The Association of BDNF Val66Met Polymorphism With Trait Anxiety in Panic Disorder
Abstract
Recent studies indicate that early-onset panic disorder (PD) may show distinct clinical characteristics. The authors compared patients with early-onset PD, patients with late-onset PD, and healthy control subjects in terms of brain-derived neurotrophic factor (BDNF), the Val66Met polymorphism, State-Trait Anxiety Inventory (STAI) scores, and the Revised NEO Personality Inventory. In patients with early-onset PD, the STAI-T score was high in the Met/Met group, whereas the STAI-T score of the Val/Val group tended to be higher for healthy control subjects. The conflicting effect of the BDNF genotype between patients with early-onset PD and healthy control subjects suggests that the BDNF Met/Met genotype may increase trait anxiety in early-onset PD.
Panic disorder (PD) is an anxiety disorder characterized by panic attacks preceded by anxiety with sudden, unanticipated fear. The incidence rate ranges from 1% to 3%, with frequent comorbidity with agoraphobia. Familial aggregation has been shown by genetic epidemiology research on anxiety disorder, and several lineage studies have been carried out, one of which showed that the proportion of PD among relatives was 24.4% in the line of direct descent from patients with PD, whereas the prevalence of PD was about 2.2% in a healthy control group.1 The incidence rate in the first-degree relatives of patients with PD was found to be eightfold compared with that of a normal control group in a large-scale investigation.2 Additionally, a twin study of the disease suggests that there is a hereditary factor that contributes to the onset of PD. The identity rate of PD in one twin study was 24% in identical twins and 11% in fraternal twins, and the estimated heritability of PD has been found to be about 43%.3
These various lines of evidence suggest that genetic factors might influence vulnerability to PD. One plausible genetic risk factor involves brain-derived neurotrophic factor (BDNF), a protein hypothesized to limit or repair the damage caused by stress. BDNF plays a key role in memory and anxiety in the brain. The concentration of serum BDNF has been found to be higher among healthy control subjects than among patients with PD,4 corresponding to lower serum levels of BDNF with high sensitivity for anxiety.5 In depressed subjects, low serum BDNF levels tend to increase with antidepressant treatment.6
BDNF is first synthesized as a proBDNF, which is converted into mature BDNF by the protease.7 ProBDNF binds to the p75 neurotrophin receptor and facilitates hippocampal long-term depression. Mature BDNF binds to TrkB receptor through which hippocampal synaptic potentiation is facilitated.7 BDNF is located in chromosome 11p3. One frequent, nonconservative polymorphism in the human BDNF gene (dbSNP number rs6265) has been identified. A single nucleotide polymorphism (SNP) at nucleotide 196 (G/A) produces an amino acid substitution (valine to methionine) at codon 66 (Val66Met) in proBDNF.8 The proportion of T allele (Met) carriers is about 41% among Japanese and Asians and about 18% among whites.9
Several recent studies on the relationship between the functional BDNF Val66Met variant and psychopathology in humans have yielded conflicting results.
A haplotype incorporating the Val allele has been associated with bipolar disorder in whites10,11 but not in Asians.12,13 The Met allele has been associated with obsessive compulsive disorder (OCD)14 and strongly associated with restrictive type anorexia nervosa.15 In a study on geriatric depression, there was a significant excess of the Met allele in depressed patients compared with the control group.16 In other studies, the Met allele appeared to be protective against bipolar disorder10 and OCD.17 Previous studies have consistently shown no association of Val66Met with PD in Japanese and Chinese populations.9,18,19
In human subjects, there is a difference in anxiety sensitivity and memory function among those with Val66Met polymorphism. The Met allele has been associated with poorer episodic memory in a stressful setting.20 It could be expected that BDNF variation might influence behavior and anxiety.
