Cerebral Blood Flow Changes After Transcranial Direct Current Stimulation for a Patient With Schizophrenia: a Case Report
To the Editor: Schizophrenia has an overall prevalence of 1%–1.5% and a chronic course. The disorder onset is typically in early adulthood, although subclinical changes may be present in childhood and adolescence.1,2 The disorder has a polymorphic feature of symptoms with relatively distinct phenomenological presentation: 1) positive symptoms, 2) impairment or negative symptoms, and 3) cognitive dysfunction. The former are characterized by hallucinations and delusions; negative symptoms by impairments in sociability, expression of affect and motivation; and cognitive dysfunction by deficits in executive functioning (attention and/or memory).3,4
The pharmacological treatment of schizophrenia involves typical and atypical antipsychotics and, in more severe and/or refractory cases, the use nonpharmacological therapies such as ECT. Recently, repetitive transcranial magnetic stimulation (rTMS) has been approved for use in the positive symptoms of schizophrenia.
Transcranial direct cranial stimulation (tDCS) is a novel noninvasive stimulation technique that can induce long-lasting changes in cortical excitability through initial alteration of membrane potentials and further changes in neurotransmitter release.5 There are electrodes in a tDCS device: the anode that is responsible for increasing cortical excitability in the area beneath it and the cathode that is responsible for decreasing cortical excitability in the area beneath it. It has been hypothesized that these changes in cortical excitability would be accessible by neuroimaging examintions.6 This hypothesis has already been investigated by different sets of neuroimaging studies such as functional MRI (fMRI)7 and SPECT.8,9 These studies have shown valuable results as to assist the understanding of tDCS biological effects.
We aim to report blood flow changes assessed by SPECT in a patient with schizophrenia treated with tDCS. The rational for using tDCS was to evaluate cerebral blood flow changes induced by tDCS.
Case Report
“Mr. R,” a 26-year-old man with schizophrenia for the last 10 years, presented with delusional thoughts, visual hallucination, problems with motivation, social withdrawal, and diminished affective responsiveness. Despite previous use of antipsychotic drugs such as haloperidol (2003–2005; higher dose of 15 mg/day), risperidone (2005–2007; maximum dose of 6 mg/day) and olanzapine (2007–2009; maximum dose of 20 mg/day), the patient had no improvement in clinical presentation. In 2009, considering the severity of symptoms and refractoriness to standard treatments, clozapine was initiated with maximum dose of 600 mg/day without success. In 2012, the medical team, decided for a tDCS treatment protocol because the patient’s family did not agree with electroconvulsive therapy. After a written informed consent was signed by a family member (patient’s mother), the patient underwent 20 sessions of tDCS for 10 days. A baseline SPECT was made to be further compared with outcome imaging. The examination showed a heterogeneous pattern of radiotracer concentration particularly in the temporal lobes, pronouncedly in the left. Thus, the cathode was positioned in the right temporoparietal transition and anode in the left temporoparietal transition. We used a direct current of 2 mA for 20 minutes. Electrodes of 35cm2 were wrapped in cotton material moistened with saline to reduce the impedance. During follow-up, a new SPECT was performed (after 30 days of final stimulation), showing overall improvement of cerebral perfusion in relation to previous SPECT, especially in the left temporal lobe (Figure 1). Patient did not show clinical improvement as assessed by rating scales [Positive and Negative Syndrome Scale (PANSS)] at follow-up.
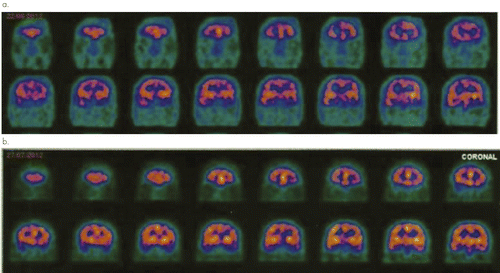
(a) Baseline SPECT 2 weeks before tDCS protocol and (b) outcome SPECT after 1 month follow-up showing improvement of cerebral perfusion mainly at left temporal lobe.
