamin concentrations. Lancet 1995; 346:85–89Crossref, Medline, Google Scholar
47 Carmel R, Karnaze DS, Weiner JM: Neurologic abnormalities in cobalamin deficiency are associated with higher cobalamin “analogue” values than are hematologic abnormalities. J Lab Clin Med 1988; 111:57–62Medline, Google Scholar
48 Moelby L, Rasmussen K, Rasmussen HH: Serum methylmalonic acid in uremia. Scand J Clin Lab Invest 1992; 52:351–354Crossref, Medline, Google Scholar
49 Lindenbaum J, Savage DG, Stabler SP, et al: Diagnosis of cobalamin deficiency, II: relative sensitivities of serum cobalamin, methylmalonic acid, and total homocysteine concentrations. Am J Hematol 1990; 34:99–107Crossref, Medline, Google Scholar
50 Carmel R, Karnaze DS: Physician response to low serum cobalamin levels. Arch Intern Med 1986; 146:1161–1165Google Scholar
51 Carmel R, Weiner JM, Johnson CS: Iron deficiency occurs frequently in patients with pernicious anemia. JAMA 1987; 257:1081–1083Google Scholar
52 Holmes JM: Cerebral manifestations of vitamin B12 deficiency. British Medical Journal 1956; 2:1394–1398Google Scholar
53 Larson EB, Reifler BV, Featherstone HJ, et al: Dementia in elderly outpatients: a prospective study. Ann Intern Med 1984; 100:417– 423Crossref, Medline, Google Scholar
54 Chatterjee A, Yapundich R, Palmer CA, et al: Leukoencephalopathy associated with cobalamin deficiency. Neurology 1996; 46:832–834Crossref, Medline, Google Scholar
55 Oishi M, Mochizuki Y: Improvement of P300 latency by treatment of vitamin B12 deficiency. J Clin Neurophysiol 1998; 15:173– 174Crossref, Medline, Google Scholar
56 Filipovic SR, Kostic VS: Utility of auditory P300 in detection of presenile dementia. J Neurol Sci 1995; 131:150–155Crossref, Medline, Google Scholar
57 Ito J: Somatosensory event-related potentials (ERPs) in patients with different types of dementia. J Neurol Sci 1994; 121:139–146Crossref, Medline, Google Scholar
58 Schellenberg R, Todorova A, Wedekind W, et al: Pathophysiology and psychopharmacology of dementia: a new study design, II: cyclandelate treatment—a placebo-controlled double-blind clinical trial. Neuropsychobiology 1997; 35:132–142Crossref, Medline, Google Scholar
59 Martin DC, Francis J, Protetch J, et al: Time dependency of cognitive recovery with cobalamin replacement: report of a pilot study. J Am Geriatr Soc 1992; 40:168–172Crossref, Medline, Google Scholar
60 Freemon FR, Rudd SM: Clinical features that predict potentially reversible progressive intellectual deterioration. J Am Geriatr Soc 1982; 30:449–451Crossref, Medline, Google Scholar
61 Nilsson Ehle H: Age-related changes in cobalamin (vitamin B12) handling: implications for therapy. Drugs Aging 1998; 12:277– 292Crossref, Medline, Google Scholar