One study has shown that the Val allele is associated with greater neuroticism,21 whereas another found that there is no difference in harm avoidance between the genotypes.22 The Met allele was found to be associated with increased harm avoidance and was most abundant in individuals with both an anxiety disorder and major depression.23 In the trait-related anxiety score of healthy subjects, a significant effect of the genotype was observed with higher levels of trait anxiety in those with the Val/Val genotype compared with those with either the Val/Met or Met/Met genotype.24 The BDNF Val/Val genotype is associated with the Revised NEO Personality Inventory (NEO-PI-R) Neuroticism domain,21 and serum levels of BDNF are conversely low at Val/Val.25
An animal study using Met mice showed that the variant BDNF Val66Met polymorphism affects the extinction of conditioned aversive memory,26 and BDNF (Met/Met) mice exhibited increased anxiety-related behaviors.27 BDNF Met carriers may play a crucial role in the increase of anxiety sensitivity, and those with Met/Met polymorphism may be more sensitive to stress-induced down-regulation of BDNF.28
Early life stress sometimes causes mental illness and may lead to a younger age at onset in PD.29 Anticipation, onset age, and familial aggregation have been studied in PD.30 The risks of PD in adult first-degree relatives of probands with PD onset at or before 20 years of age and after 20 years of age were found to be increased 17-fold and sixfold, respectively, compared with adult first-degree relatives of normal control subjects,30 and the age at onset of PD in parents and children was 30.1 and 20.8 years old, respectively.31 The association with a high frequency of suicide attempts in those with early-onset PD (age at onset ≤25 years) compared with late-onset PD (age at onset >25 years) indicates clinical severity in early-onset PD.32 In a study that compares early-onset panic (panic onset <50 years of age) with late onset (onset ≥50 years of age), late-onset panic attacks were associated with less utilization of mental health services, lower levels of comorbidity, and less hypochondriasis.33 In patients with early-onset PD, increased severity of clinical symptoms and higher prevalence of agoraphobia were found.30
In this context, we hypothesized that the complex regulation of the BDNF genotype is related to the characteristics of PD that might be modulated with onset age. In the present study, we examined the association between BDNF polymorphism and trait anxiety in patients with early- and late-onset PD and healthy control subjects. We considered a cutoff age of 30 years suitable because the phenotype of anxiety-related personality traits would be established around this age and identifiable on examination. Together with previous literature reports,32,33 the sample was subdivided in two groups according to age at onset: an early-onset PD group (<30 years) and late-onset PD group (≥30 years).
Methods
Participants
The subjects in the present study were 252 patients with PD (83 men and 169 women) divided into early- (N=152) and late-onset (N=100) groups and 191 healthy control subjects (78 men and 113 women). All subjects provided their written informed consent. In the healthy control group, the inclusion criteria were as follows: drug free, no previous diagnosis with a psychiatric disorder, and no family history of psychiatric disorder. We excluded those who had a history of a major physical illness, neurological disorder, alcohol abuse, substance abuse, or loss of consciousness due to head injury. The healthy control group was screened for the presence or absence of a DSM-IV axis I disorder using the Japanese version of the Mini International Neuropsychiatric Interview 5.0.0. All patients with a DSM-IV diagnosis of PD were outpatients at the Nagoya Mental Clinic in Japan and were diagnosed by at least two doctors. Most patients were on medication.
This study was approved by the institutional ethics committees of the Mie University Graduate School of Medicine and the Warakukai Nagoya Mental Clinic.
