Olfaction and Cognition in Schizophrenia: Sex Matters
Abstract
Cognitive and olfactory deficits occur in schizophrenia, but little is known whether sex modifies these deficits. We examined the relationship between olfaction and cognition in 55 schizophrenia patients and 32 healthy controls. Patients and controls demonstrated significant differences performing cognitive tasks. In patients, sex modified all relationships of odor identification to cognition. Female patients showed significantly stronger trends than male patients correlating better smell identification with higher scores on intelligence, memory, and attention, whereas their correlations of odor identification with executive functioning contradicted those of male patients. Odor acuity significantly correlated with several cognitive measures, especially in male patients, in whom better acuity was generally associated with better cognition. Female patients again differed significantly from males; odor acuity correlations with cognitive measures were weaker, or contradicted, those of male patients. These findings indicate significant sex differences in olfactory processing in schizophrenia. Combining the sexes in research analyses may obscure important differences.
Deficits in both cognition and olfaction are associated with schizophrenia.1,2 A large body of research in olfaction has shown unequivocally that schizophrenia patients have impaired ability to identify smells (reviewed by Atanasova3). We and others have shown this impairment to be correlated with deficits in motivated behavior and emotional expression4–8 as well as with impaired verbal and nonverbal memory.6,9–11 It is not clear, however, whether these impairments reflect a general deficit in cognition or whether they relate to specific pathways of olfactory processing. This question might be illuminated though comparisons of results in different sexes, given that brain structures, chemistry, and function are sexually dimorphic;12,13 however, few researchers have explicitly examined whether sex modifies how odor identification relates to cognitive functioning.
Other aspects of olfaction studied in schizophrenia include acuity (or sensitivity or threshold), discrimination, familiarity/recognition, intensity and pleasantness; however, the findings have been variable (reviewed by Atanasova3). For example, patients with schizophrenia have shown decreased,14–17 increased17,18 or unchanged mean values for odor acuity,2,10,19–23 leading some to suggest that odor acuity is not altered in schizophrenia.3 One interpretation of this could be that the deficits in smell identification in schizophrenia reflect abnormalities in central processing, whereas the supposedly unchanged acuity reflects normal peripheral processing.3 This view would be supported if acuity were found to be unrelated to cognition and if results were true for both sexes. Little is known, however, about how olfactory acuity relates to cognition and to sex in those with schizophrenia. Furthermore, given sex differences in structure, connectivity and function in regions of the brain involved in olfaction in humans,24 surprisingly little is known about how sex modifies olfactory processing in humans, either in normal people or schizophrenia patients. Research examining olfaction and cognitive processes in schizophrenia rarely includes sex stratification. One study that did address sex differences did not assess odor acuity.25 Another study suggested that there was no relationship between acuity and cognition in schizophrenia,10 but did not separate the sexes.
The vast majority of schizophrenia research has not stratified the results according to sex. Instead, female findings have been generally added to those of the males. Although there may be obvious reasons for analyzing both sexes together (e.g. small sample size), it is also surprising given the burgeoning literature on sex influences on the brain,13 and the growing recognition that olfactory probes hold great promise as biomarkers for understanding schizophrenia.26 In the current study we examine how measures of cognition, with particular focus on memory and attention, relate both to smell identification and olfactory acuity in men and women with schizophrenia. While the emphasis is on sex differences in schizophrenia patients, we also included a smaller sample of healthy control subjects.
Method
Subjects
Patients (N=55, males=54.5%) with schizophrenia or schizoaffective disorder (Diagnostic and Statistical Manual of Mental Disorders – Fourth Edition27) were recruited from inpatient and outpatient research centers at the New York State Psychiatric Institute (NYSPI). All were clinically stable for at least one month with no changes in medication. Healthy controls (N=32, males=53.1%) were recruited from postings at the medical center and internet advertisements. Patients and controls were excluded if they were pregnant or on oral contraceptives, had any substance dependence disorder or had a history of epilepsy, rhinoplasty or a major head injury requiring medical treatment. The study was approved by the Institutional Review Board (IRB) at the NYSPI and all participants gave informed consent.
Diagnoses, Olfactory Measures and Cognitive Tests
We assessed psychiatric diagnoses for all patients and controls, using the Diagnostic Interview for Genetic Studies (DIGS28). To assess ability to identify odors we used the University of Pennsylvania Smell Identification Test (UPSIT29), in which subjects choose one of four possible answers for each of 40 odor-impregnated strips. To assess acuity we used the Smell Threshold Test (STT; Sensonics Inc.), measuring ability to detect various concentrations of a single odor, phenyl ethyl alcohol (PEA), which has a rose odor. Subjects were asked not to smoke, use cosmetics or perfume on the day of testing and did not eat or drink for at least 2 hours before testing; furthermore they were screened prior to testing to rule out colds or allergies. For the STT, we used a single staircase forced choice procedure, applying the odorant to each nostril separately, while occluding the opposite nostril with foam tape. The first dilution to be tested was a −6.00 log concentration; then we applied the odorant in increasingly higher concentrations in full log steps until five consecutive correct detections occurred in a given trial. The staircase was then reversed and moved down or up in half log increments with two pairs of trials at each concentration. The mean of the last four staircase reversal points was used as the measure of threshold. Higher raw STT scores correspond to a stronger concentration required for odor detection (i.e. less sensitive acuity).