Discussion
In the present case, we demonstrate cerebral blood flow changes after a tDCS protocol for a patient with schizophrenia. During follow-up (after 30 days of final stimulation), there was overall improvement of cerebral perfusion mainly in the left temporal lobe in relation to previous neuroimaging assessment. SPECT was able to assess tDCS biological effects for a patient with schizophrenia.
Studies combining tDCS with brain imaging methods, such as SPECT, promise to provide invaluable insights on the correlation between modification of behavior and its underlying neurophysiologic underpinnings.10 The results of clinical research accessing neuroimaging findings for tDCS protocols have predominately focused on motor cortical stimulation instead of stimulation of brain areas relevant to the treatment of specific mental disorders, and there has been some inconsistency within the findings. For example, Lang et al using PET found that both anodal and cathodal stimulation increased underlying regional blood flow, whereas an earlier fMRI study reported a decrease in activity with cathodal tDCS and no change with anodal stimulation.11
To date, only one trial assessed tDCS in the treatment of schizophrenia. Thirty patients with schizophrenia with persistent auditory hallucinations were randomized to receive either active stimulation or sham. The cathode was placed on the left temporo-parietal region and the anode on the left dorsolateral prefrontal cortex. The rationale of this position was at the same time perform an inhibitory stimulation over the area related to positive symptoms (auditory hallucinations) and an excitatory stimulation over the area correlated with negative symptoms. tDCS was applied twice daily for 5 days, and the authors showed an improvement of hallucinatory symptoms (primary endpoint) after the end of stimulation, with sustained clinical response after 1 and 3 months of treatment.12
Conclusions
In the current case study, SPECT was a valuable tool to provide data regarding neurobiological changes induced by tDCS intervention for the treatment of a mental disorder as shown by improvement in perfusion of compromised brain areas.
1 : Sex difference in age at onset of schizophrenia. Arch Gen Psychiatry 1984; 41:157–161Crossref, Medline, Google Scholar
2 : Early detection and intervention with schizophrenia: rationale. Schizophr Bull 1996; 22:201–222Crossref, Medline, Google Scholar
3 : Symptoms, signs, and diagnosis of schizophrenia. Lancet 1995; 346:477–481Crossref, Medline, Google Scholar
4 : Schizophrenia: from phenomenology to neurobiology. Neurosci Biobehav Rev 2003; 27:269–306Crossref, Medline, Google Scholar
5 : Rational modulation of neuronal processing with applied electric fields. Conf Proc IEEE Eng Med Biol Soc 2006; 1:1616–1619Crossref, Medline, Google Scholar
6 : Making sense of neuroimaging in psychiatry. Acta Psychiatr Scand 2008; 117:100–117Medline, Google Scholar
7 . Modulation of cortical activity after anodal transcranial direct current stimulation of the lower limb motor cortex: A functional MRI study. Brain Stimulation 2012; 5:462‒467.Google Scholar
8 : Myoinositol content in the human brain is modified by transcranial direct current stimulation in a matter of minutes: a 1H-MRS study. Magn Reson Med 2008; 60:782–789Crossref, Medline, Google Scholar
9 : Transcranial direct current stimulation (tDCS) produces localized and specific alterations in neurochemistry: a ¹H magnetic resonance spectroscopy study. Neurosci Lett 2011; 500:67–71Crossref, Medline, Google Scholar
10 : Transcranial direct current stimulation: State of the art 2008. Brain Stimulat 2008; 1:206–223Crossref, Medline, Google Scholar
11 : Regional modulation of BOLD MRI responses to human sensorimotor activation by transcranial direct current stimulation. Magn Reson Med 2001; 45:196–201Crossref, Medline, Google Scholar
12 : Examining transcranial direct-current stimulation (tDCS) as a treatment for hallucinations in schizophrenia. Am J Psychiatry. 2012;169:719–724.Google Scholar