Psychological Tests
Psychological tests were administered to the participants by questionnaire and by interview. The questionnaire was used to collect clinical information including basic data on the related PD and genetic factors, and the tests administered were as follows:
| 1. | Mini International Neuropsychiatric Interview 5.0.0: a structured interview used to assess psychiatric illness. Psychiatric illnesses including PD were screened. | ||||
| 2. | Fact sheet (sex, age, height, weight, blood type, birth place, growth history, body weight at birth, marital status, drinking habits, smoking habits, menstruation, medical past history, family medical history, disease under treatment). | ||||
| 3. | Questionnaire about PD (symptoms of first panic attack, frequency of panic attacks, avoidance of situations in which a panic attack may occur during the previous month). | ||||
| 4. | State-Trait Anxiety Inventory (STAI). An assessment of state anxiety and trait anxiety34 consisting of 20 items each. The participants answered the questions by marking the total 40 items as “very much,” “likely,” “not so,” or “not at all.” State anxiety reflects a transitory emotional state or “right now” condition. Trait anxiety represents stable individual differences in anxiety proneness and refers to a general tendency to respond with general anxiety. | ||||
| 5. | The NEO-PI-R developed by Costa and McCrae.35 The NEO-PI-R is a standard instrument for measuring the personality traits of individuals of widely ranging ages, from the elderly to the young, based on a five-factor model of character: neuroticism (N), extroversion (E), openness to experience (O), agreeableness (A), and conscientiousness (C). The participants responded to the 240 items with “very much,” “likely,” “not both,” “not so,” or “not at all.” N signifies the subject’s level of adjustment relative to the level of maladaptiveness or the subject’s level of emotional security relative to the level of neuroticism. A person with a high N score tends to have unrealistic ideas, cannot control his or her anger easily, and is not adept at coping with stress induced by others. | ||||
Genotyping BDNF Val66Met Polymorphism
A 7-mL blood sample was centrifuged at 2000 rpm for 10 minutes in a blood-collecting tube and separated into the plasma layer, the buffy-coat layer (which contains white blood corpuscles), and the red blood corpuscle layer. A 150-μL sample of the buffy coat was used for automatic blood DNA extraction. DNA was extracted using a BioRobot EZ1 (Qiagen, Hilden, Germany). BDNF G196A (Val66Met) polymorphisms (rs6265) were genotyped using allelic discrimination by TaqMan real-time polymerase chain reaction (PCR) assay. The assay includes a sequence-specific fluorogenic minor groove binder probe for each allele. Each probe is 5′-labeled with a different reporter fluorescent dye (4,7,2′-trichloro-7′-phenyl-6-carboxyfluorescein and 6-carboxyfluorescein) to differentiate the amplification of each allele. Primer−probe sets for the detection of the polymorphisms were purchased from Applied Biosystems (Foster City, CA). PCR was carried out on an ABI StepOnePlus Real-Time PCR System according to the manufacture’s protocol (Applied Biosystems).
Statistics
Statistical calculations were carried out using SPSS for Windows (Release 17.0; Armonk N.Y., IBM). The chi-square test or Fisher’s exact test was used for the analysis of categorical data, and the Student t test and analysis of variance (ANOVA) were used for continuous variables with normal distributions followed by a post hoc Scheffe test. The effects of genotype and onset age on personality variables were computed with a two-way ANOVA. All tests were performed with a two-tailed type-I error rate of p<0.05.
Results
There was no significant difference in the sex ratio between the PD (N=252: 83 men and 169 women) and health control (N=191: 78 men and 113 women) groups (χ2=2.93, p=0.087). The mean ages and sex ratios did not differ significantly among the three groups (Table 1). The average age of the PD group was 36.21±8.69 years old, and the average age of the health control group was 34.85±10.78 years old (t=1.424, p=0.155; Table 1). The average age at onset of PD was 27.40±8.76 years old. For statistical analysis, the sample was subdivided into two groups according to age at onset: the early-onset PD group (<30 years) and the late-onset PD group (≥30 years). The cutoff age between 29 and 30 years is close to the average age at onset. The 252 patients with PD were thus divided into 152 patients with early-onset PD and 100 patients with late-onset PD. The average onset ages of the early- and late-onset PD groups were 21.99±5.17 and 35.62±6.37 years, respectively. The average age of the early-onset PD group was 32.73±8.28 years, which was younger than that of the health control group (t=−2.063, p=0.04), and that of the late-onset PD group was 41.50±6.34 years, which was older than health control subjects (t=−9.488, p<0.001).
