Oxytocin and Behavior: Evidence for Effects in the Brain
Abstract
Knowledge about the oxytocin (OT) system in the brain has increased greatly over the past decade.15 Although this neuropeptide is best known for its peripheral effects, direct modulation of central nervous system (CNS) areas has also been implicated in OT’s actions, which include a major role in a wide range of affiliative behaviors.16–24 Often referred to as the “social bonding” hormone, speculations are being made as to its applications and potential uses in enhancing human relationships. Alterations in the OT system have been implicated in several neuropsychiatric disorders.25 Multiple types of psychopathology manifest in deficits in social functioning, including inability to maintain interpersonal relationships and engage in socially appropriate behavior. The OT system may influence the efficacy of psychotherapy, as research has repeatedly shown that the therapeutic relationship is one of the largest predictors of therapeutic change.26 OT may also have value as a therapeutic intervention.
CNS Anatomy
OT is a nine amino acid peptide, synthesized primarily in the supraoptic and paraventricular nuclei (SON and PVN) of the hypothalamus.1,18 Neurons in both the SON and PVN project to the posterior pituitary gland, where OT is released into the bloodstream in response to specific physiological events (e.g., sexual stimulation, nursing, stress) and exerts multiple peripheral effects. OT neurons also send projections to (as indicated by staining of fibers) and OT receptors are present in (as indicated by specific binding) other regions of the CNS in species-specific distributions.1,2 The few studies of OT receptor binding in human brain (Figure 1) indicate considerable differences from rodent brain.1,3,4 Of particular relevance is the absence in human brain of the extensive binding in limbic-related areas (e.g., amygdala, hippocampus, subiculum, entorhinal cortex, bed nucleus of the stria terminalis) that is prominent in the rodent. In contrast, high levels of binding were present in parts of the human basal forebrain (e.g., basal nucleus of Meynert, vertical limb of the diagonal band of Broca) and in substantia nigra that are not found in rodent. Both species have binding in the lateral septal nucleus, ventral striatum, and in multiple areas of brainstem and spinal cord. Animal studies indicate that OT receptor distribution is both species- and strain-specific and correlates with behavioral differences.18,23,27 The few available studies of OT fiber staining in human brain (Figure 1) suggest a generally similar pattern to the receptor mapping, with fibers present in the basal forebrain (e.g., septal nucleus, diagonal band of Broca, bed nucleus of the stria terminalis) and brainstem, but not in amygdala or hippocampus.5–7 These differences should be borne in mind when applying results from other species to interpretation of human studies or application to human behaviors.
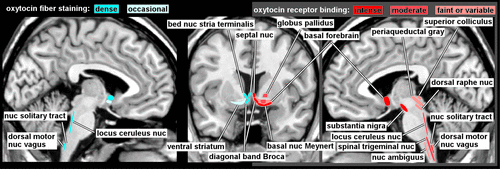
An intriguing aspect of the OT system in the CNS is the presence of areas with substantial OT receptor binding but low levels of fibers staining for OT, such as the substantia nigra pars compacta.1,5 It has been hypothesized that such mismatches indicate the presence of volume transmission as an important mode of OT action.28,29 OT, like other neuropeptides, is stored in large dense-core vesicles which are found in and can release OT from multiple locations (e.g., nerve endings, dendrites, soma, axonal varicosities).23 Dendritic release by hypothalamic neurons is believed to be a major source of OT in the cerebral spinal fluid (CSF).23,28 CSF and plasma OT levels are different and appear to be mostly independently regulated. The half-life of OT is much longer in CSF (∼28 minutes) than in the blood (1–2 minutes), suggesting a very different time-scale for CNS effects.28 Unlike circulating OT, which does not easily cross the blood–brain barrier, OT in CSF has been suggested to reach multiple brain areas and to thereby alter physiology and behavior.28
Peripheral Oxytocin
Numerous studies in humans have reported correlations between peripheral levels of OT (i.e., in blood, saliva, or urine) and socially-relevant behaviors.16,19,22,24,25 No correlation between urinary and plasma OT has been reported, suggesting that urine sampling may not be reliable marker of circulating OT.30,31 In most studies, OT is measured by assay of OT concentration in the blood or saliva, and the two are shown to correlate to a certain extent.30–32 However, OT has also been shown to be synthesized and released at peripheral sites, including the heart, thymus gland, gastrointestinal tract, uterus, placenta, amnion, corpus luteum, and testes.1,20
In humans, the most notable peripheral OT release is related to childbirth, breast-feeding, and copulation.16,22,33 Peripheral OT has been correlated with many aspects of parent–infant bonding.22 Plasma OT levels of pregnant women in the first trimester predict the amount of postpartum maternal–infant bonding behavior, and the increase in OT from the first to third trimester predicts third-trimester strength of maternal bonding (Maternal–Fetal Attachment Scale), suggesting that OT in gestation functions to prime the expression of maternal behavior.22 Emerging research suggests that disruptions in maternal–infant bonding are marked by dysregulation of the OT system. Mothers at higher risk for postpartum depression, based on self-reported symptoms pre- and postpartum, had lower plasma OT during pregnancy.34 Measures of adult attachment and temperament correlated with maternal differences in the increase in OT induced by interacting with their infants, and with brain activation patterns (measured with functional magnetic resonance imaging, fMRI) while viewing pictures of their own or other infants.35,36 When viewing their own infant’s happy face, secure mothers showed greater activation in ventral striatum, orbitofrontal cortex, and medial