The Wechsler Adult Intelligence Scale–Third Edition (WAIS-III30) assessed verbal, performance, and full scale IQs, as well as working memory, processing speed, verbal comprehension and perceptual organization. The Wechsler Memory Scale–Revised (WMS-R31) evaluated verbal and visual memory, attention, delayed recall and general memory. Executive functioning and set-switching were assessed with the Wisconsin Card Sorting Test (WCST32). Visual attention and processing speed were measured with the Trail Making Tests A and B (TMT33). Premorbid intelligence was estimated with the National Adult Reading Test (NART34). All assessments were completed by trained clinicians with a master’s degree or higher.
Data Analysis
Data were entered and verified using the SIR Database Management Software (SIR 2002, SIR Pty Ltd). SPSS (PASW Statistics 17) was used for analyses. Descriptive statistics of all measures were examined to identify key features of the data (e.g. nonnormal distribution, outliers, skewness) that might influence inferential methods. We examined demographic characteristics, age, education, WAIS full, verbal and performance IQs using multivariate ANOVA to assess effects of group membership (patients versus controls) and sex (male versus female) and to test for interaction between group and sex. Similarly, analyses of the other cognitive measures were performed using multivariate ANOVAs to assess the main effects of diagnostic group and sex, and their potential interaction.
We assessed odor acuity in the left and right nostril; however, preliminary results indicated nonsignificant differences between them, thus the data for both sides were combined into a mean odor threshold. To avoid confusion in interpreting the results, we analyzed the absolute values of odor acuity: better acuity is represented by a higher value.
To assess sex differences in patients, we used multiple regression analyses in which the dependent variable was either measure of olfaction, and the sample tested included patients of both sexes. Three variables were entered for each model: the cognitive measure, an interaction term constructed from its product with gender, and gender alone. A backward elimination strategy forced retention of the first two variables and allowed retention or elimination of the third at the level of p<0.05. Further analyses were done in which the other variables were forced into the models at the first step; these included the other measure of olfaction, patient’s age at testing and age at diagnosis.
Results
Subjects’ Characteristics and Olfactory Scores
Table 1 shows characteristics of the schizophrenia patients and controls and a summary comparison of the distributions of the two olfactory measures for these two groups, by sex. Patients and controls were of similar age with sexes combined; however, while male patients were somewhat older than male controls, the reverse was true for females, so that male controls were about 10 years younger than female controls. Patients were significantly less educated than controls and in both groups females were more educated than males. Not shown in the table, the 87 subjects included 41% Whites, 23% Blacks, 24% other specific races and 11% other/unknown; there were no significant differences in the distributions of these between patients and controls, males and females or the four groups.
| Schizophrenia patients | Healthy controls | Statistics | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Combined Mean (SD) N=55 | Males Mean (SD) N=30 | Females Mean (SD) N=25 | Combined Mean (SD) N=32 | Males Mean (SD) N=17 | Females Mean (SD) N=15 | Diagnosis F | Gender F | Interaction Diag./Gen. F | |
| Age | 32.7 (9.3) | 32.3 (10.2) | 33.2 (8.3) | 33.4 (12.4) | 28.6 (7.7) | 38.9 (14.5) | 0.21 | 6.08* | 4.39* |
| Age at Onset | 23.4 (7.1) | 22.0 (6.8) | 25.3 (7.1) | — | — | — | — | t = 1.68 | — |
| Education (category)c | 3.7 (1.5) | 3.3 (1.6) | 4.3 (1.2) | 4.6 (0.83) | 4.4 (0.94) | 4.9 (0.64) | 10.04** | 7.21** | 1.04 |
| Smell identification (UPSIT total) | N = 51 | N = 27 | N = 24 | N = 30 | N = 16 | N = 14 | |||
| 31.6 (4.1) | 30.5 (4.0) | 32.8 (4.0) | 32.3 (4.4) | 31.4 (3.7) | 33.3 (5.0) | 0.55b | 4.67*,b | 0.05b | |
| Odor Acuity (STT) | N = 43 | N = 22 | N = 21 | N = 30 | N = 16 | N = 14 | 0.03a | 1.43a | 0.50a |
| −4.68 (1.7) | −4.34 (1.4) | −5.02 (2.0) | −4.71 (1.3) | −4.66 (1.5) | −4.78 (1.1) | 0.01b | 1.13b | 0.56b | |
Regarding smell identification (UPSIT) shown in Table 1, the female patients and controls demonstrated slightly higher scores than their male counterparts (p=0.034). There were no statistically significant differences between patients and controls, but patients of both sexes tended to achieve lower UPSIT scores than their sex-matched controls. Regarding odor acuity, more negative scores indicate lower thresholds for detection and a higher acuity; male schizophrenia patients required somewhat higher concentrations of the test odor before detecting it. Females’ acuity was better both in patients and controls, and female patients demonstrated a larger standard deviation and a lower mean threshold for detection. These differences were subtle, however, and none was statistically significant.