| Group | N | Age, Years (Mean±Standard Deviation) | Sex, N (%) | Genotypes, N (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| pb | Men | Women | pb | V/V | V/M | M/M | pb | |||
| Control subjects | 191 | 34.85±10.78 | NA | 78 (40.8%) | 113 (59.2%) | NA | 65 (34%) | 90 (47%) | 36 (19%) | NA |
| Patients with PD | 252 | 36.21±8.69 | 0.155 | 83 (32.9%) | 169 (67.1%) | 0.087 | 83 (33%) | 115 (46%) | 54 (21%) | 0.799 |
| Patients with early-onset PD (<30) | 152 | 32.73±8.28 | 0.040 | 42 (27.6%) | 110 (72.4%) | 0.01 | 50 (33%) | 65 (43%) | 37 (24%) | 0.451 |
| Patients with late-onset PD (≥30) | 100 | 41.50±6.34 | <0.001 | 41 (41%) | 59 (59%) | 0.97 | 33 (33%) | 50 (50%) | 17 (17%) | 0.879 |
TABLE 1. Comparison of the Mean Age, Sex, BDNF Val66Met Genotype Distribution, and Allele Frequency Between the Patient and Control Groupsa
The comorbidity rates of agoraphobia in the early-onset group and late-onset group were 74.34% and 67.65%, respectively, which showed no statistical significance as determined by the chi-square test (p=0.333). The comorbidity rates in these two groups for past depression were 12.39% and 4.41% (p=0.113), respectively, and for present ,depression they were 19.47% and 14.71% (p=0.416), respectively. For OCD, the comorbidity rates were 8.85% and 2.94% (p=0.215) for the early-onset group and late-onset group, respectively. For posttraumatic stress disorder (PTSD), the comorbidity rates were 3.54% and 0% (p=0.299) for these two groups, respectively,
The PD group showed allele frequencies of 33% Val/Val, 46% Val/Met, and 21% Met/Met, and the health control group showed 34% Val/Val, 47% Val/Met, and 19% Met/Met, giving a genotype distribution that was within Hardy-Weinberg equilibrium (p=0.233 for patients with PD, p=0.625 for health control subjects). No significant difference was observed in the frequency of gene polymorphism between the PD and health control groups (χ2=0.446, p=0.799).
The genotype frequencies of the PD subgroups were 33% Val/Val, 43% Val/Met, and 24% Met/Met for the early-onset group and 33% Val/Val, 50% Val/Met, and 17% Met/Met for the late-onset group. A comparison between the early- and late-onset groups found no statistically significant difference (χ2=2.20, p=0.331), nor were significant differences observed between the early-onset PD and health control groups (χ2=1.58, p=0.451) or between the late-onset PD and health control groups (χ2=0.256, p=0.879). The interaction between BDNF polymorphism and anxiety sensitivity score was evaluated in an ANOVA based on a factorial design, with the polymorphism being the independent variable and STAI trait score being the dependent variable (Table 2).
| STAI Trait | Val/Val | Genotype | F Value | p Value | Two-Way ANOVA | ||
|---|---|---|---|---|---|---|---|
| Val/Met | Met/Met | F Value | p Value | ||||
| Patients with early-onset PD | 50.61±14.63 | 52.61±11.54 | 58.28±12.95 | 3.72 | 0.026b | ||
| Patients with late-onset PD | 48.58±11.24 | 47.66±12.51 | 50.26±12.44 | 0.275 | 0.76 | ||
| Control subjects | 42.25±9.60 | 40.24±10.51 | 37.69±8.37 | 2.506 | 0.084 | 3.109 | 0.015b |
TABLE 2. Comparison of STAI Trait Between Patients With PD (Early-Onset and Late-Onset) and Healthy Control Subjects With Three Different BDNF Val66Met Genotype Groupsa
The ANOVA showed that the dependent variable of STAI trait score was significantly affected by genotype factor in the early-onset PD group (F=3.72, p=0.026). In the early-onset PD group, a significant difference was observed between Val/Val and Met/Met by post hoc Scheffe test (p=0.031) (Table 2; Figure 1). Considering group and BDNF genotype as two independent variables, two-way ANOVA revealed that STAI trait score was significantly affected by the interaction between subgroup and genotype (F=3.109, df=4, p=0.015; Table 2). With respect to the STAI state score and the five NEO-PI-R factor scores, two-way ANOVA showed no significant effect of genotype factor (STAI state: F=1.064, df=4, p=0.374; neuroticism: F=2.186, df=4, p=0.070; extroversion: F=1.743, df=4, p=0.140; openness: F=0.244, df=4, p=0.913; agreeableness: F=1.057, df=4, p=0.378; conscientiousness: F=0.414, df=4, p=0.798).
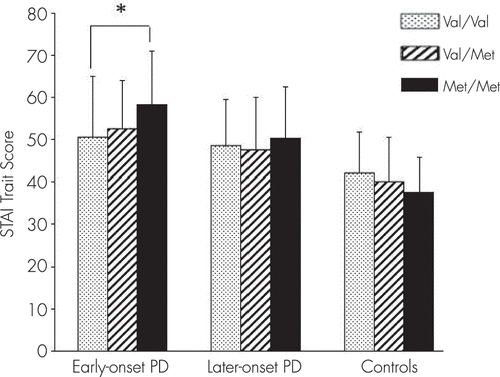
FIGURE 1. Comparison Between Genotype and Subgroups (Early-Onset PD, Late-Onset PD, Control Subjects) With Two-Way ANOVA for STAI Trait Score (p=0.015)a
aNo significant association between Val/Val and Met/Met in control subjects by Scheffe test (p=0.087). Note the trend of subgroups × genotype interaction by two-way ANOVA. ANOVA: analysis of variance; PD: panic disorder; STAI: State-Trait Anxiety Inventory. *p=post hoc Scheffe test (p=0.031).
STAI trait score was not affected by genotype factor in the late-onset PD group (p=0.76) according to ANOVA. However, a trend (p=0.084) of genotype effect on STAI trait was found in the health control group by ANOVA. In the health control group, the STAI trait score of Met/Met tended to be lower than that of Val/Val or Val/Met (Figure 1).
Discussion
STAI trait scores are related to earlier age of onset in anxiety disorders such as obsessive compulsive disorder.36 Trait anxiety as determined by STAI trait score is notably related to the hereditary influence by BDNF genotype.24 We studied the relationship between age of onset in PD and STAI trait scores. A comparison of genotype frequency between PD and health control subjects and between the early- and late-onset PD groups found no statistically significant differences. Furthermore, the genotype effect of BDNF Val66Met on PD was not remarkable; however, the present results showed different effects of BDNF genotype on STAI trait score for patients with early- and late-onset PD and health control subjects. Additional psychological tests such as STAI state and five factors of NEO-PI-R were conducted, but no significant relationships were observed.
In the early-onset PD group, the Met/Met STAI trait score was higher than that of Val/Val or Val/Met, whereas the Val/Val STAI trait score was higher in the health control group. With regard to STAI state score and the five NEO-PI-R domain scores, ANOVA found a less significant effect of genotype factor compared with STAI trait score. The present results show that the anxiety STAI trait score in health control subjects tended to be higher in the Val/Val group than in the Val/Met and Met/Met groups. Frustaci et al. carried out meta-analyses to evaluate the relationship between BDNF Val66Met polymorphism and anxiety-related personality traits of healthy people, finding that both Met/Met and Val/Met individuals, compared with Val/Val, showed a statistically significant lower neuroticism score.37 The Val/Val BDNF genotype is associated with the NEO-PI-R neuroticism domain in healthy subjects.21 Our results also showed a trend of higher neuroticism score in health control subjects. In the STAI study of personality traits and anxiety, the Val/Val group showed higher STAI trait anxiety scores than Val/Met or Met/Met among health control subjects.24 Additionally, Joffe et al. reported in a study on health control subject Met carriers that elevations in neuroticism, trait depression, and stress were associated with lower mean hippocampal volume,38 but there were no such associations in Val homozygotes. These results thus show contradictory effects among BDNF Val66Met genotypes.