Cognitive performance in cases and controls of both sexes
Table 2 shows scores for the cognitive tests, comparing patients and controls, with sexes combined and separated. The statistics shown in the right hand columns of the table compare patients of both sexes with controls of both sexes; similarly, the statistics columns compare males versus females with the patients and controls combined, together with tests for interactions. Most test scores indicated lower scores on cognitive measures in patients of either sex, compared with their respective controls. Notable differences between patients and controls were observed in the WAIS-III indices (p=0.001), due to assessments of working memory (p=0.001) and processing speed (p=0.001); and in the Trail Making Test (p=0.027), due to the Trail A test (p=0.007). The patients’ reduced cognitive abilities were also reflected in the WAIS-III assessment of Verbal IQ (p=0.046) and the General Index of the WMS-R (p=0.039); furthermore, the schizophrenia patients exhibited significant deficits in executive functioning, making more errors (p=0.024), more perseverative responses (p=0.030) and more perseverative errors (p=0.038) on the WCST. Differences between the sexes were minimal on the NART, and none was statistically significant, though the male patients and male controls tended to make more errors than the females. Similarly, there were no significant interactions between sex and diagnosis, with the exception of the assessment of working memory (p=0.043); in this test the male patients scored 16 points lower than the male controls, while the female patients were only 4 points lower than the controls.
| Schizophrenia patients | Healthy controls | Statistics | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Combined Mean (SD) N = 47 | Males Mean (SD) N = 25 | Females Mean (SD) N = 22 | Combined Mean (SD) N = 31 | Males Mean (SD) N = 17 | Females Mean (SD) N = 14 | Diagnosis F | Gender F | Interaction Diag./Gen. F | |
| WAIS IQ Indices | 2.15a | 0.01a | 2.64a | ||||||
| Verbal IQ | 101.7 (15.0) | 99.8 (16.2) | 103.7 (13.6) | 108.6 (12.9) | 110.4 (13.1) | 106.4 (12.9) | 4.01* | 0.00 | 1.40 |
| Performance IQ | 95.6 (16.4) | 97.0 (19.2) | 94.1 (12.7) | 98.8 (13.2) | 97.8 (12.6) | 99.9 (14.3) | 0.89 | 0.01 | 0.49 |
| Full Scale IQ | 99.0 (15.5) | 98.6 (17.9) | 99.6 (12.7) | 104.4 (12.0) | 105.2 (12.3) | 103.4 (12.1) | 2.40b | 0.01b | 0.18b |
| WAIS Indices | 8.80***,a | 1.78a | 2.13a | ||||||
| Verbal Comprehension Index | 104.8 (16.3) | 102.3 (17.8) | 107.7 (14.3) | 110.6 (14.2) | 110.3 (14.0) | 110.9 (15.0) | 2.40 | 0.71 | 0.44 |
| Perceptual Organization Index | 97.7 (16.6) | 99.9 (18.9) | 95.2 (13.5) | 97.9 (15.2) | 97.1 (15.1) | 98.9 (15.8) | 0.01 | 0.15 | 0.75 |
| Working Memory Index | 95.9 (11.7) | 94.8 (13.2) | 97.1 (9.8) | 106.3 (14.2) | 110.7 (16.2) | 101.0 (9.3) | 11.70*** | 1.67 | 4.22* |
| Processing Speed Index | 89.6 (13.8) | 88.2 (13.7) | 91.1 (14.0) | 103.8 (11.2) | 103.7 (10.7) | 104.0 (12.2) | 22.29*** | 0.31 | 0.19 |
| Wechsler Memory Scale – Revised Indices | N = 48 | N = 25 | N = 23 | N = 27 | N = 13 | N = 14 | 1.71a | 1.26a | 0.60a |
| Verbal Index | 90.0 (15.3) | 89.4 (17.1) | 90.6 (13.6) | 95.3 (14.0) | 98.8 (14.0) | 92.1 (13.8) | 2.25 | 0.59 | 1.20 |
| Visual Index | 100.6 (18.1) | 99.3 (17.9) | 102.0 (18.6) | 99.0 (15.2) | 97.5 (13.7) | 100.4 (16.9) | 0.17 | 0.45 | 0.00 |
| Attention Index | 91.3 (16.5) | 94.7 (17.9) | 87.7 (14.3) | 96.8 (17.0) | 99.9 (17.6) | 93.9 (16.6) | 2.08 | 2.69 | 0.02 |
| Delayed Recall Index | 94.5 (14.5) | 93.5 (15.4) | 95.7 (13.7) | 99.5 (15.0) | 102.1 (18.7) | 97.1 (10.6) | 2.00 | 0.16 | 0.99 |
| General Index | 91.9 (16.4) | 90.6 (18.5) | 93.4 (14.0) | 100.4 (17.1) | 99.0 (14.6) | 101.6 (19.7) | 4.41*b | 0.43b | 0.00b |
| Wisconsin Card Sort Test | N = 45 | N = 24 | N = 21 | N = 19 | N = 12 | N = 7 | 1.86a | 0.07a | 0.74a |
| Error % | 30.0 (16.1) | 30.2 (13.6) | 30.9 (19.2) | 20.6 (13.7) | 21.4 (16.0) | 19.2 (9.2) | 5.33* | 0.03 | 0.11 |
| Perseverative response % | 15.6 (9.5) | 17.2 (8.7) | 14.7 (10.9) | 10.2 (6.9) | 9.9 (7.4) | 10.5 (6.6) | 4.97* | 0.15 | 0.36 |
| Perseverative error % | 17.7 (12.1) | 19.7 (10.9) | 16.3 (13.6) | 11.2 (8.0) | 10.8 (8.6) | 11.8 (7.7) | 4.52* | 0.14 | 0.48 |