In patients with early-onset PD, increased severity of clinical symptoms and a higher prevalence of agoraphobia have been shown.30 The early-onset PD group showed a higher rate of comorbidity, a finding that is consistent with a previous report showing that the early-onset PD group had higher rates of comorbidity with depression or bipolar disorder.39 The association with a high frequency of suicide attempts in early-onset PD (age at onset ≤25 years) compared with late-onset PD (>25 years) indicates clinical severity in early-onset PD.32 In a study that compared early- (<50 years) and late-onset (≥50 years) PD, late-onset panic attacks were found to be associated with less utilization of mental health services, lower levels of comorbidity, and less hypochondriasis.34 Additionally, early life stress sometimes causes mental illness and might lead to a younger age at onset in PD.29
A recent study showed the interactions between the BDNF Val66Met polymorphism and early life stress.40 The combination of the BDNF Val/Val genotype and early life stress predicted increases in the gray matter of the amygdala and associated medial prefrontal cortex, which in turn predicted startle-elicited heart rate variability and higher anxiety. In contrast, the combination of Met carrier status and exposure to early life stress predicted reduced gray matter in the hippocampus, a decline in working memory, hypofunction of the prefrontal lobe, and higher depression.40 Higher anxiety was linked to verbal memory and to impulsivity. The BDNF Met–early life stress interaction predicted elevated neuroticism and higher depression and anxiety by elevations in body arousal. Furthermore, the BDNF Met allele and stress were found to play an additional role in predicting syndromal depression and associated anxiety.40 Vulnerability to stress may be enhanced by the Met allele, which modulates the psychiatric symptoms of mental illness. The association of the BDNF genotype with early-onset anxiety disorder has been observed in PTSD and bipolar disorder, partly due to the experience of childhood abuse.41 Childhood abuse is also reported to be related with the early onset of generalized anxiety disorder.42 It would be worthwhile to study the association of early life stress with age at onset in PD to gain further understanding of the disease.
The results discussed above imply that the BDNF Val/Val genotype promotes anxiety as a result of a reaction against life stress in healthy people and that BDNF polymorphism could be associated with resilience. The BDNF Met allele might be protective for health control subjects but a risk for patients with early-onset PD under high stress.43 In the early-onset PD group, carriers of BDNF Met/Met were found to be more sensitive to stress,28 suggesting that genetic control of sensitivity to the environment should be considered.44
Taken together, our results showed that subjects with the Met/Met polymorphism of the BDNF Val66Met genotype were more sensitive to anxiety in the early-onset PD group, suggesting the importance of further examination concerning increased trait anxiety by the BDNF Met allele in a subgroup of patients with early-onset PD.
The present study had certain limitations. The sample size was relatively small. Therefore, subdividing the patient population according to family history and sex differences resulted in a loss of statistical power and the finding that the BDNF Val66Met genotypes show an association between anxiety-related personality traits and the onset age of PD should be interpreted cautiously. Another limitation is that we evaluated psychopathology at a single point in time during treatment. Long-term psychopathology may not be reflected by a single point assessment because psychopathology may vary between different episodes and during the course of illness of each patient. Therefore, collecting larger samples will be the next step.
Conclusions
In early-onset PD, the STAI trait score was higher in the Met/Met group, but a trend of higher scores was also shown in the health control subject Val/Val group. It is assumed that BDNF polymorphism has a conflicting effect between psychological illness and health control subjects and that the effect of this genotype manifests itself as increased trait anxiety in early-onset PD.
1 : A family study of panic disorder. Arch Gen Psychiatry 1983; 40:1065–1069Crossref, Medline, Google Scholar
2 : Family genetic studies of panic disorder. J Psychiatr Res 1993; 27(Suppl 1):69–78Crossref, Medline, Google Scholar
3 : A review and meta-analysis of the genetic epidemiology of anxiety disorders. Am J Psychiatry 2001; 158:1568–1578Crossref, Medline, Google Scholar
4 : Acute exercise ameliorates reduced brain-derived neurotrophic factor in patients with panic disorder. Psychoneuroendocrinology 2010; 35:364–368Crossref, Medline, Google Scholar
5 : Decreased serum brain-derived neurotrophic factor levels in major depressed patients. Psychiatry Res 2002; 109:143–148Crossref, Medline, Google Scholar
6 : Serum BDNF levels before treatment predict SSRI response in depression. Prog Neuropsychopharmacol Biol Psychiatry 2011; 35:1623–1630Crossref, Medline, Google Scholar
7 : Pro-region of neurotrophins: role in synaptic modulation. Neuron 2003; 39:735–738Crossref, Medline, Google Scholar
8 : BDNF variant linked to anxiety-related behaviors. Bioessays 2007; 29:116–119Crossref, Medline, Google Scholar
9 : No association of the brain-derived neurotrophic factor (BDNF) gene polymorphisms with panic disorder. Prog Neuropsychopharmacol Biol Psychiatry 2005; 29:708–712Crossref, Medline, Google Scholar
10 : Family-based association study of 76 candidate genes in bipolar disorder: BDNF is a potential risk locus. Brain-derived neutrophic factor. Mol Psychiatry 2002; 7:579–593Crossref, Medline, Google Scholar
11 : Genetic variation of brain-derived neurotrophic factor (BDNF) in bipolar disorder: case-control study of over 3000 individuals from the UK. Br J Psychiatry 2006; 188:21–25Crossref, Medline, Google Scholar
12 : Association study of a brain-derived neurotrophic-factor genetic polymorphism and mood disorders, age of onset and suicidal behavior. Neuropsychobiology 2003; 48:186–189Crossref, Medline, Google Scholar
13 : No association between the Val66Met polymorphism of the brain-derived neurotrophic factor gene and bipolar disorder in a Japanese population: a multicenter study. Biol Psychiatry 2004; 56:376–378Crossref, Medline, Google Scholar
14 : Positive association between MET allele (BDNF Val66Met polymorphism) and obsessive-compulsive disorder. Rev Bras Psiquiatr 2010; 32:323–324Crossref, Medline, Google Scholar
15 : Met66 in the brain-derived neurotrophic factor (BDNF) precursor is associated with anorexia nervosa restrictive type. Mol Psychiatry 2003; 8:745–751Crossref, Medline, Google Scholar
16 : The Val66Met polymorphism of the brain-derived neurotrophic-factor gene is associated with geriatric depression. Neurobiol Aging 2006; 27:1834–1837Crossref, Medline, Google Scholar
17 : Sequence variants of the brain-derived neurotrophic factor (BDNF) gene are strongly associated with obsessive-compulsive disorder. Am J Hum Genet 2003; 73:370–376Crossref, Medline, Google Scholar
18 : No association between the brain-derived neurotrophic factor gene and panic disorder in Japanese population. J Hum Genet 2009; 54:437–439Crossref, Medline, Google Scholar
19 : Association study of a brain-derived neurotrophic factor (Val66Met) genetic polymorphism and panic disorder. Neuropsychobiology 2004; 49:178–181Crossref, Medline, Google Scholar
20 : The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell 2003; 112:257–269Crossref, Medline, Google Scholar
21 : A BDNF coding variant is associated with the NEO personality inventory domain neuroticism, a risk factor for depression. Neuropsychopharmacology 2003; 28:397–401Crossref, Medline, Google Scholar
22 : Association study of a brain-derived neurotrophic factor (BDNF) Val66Met polymorphism and personality trait and intelligence in healthy young females. Neuropsychobiology 2004; 49:13–16Crossref, Medline, Google Scholar