| Nonperseverative error % | 14.4 (10.9) | 13.0 (6.6) | 16.2 (14.1) | 10.4 (7.8) | 11.4 (9.3) | 8.6 (4.3) | 2.60 | 0.01 | 1.13 |
| Trail Making Test | N = 50 | N = 30 | N = 20 | N = 29 | N = 14 | N = 15 | 3.80*a | 0.32a | 0.07a |
| Trail A Time | 41.2 (22.3) | 39.4 (13.7) | 44.0 (31.3) | 29.1 (12.0) | 27.6 (12.4) | 30.5 (11.8) | 7.70** | 0.65 | 0.04 |
| Trail B Time | 98.1 (68.0) | 93.8 (42.7) | 104.5 (95.2) | 77.5 (45.5) | 77.5 (60.8) | 77.4 (26.7) | 2.25 | 0.14 | 0.14 |
| NART Errors | 28.9 (14.7) | 31.4 (14.1) | 25.8 (15.1) | 27.0 (10.9) | 29.9 (10.3) | 24.1 (11.1) | 0.27b | 3.29b | 0.00b |
Olfaction and Cognition
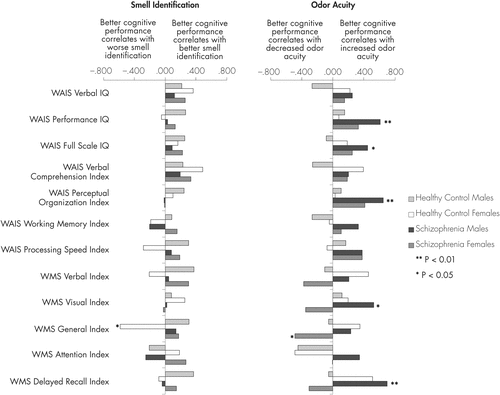
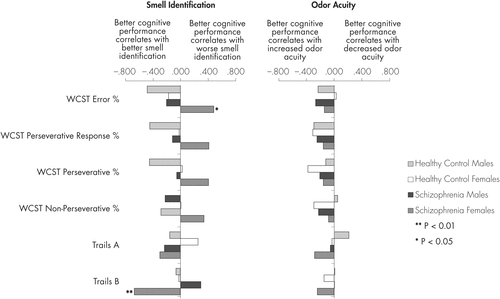
Associations between the two measures of olfaction and each of the cognitive tests are shown in Figures 1 and 2, together with Table 3. The figures show the correlation coefficients for patients and controls, with the sexes separated. Table 3 shows the correlation coefficients for the patients with the sexes combined, and tests whether the associations differ between the sexes. In a parallel analysis of the controls with the sexes combined (data not shown) there were no statistically significant findings.
| Smell Identification | Odor Acuity | |||||||
|---|---|---|---|---|---|---|---|---|
| N | Cognitive variable alone | Interaction with sexa | N | Cognitive variable alone | Interaction with sexa | |||
| r | p | p | r | p | p | |||
| WAIS | ||||||||
| Verbal IQ | 45 | 0.195 | - | 0.020 | 38 | 0.220 | - | - |
| Performance IQ | 45 | 0.027 | - | 0.015 | 38 | 0.419 | 0.002 | - |
| Full Scale IQ | 45 | 0.134 | - | 0.015 | 38 | 0.338 | 0.022 | - |
| Verbal Comprehension Index | 46 | 0.286 | - | 0.021 | 38 | 0.184 | - | - |
| Perceptual Organization Index | 45 | −0.062 | - | 0.021 | 38 | 0.461 | 0.0005 | 0.048 |
| Working Memory Index | 45 | −0.050 | - | 0.009 | 38 | 0.220 | - | - |
| Processing Speed Index | 45 | 0.153 | - | 0.020 | 38 | 0.386 | 0.019 | - |
| WMS | ||||||||
| Verbal Index | 45 | 0.161 | - | 0.020 | 39 | 0.083 | - | - |
| Visual index | 45 | 0.025 | - | 0.036 | 39 | 0.002 | - | 0.006 |
| General Index | 46 | 0.180 | - | 0.038 | 39 | −0.113 | - | 0.022 |
| Attention Index | 45 | −0.094 | - | 0.019 | 39 | 0.101 | - | - |
| Delayed Recall Index | 45 | 0.072 | - | 0.028 | 39 | 0.133 | - | 0.001 |
| WCST | ||||||||
| Error % | 45 | 0.161 | - | 0.008 | 39 | −0.186 | - | - |
| Perseverative Response % | 45 | 0.092 | - | 0.010 | 39 | −0.220 | - | - |
| Perseverative Error % | 41 | 0.123 | - | 0.018 | 36 | −0.207 | - | - |
| Non-Perseverative Error % | 45 | 0.150 | - | 0.013 | 39 | −0.084 | - | - |
| Trails A | 48 | −0.213 | - | 0.030 | 42 | −0.202 | - | - |
| Trails B | 46 | −0.224 | - | 0.006 | 41 | −0.155 | - | - |
Smell identification and cognition
Correlations between cognitive variables and odor identification (UPSIT) are shown on the left side of Figure 1 and Figure 2 and the left side of Table 3. A salient feature seen in both of the figures is the consistency of the differences between the male and female patients. In Figure 1, the female patients showed larger correlation coefficients than those of the male patients for almost all of the cognitive measures, their direction being positive (i.e. better cognitive performance correlating with better odor identification). Although none of the individual correlation coefficients in patients was statistically significant, either for males or females (Figure 1) or for the sexes combined (Table 3), the cognitive scores’ relation to UPSIT differed significantly between male and female patients when tested in a multiple regression analysis (Table 3). In Figure 1, findings in the controls were more variable. A few of the correlations for female controls were in the opposite direction from those of male controls, notably those for working, verbal, and general memory, and processing speed, where better cognitive performance in females correlated with poorer odor identification. There was, however, no consistent pattern comparing the sexes in controls; there were no significant sex differences in controls; and sex-specific correlations in the controls were not significant, with the exception of the General Index for the WMS-R (p=0.002).