23 : BDNF variation and mood disorders: a novel functional promoter polymorphism and Val66Met are associated with anxiety but have opposing effects. Neuropsychopharmacology 2005; 30:1353–1361Crossref, Medline, Google Scholar
24 : Association of a functional BDNF polymorphism and anxiety-related personality traits. Psychopharmacology (Berl) 2005; 180:95–99Crossref, Medline, Google Scholar
25 : BDNF serum levels, but not BDNF Val66Met genotype, are correlated with personality traits in healthy subjects. Eur Arch Psychiatry Clin Neurosci 2011; 261:323–329Crossref, Medline, Google Scholar
26 : Variant BDNF Val66Met polymorphism affects extinction of conditioned aversive memory. J Neurosci 2009; 29:4056–4064Crossref, Medline, Google Scholar
27 : Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science 2006; 314:140–143Crossref, Medline, Google Scholar
28 : The impact of childhood abuse and recent stress on serum brain-derived neurotrophic factor and the moderating role of BDNF Val66Met. Psychopharmacology (Berl) 2011; 214:319–328Crossref, Medline, Google Scholar
29 : Premorbid conditions and precipitating events in early-onset panic disorder. Compr Psychiatry 2002; 43:28–36Crossref, Medline, Google Scholar
30 : Familial aggregation and phenomenology of ‘early’-onset (at or before age 20 years) panic disorder. Arch Gen Psychiatry 1997; 54:271–278Crossref, Medline, Google Scholar
31 : Age-at-interview bias in anticipation studies: computer simulations and an example with panic disorder. Psychiatr Genet 1996; 6:61–66Crossref, Medline, Google Scholar
32 : Patterns of axis II comorbidity in early-onset versus late-onset panic disorder in Japan. Compr Psychiatry 2004; 45:114–120Crossref, Medline, Google Scholar
33 : A comparison of persons with early-versus late-onset panic attacks. J Clin Psychiatry 2000; 61:422–427Crossref, Medline, Google Scholar
34 : Manual for the State-Trait Anxiety Inventory, Revised edition. Palo Alto, CA, Consulting Psychologists Press, 1983Google Scholar
35 : Revised NEO Personality Inventory and NEO Five-Factor Inventory: Professional Manual. Odessa, FL, Psychological Assessment Resources, 1992Google Scholar
36 : Cross-sectional comparison of the clinical characteristics of adults with early-onset and late-onset obsessive compulsive disorder. J Affect Disord 2012; 136:498–504Crossref, Medline, Google Scholar
37 : Meta-analysis of the brain-derived neurotrophic factor gene (BDNF) Val66Met polymorphism in anxiety disorders and anxiety-related personality traits. Neuropsychobiology 2008; 58:163–170Crossref, Medline, Google Scholar
38 : Brain derived neurotrophic factor Val66Met polymorphism, the five factor model of personality and hippocampal volume: Implications for depressive illness. Hum Brain Mapp 2009; 30:1246–1256Crossref, Medline, Google Scholar
39 : The early-onset fearful panic attack as a predictor of severe psychopathology. Psychiatry Res 2002; 109:71–79Crossref, Medline, Google Scholar
40 : Interactions between BDNF Val66Met polymorphism and early life stress predict brain and arousal pathways to syndromal depression and anxiety. Mol Psychiatry 2009; 14:681–695Crossref, Medline, Google Scholar
41 : BDNF function as a potential mediator of bipolar disorder and post-traumatic stress disorder comorbidity. Mol Psychiatry 2012; 17:22–35Crossref, Medline, Google Scholar
42 : Sooner or later: age at onset of generalized anxiety disorder in older adults. Depress Anxiety 2012; 29:39–46Crossref, Medline, Google Scholar
43 : Negative affectivity moderated by BDNF and stress response. J Affect Disord 2011Medline, Google Scholar
44 : Models for the joint effect of genotype and environment on liability to psychiatric illness. Am J Psychiatry 1986; 143:279–289Crossref, Medline, Google Scholar