For the Wisconsin Card Sorting Test scores (Figure 2), the direction of the association with UPSIT differed between the sexes. While for male patients (and even more, the male controls) those with better executive function tended to have better odor identification, while for female patients the reverse was true. On the other hand, on the Trails Making Test (Figure 2) the female patients showed stronger correlations between better cognitive performance and better odor identification; for the Trails B the individual correlation coefficient was highly significant for the female patients (p=0.002), the coefficient for male cases was in the reverse direction and this sex difference was unlikely to be due to chance, as seen in Table 3.
Odor acuity and cognition
Correlations between the cognitive tests and odor acuity are shown on the right side of the Figures and the right side of Table 3. More sensitive odor acuity predicted better scores for Performance IQ (p=0.004) and Full Scale IQ (p=0.047), and Perceptual Organization Index (p=0.002) in schizophrenia males (Figure 1). In female patients, these scores’ relations with odor acuity were only half as strong. They were in the same direction as the males’ scores, however, and the corresponding scores for the sexes combined were highly significant (Table 3). Furthermore, the difference between sexes was significant for the Perceptual Organization Index. Both male and female patients with more sensitive odor acuity had evidence of faster processing speed, this being significant in the combined group of patients (p=0.019).
Regarding the memory scores in the schizophrenia patients (Figure 1), males’ and females’ correlations with odor acuity were all in opposite directions and tended to differ significantly. While none of the scores calculated with the sexes combined was significant in the patients (Table 3), Figure 1 shows that better memory scores in male patients correlated with better odor threshold, notably in the Visual Memory Index (p=0.024) and Delayed Recall (p=0.001); for both of these, a Fisher’s test using r to z transformation showed that the sex difference was significant (p<0.007). Similarly, the General Memory Index in female patients was significant alone (p=0.024) and significantly different from the opposing score in males (p<0.03, Fisher’s r to z transformation). These sex differences are confirmed in Table 3 where the estimates for interactions of sex with the cognitive measures were derived from multiple regression analyses.
In contrast to the findings for patients, the relation between the cognitive and the olfaction tasks was more variable in controls. Differences between the sexes were inconsistent in controls and none of the sex-specific correlations was significant. Both male and female controls showed a negative correlation between odor acuity and attention, i.e. better attention correlated with poor odor acuity. With the sexes combined, this was significant (r=-0.457, p=0.016).
Correlations between odor acuity and executive function (WCST) are shown in Figure 2. Male and female patients’ correlations were in the same direction, better executive functioning correlating with better odor threshold; generally, the controls showed the same trend. In the patients, the males’ correlations were slightly stronger than those of the females, but none of these findings was significant. On the Trails Making Test A and B, females’ correlations with odor threshold were slightly stronger than those of the males, but again, none was significant.
Conclusions were unchanged in multiple regression models controlling each olfactory measure for the other measure, or controlling for patients’ ages, age at onset or education.
Discussion
The results of this study suggest that odor detection and smell identification are both associated with cognitive performance in patients with schizophrenia. However, this association is dissimilar and significantly modified by sex.
Female and male patients’ correlations between olfactory tasks and cognition significantly differed in the strength of the association for some measures, but were associated by sex for other measures. The most notable contradiction between the sexes occurred for the association of odor threshold with memory (WMS-R), showing opposing correlations in male and female patients (Figure 1). Another contradiction was found in smell identification and attention; male patients’ scores were positively associated with better performance on an executive functioning task (WCST) and faster processing speed (Trail Making Test B), whereas better smell identification scores in females were related to more errors on the executive functioning task (Figure 2). The female patients’ results seem to be counter-intuitive in that better cognitive performance correlated with lesser performance on the olfactory tasks. This may be due to females de-emphasizing olfactory processing in proportion to certain cognitive abilities, such as attention.
Sex differences were also observed in olfactory performance and other cognitive tasks. For smell identification, female patients had stronger correlations to intelligence scales (WAIS-III) than male patients, whereas for odor threshold the associations were weaker in the females than the males (Figure 1). Given that the sex differences, tested as statistical interactions, were highly significant (Table 3), these findings may imply fundamental differences in the mechanisms of olfactory processing between male and females. A differential modulation of olfactory processing in light of higher cognitive processing may have evolved in women to optimize sex-specific outcomes of mating, reproduction, and childrearing.
Our results comparing the means and distributions of the two olfactory measures in male and female patients and the results of comparisons between patient and control groups are compatible with previous studies (Table 1). We found that females performed better on smell identification and had somewhat greater acuity than males; schizophrenia patients of both sexes had lower smell identification scores than their sex-matched controls. For odor acuity, we found that the female patients’ scores were higher than those of the controls, but they also demonstrated a larger variance.
Studies on odor acuity in schizophrenia have revealed contrasting results. Some reports that acuity is elevated, whereas others indicate that acuity is decreased or similar to that of controls, leading to a consensus that it is unaltered in schizophrenia.3 Our finding of increased variability in odor acuity sheds light on this controversy. Enhanced odor acuity detection may reflect the reduced gating of sensory stimuli that is well described in the disease (see Martin et al35). The large variability in the measure is consistent with the presumed heterogeneity of schizophrenia.36
The negative association between odor threshold and attention in the controls (WMS-R attention index in Figure 1) was surprising as it suggests that decreased attention in healthy people might be related to hyper-acuity for odor detection. However, a compatible finding was reported in people with attention deficit disorder.37 Attention was not related to odor acuity in our schizophrenia patients, perhaps because of defective attention in the disease. Defective attention and failed inhibitory processes are closely associated with the pathogenesis of schizophrenia.38 Although historical precedents have traditionally considered odor identification and odor acuity to reflect central and peripheral processes respectively,39 the latter measure of olfaction also depends on the synapses of olfactory receptor neuron axons in the olfactory bulb, which is under the control of higher centers.40
Moreover, the fact that patients with schizophrenia show sex differences in the relation of olfaction to memory, attention, and other cognitive abilities corroborate results from other studies that have found sex differences in the relative size of brain structures involved in olfaction, and in their connectivity and function. A number of the same structures are abnormal in schizophrenia, on the one hand, and are functionally important in cognitive functioning, on the other hand. For example, females have greater gray matter in the orbital prefrontal cortex,41 a high level multimodal olfactory system that can inhibit subcortical regions; whereas males have greater gray matter in the entorhinal cortex that receives direct input from the olfactory bulb.24 Research shows that sex differences help explain why larger regional olfactory brain volumes variably predict enhanced or reduced olfactory function.42 One study found that stimulation from background odors impaired the reaction time performance of healthy males, but not females.43 Other variability may be related to the etiological heterogeneity of schizophrenia. Smell identification has been associated with both the orbitofrontal and the dorsolateral prefrontal circuitry in patients with schizophrenia.4,8,44 These regions play greater roles in metacognitive executive function versus emotional/motivational function, respectively.45 Dysfunction in either brain circuit is reported in individual patients with schizophrenia.46
A particular strength of our study is that the patients and the controls were as well matched cognitively as may be possible. They differed in working memory, processing speed, verbal intelligence and general memory; deficits which are part and parcel of neurocognitive impairment profiles in schizophrenia.47,48 It is noteworthy that olfaction was significantly associated with these subtle cognitive deficits, even though the mean threshold and smell identification scores did not significantly distinguish between cases and controls in this sample.
There are several limitations with this study that bear some discussion. We used only phenyl ethyl alcohol (PEA) to test acuity. Although the thresholds for detecting different odorants are highly correlated in humans,49 other odorants should be examined in future studies. It should also be noted that our cases were all on stable medications and in an optimized clinical state. Although there is no evidence linking medication status to performance on smell identification,50 a medication effect has been reported for causing asymmetrical olfactory thresholds in schizophrenia.51 With regard to verbal cognitive performance, we and other groups have reported significant links between smell identification and verbal ability.11,25,52 These associations were positive in all of our groups, although the relationships did not reach significance. Also, studies in nonschizophrenia populations have shown that reduced olfactory functioning in healthy subjects is related to poor cognitive processing speed, attention and working memory, vocabulary level, reasoning ability, confrontation naming, verbal memory, and general cognitive impairment (e.g. Dulay et al53). Our sample size of healthy subjects was too small to uncover any of these relationships in our data. Despite less education in the schizophrenia patients, their similar intelligence scores suggest that less education in the schizophrenia patients may be an artifact of the illness, likely due to avolition or other symptoms. Therefore, we did not adjust the analyses for education; although doing so produced similar results (data not shown). We also did not separately analyze threshold data from right and left nostrils, since we were examining cognitive associations and information from both nostrils converge at the anterior olfactory nucleus.54 Event-related potential studies of central olfactory processing also do not reveal main effects for right versus left nostril odor presentations.55,56 We did not control for menstrual cycle, which could theoretically modulate differential relationships of odor threshold and smell identification to cognitive functioning between males and females. Lastly, we included both patients with schizophrenia and schizoaffective disorder in the study. As both disorders are heterogeneous, future studies should examine olfaction, cognitive functioning, and sex differences in more homogeneous schizophrenia groups.
In summary, olfactory processing is implicated in social, sexual and other goal-directed human behaviors, including cognitively demanding tasks. This link between olfaction and cognitive functioning, such as memory and attention, is sexually dimorphic. Sexually dimorphic features are present in individuals with schizophrenia as well. The failure of research studies to account for these dimorphic features may underlie some of the roadblocks that have been encountered in etiological research and treatment studies (e.g. treatment of negative symptoms). Our present study highlights that olfactory processing is an appealing model in which to study the links between perception and cognitive functioning in schizophrenia, a disorder that includes deficits in both olfactory processing and cognitive abilities. By combining male and female groups to study olfaction, real and important differences between the patient and control groups may be masked. Our findings make it clear that research aiming to discover differences between schizophrenia patients and controls should separately consider the sexes. Combining them is likely to obscure the observation of abnormalities that are sexually dimorphic. Sex stratification may similarly enhance the utility of olfactory research in other neuropsychiatric disorders as well.
1 : Scent of a disorder: olfactory functioning in schizophrenia. Curr Psychiatry Rep 2003; 5:311–319Crossref, Medline, Google Scholar
2 : Sex differences in olfactory function in schizophrenia. Am J Psychiatry 1989; 146:1320–1322Crossref, Medline, Google Scholar
3 : Olfaction: a potential cognitive marker of psychiatric disorders. Neurosci Biobehav Rev 2008; 32:1315–1325Crossref, Medline, Google Scholar
4 : Neuropsychological, olfactory, and hygiene deficits in men with negative symptom schizophrenia. Biol Psychiatry 1996; 40:1021–1031Crossref, Medline, Google Scholar
5 : Olfactory deficits, cognition and negative symptoms in early onset psychosis. Schizophr Res 2005; 80:283–293Crossref, Medline, Google Scholar
6 : Olfaction and social drive in schizophrenia. Arch Gen Psychiatry 2003; 60:578–584Crossref, Medline, Google Scholar
7 : Odor identification, eye tracking and deficit syndrome schizophrenia. Biol Psychiatry 2002; 51:809–815Crossref, Medline, Google Scholar
8 : Odor discrimination deficits in schizophrenia: association with eye movement dysfunction. J Neuropsychiatry Clin Neurosci 1994; 6:273–278Link, Google Scholar
9 : Associations between olfactory identification and verbal memory in patients with schizophrenia, first-degree relatives, and non-psychiatric controls. Schizophr Res 2006; 86:154–166Crossref, Medline, Google Scholar
10 : Olfactory functioning in schizophrenia: relationship to clinical, neuropsychological, and volumetric MRI measures. J Clin Exp Neuropsychol 2006; 28:1444–1461Crossref, Medline, Google Scholar
11 : Olfactory identification and WAIS-R performance in deficit and nondeficit schizophrenia. Schizophr Res 2004; 69:55–65Crossref, Medline, Google Scholar
12 : Sex bias in neuroscience and biomedical research. Neurosci Biobehav Rev 2011; 35:565–572Crossref, Medline, Google Scholar
13 : Why sex matters for neuroscience. Nat Rev Neurosci 2006; 7:477–484Crossref, Medline, Google Scholar
14 : Olfactory sensitivity to androstenone in schizophrenic patients. Biol Psychiatry 1987; 22:922–925Crossref, Medline, Google Scholar
15 : Various bilateral olfactory deficits in male patients with schizophrenia. Schizophr Bull 2005; 31:155–165Crossref, Medline, Google Scholar
16 : Olfactory sense in psychoses. Biol Psychiatry 1990; 28:830Crossref, Medline, Google Scholar
17 : Increased olfactory sensitivity in first episode psychosis and the effect of neuroleptic treatment on olfactory sensitivity in schizophrenia. Psychiatry Res 1999; 86:143–153Crossref, Medline, Google Scholar
18 : Olfactory acuity to a pheromonal substance and psychotic illness. Biol Psychiatry 1984; 19:899–905Medline, Google Scholar
19 : Olfactory acuity in the positive and negative syndromes of schizophrenia. Biol Psychiatry 1991; 29:774–778Crossref, Medline, Google Scholar
20 : Left nostril olfactory identification impairment in a subgroup of male patients with schizophrenia. Schizophr Res 1998; 33:35–43Crossref, Medline, Google Scholar
21 : Olfactory dysfunction in schizophrenia and temporal lobe epilepsy. Neuropsychiatry Neuropsychol Behav Neurol 2001; 14:83–88Medline, Google Scholar
22 : Olfactory deficits in neuroleptic naive patients with schizophrenia. Schizophr Res 1993; 8:245–250Crossref, Medline, Google Scholar
23 : Left temporo-limbic and orbital dysfunction in schizophrenia during odor familiarity and hedonicity judgments. Neuroimage 2006; 29:302–313Crossref, Medline, Google Scholar
24 : Sex differences in the human olfactory system. Brain Res 2006; 1116:103–111Crossref, Medline, Google Scholar
25 : Sex differences in olfactory identification and Wisconsin Card Sorting performance in schizophrenia: relationship to attention and verbal ability. Biol Psychiatry 1997; 42:104–115Crossref, Medline, Google Scholar
26 : Scents and nonsense: olfactory dysfunction in schizophrenia. Schizophr Bull 2009; 35:1117–1131Crossref, Medline, Google Scholar
27 Association AP: Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition-Text Revision). Washington D.C., APA, 2000.Google Scholar
28 Nurnberger JI, Jr., Blehar MC, Kaufmann CA, et al. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH Genetics Initiative. Arch Gen Psychiatry. 1994;51(11):849-859; discussion 863-844.Google Scholar
29 : Development of the University of Pennsylvania Smell Identification Test: a standardized microencapsulated test of olfactory function. Physiol Behav 1984; 32:489–502Crossref, Medline, Google Scholar
30 Wechsler D: Wechsel Adult Intelligence Scale, Third Edition (WAIS-III) administration and scoring manual. San Antonio, TX, The Psychological Corporation, 1997.Google Scholar
31 : Wechsler Memory Scale, Revised (WMS-R). San Antonio, TX, Psychological Corporation, 1987Google Scholar
32 : Wisconsin Card Sorting Test: Computer version-2 research edition. Odessa, FL, Psychological Assessment Resources, 1993Google Scholar
33 : Validity of the Trail Making Test as an indication of organic brain damage. Percept Mot Skills 1958; 8:271–276Crossref, Google Scholar
34 : National Adult Reading Test (NART): Test Manual. Windsor, UK, NFER Nelson, 1982Google Scholar
35 : Alpha-7 nicotinic receptor agonists: potential new candidates for the treatment of schizophrenia. Psychopharmacology (Berl) 2004; 174:54–64Crossref, Medline, Google Scholar
36 : Schizophrenia, “just the facts” 4. Clinical features and conceptualization. Schizophr Res 2009; 110:1–23Crossref, Medline, Google Scholar
37 : Improved odor sensitivity in attention-deficit/hyperactivity disorder. Biol Psychiatry 2008; 64:938–940Crossref, Medline, Google Scholar
38 : The P50 auditory event-evoked potential in adult attention-deficit disorder: comparison with schizophrenia. Biol Psychiatry 2000; 47:969–977Crossref, Medline, Google Scholar
39 : Olfactory dysfunction in neuropsychiatric disorders: review and methodological considerations. Biol Psychiatry 1997; 42:721–732Crossref, Medline, Google Scholar
40 : Olfactory plasticity: one nostril knows what the other learns. Nature 2002; 419:802Crossref, Medline, Google Scholar
41 : Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cereb Cortex 2001; 11:490–497Crossref, Medline, Google Scholar
42 : Odor localization and sniffing. Chem Senses 2009; 34:139–144Crossref, Medline, Google Scholar
43 : A gender difference related to the effect of a background odor: a magnetoencephalographic study. J Neural Transm 2009; 116:1227–1236Crossref, Medline, Google Scholar
44 : Olfactory identification deficiency and WCST performance in men with schizophrenia. Psychiatry Res 1998; 81:251–257Crossref, Medline, Google Scholar
45 : On the evolutionary origins of executive functions. Brain Cogn 2008; 68:92–99Crossref, Medline, Google Scholar
46 : Divergent plasticity of prefrontal cortex networks. Neuropsychopharmacology 2008; 33:42–55Crossref, Medline, Google Scholar
47 : The assessment of neuropsychological functioning in schizophrenia. Dialogues Clin Neurosci 2010; 12:383–392Medline, Google Scholar
48 : Neuropsychological functioning in first-episode schizophrenia. Br J Psychiatry 2009; 195:336–345Crossref, Medline, Google Scholar
49 : Human olfaction: from genomic variation to phenotypic diversity. Trends Genet 2009; 25:178–184Crossref, Medline, Google Scholar
50 : Olfactory dysfunction in schizophrenia: a qualitative and quantitative review. Neuropsychopharmacology 1999; 21:325–340Crossref, Medline, Google Scholar
51 : Asymmetrical olfactory acuity and neuroleptic treatment in schizophrenia. Schizophr Res 2000; 44:221–232Crossref, Medline, Google Scholar
52 : Neuropsychological probes of fronto-limbic system dysfunction in schizophrenia. Olfactory identification and Wisconsin Card Sorting performance. Schizophr Res 1991; 6:55–65Crossref, Medline, Google Scholar
53 : Assessment of the influence of cognition and cognitive processing speed on three tests of olfaction. J Clin Exp Neuropsychol 2008; 30:327–337Crossref, Medline, Google Scholar
54 : Compensatory rapid switching of binasal inputs in the olfactory cortex. J Neurosci 2008; 28:11989–11997Crossref, Medline, Google Scholar
55 : Chemosensory event-related potentials in relation to side of stimulation, age, sex, and stimulus concentration. Clin Neurophysiol 2006; 117:1367–1375Crossref, Medline, Google Scholar
56 : Laterality of the olfactory event-related potential response. Chem Senses 2006; 31:699–704Crossref, Medline, Google Scholar