prefrontal cortex, whereas insecure mothers showed greater activation in the dorsolateral prefrontal cortex.35 The authors suggested that these differences indicate inhibition of negative affect by the insecure mothers, which may correspond with a disruption of the OT system that presumably supports parent–child empathy. Other studies have shown that both basal levels as well as reactivity OT measured in plasma and saliva of parents is associated with gender-specific parental behaviors such as affectionate touch in mothers and stimulatory touch in fathers, suggesting that touch-based interventions might be efficacious when the OT system is dysfunctional (e.g., maternal postpartum depression).22 Similarly, a recent study found that increases in key paternal parenting behaviors (e.g., touch, social gaze, social reciprocity) were paralleled by increases in infants’ engagement behaviors (e.g., social gaze, exploration, social reciprocity) and peripheral OT levels, suggesting possible translational implications for an indirect neuropeptide treatment of infants at risk for social dysfunctions, such as siblings of children with autism-spectrum disorders.37 Parents who are unable to attune to their child may contribute to dysfunctional development of the child’s OT system. Lower levels of OT in response to interactions with caregivers were found among adopted children with a history of disrupted caregiving, although basal levels were normal.38 A recent study of personality traits, that included neuroimaging, found a positive correlation with extraversion (higher OT, higher extraversion) and a negative correlation with volume of the right amygdala/hippocampus complex (higher OT, smaller lateral amygdala and anterior hippocampus).39 The authors noted that the lateralization is consistent with preferential involvement of the right hemisphere in modulation of social and emotional behavior, and they suggest that interaction of social experience with genetic factors may be involved.
Some studies have found that peripheral OT levels are significantly increased in the early stages of romantic attachment and that OT levels correlated with positive behaviors (e.g., positive communication, emotional matching, affectionate touch, interpersonal focus, and emotional support between partners).40–42 Interestingly, OT levels among new lovers were significantly higher even than those observed in new parents and were modestly predictive of relationship stability at 6-month follow-up.42 However, plasma OT also correlated with new lovers' preoccupations and worries regarding the partner and the relationship, providing evidence for a possible role of OT in anxious attachment. Similarly, several studies have reported positive correlations between OT and relational distress (e.g., anxiety, anxious attachment, distressful romantic relationships).43–45 In a recent large, healthy-cohort study (N=473), trait anxiety and attachment anxiety were negatively correlated with plasma OT levels in men, consistent with its proposed anxiolytic effects.46 In contrast, attachment anxiety was positively correlated with OT in women, and no association was found between OT and trait anxiety. Also, women with extreme values of OT were much more likely to self-report being highly anxious on a daily basis, suggesting that severe anxiety symptomology in women may be linked with an over-activation of the OT system.46
Altered levels of peripheral OT have also been correlated with presence of several neuropsychiatric disorders. Lower levels have been reported in some studies of depression, autism-spectrum disorders (ASD), and schizophrenia, although findings do vary.19,24,25,47,48 In contrast, a positive correlation was found between OT and symptom severity in patients with social anxiety disorder (SAD).25 It has been suggested that peripheral OT level may indicate an individual’s sensitivity to socially-relevant information.49 Although studies finding significant correlations between peripheral OT and many aspects of human affiliative behaviors are intriguing, disentangling peripheral and central sites of action is complex.16,20 The finding that intranasally-administered neuropeptides (melanocortin, vasopressin, insulin) reach the CSF led to a surge of studies demonstrating the impact of exogenous OT administration on a host of social behaviors and cognitions in humans.50
Intranasal Oxytocin Administration
Intranasal administration of OT has been found to influence a variety of human behaviors. It is important to bear in mind that the original study demonstrating uptake of vasopressin into CSF found that peripheral vasopressin also increased, complicating interpretation of behavioral changes.50 Prolonged increase in peripheral OT after intranasal administration has been demonstrated in humans.52,53 However, a study in humans comparing the effects of intranasal and intravenous administration of vasopressin reported that only intranasal administration altered event-related potentials evoked by an auditory attention task.51 Recently, a study in nonhuman primates confirmed both an increase in CSF levels of OT after intranasal administration and altered prosocial behaviors.54 At present, route(s) of uptake into the CSF have not been determined, and penetration of neuropeptides into relevant brain areas after intranasal administration has not been experimentally verified.20,55
The ability of exogenous OT to modulate many aspects of social-emotional behavior is strongly supported by recent reviews.21,24,25,49,55–59 Administration of OT can improve multiple measures related to social perception (e.g., faces versus houses, social versus nonsocial stimuli, biological versus nonbiological motion) without improving detection, suggesting that OT enhances a later stage in processing. More complex aspects of social cognition (e.g., emotion recognition and categorization, memory for socially-relevant emotional information, parent–infant interactions, trust-related behaviors) may also be enhanced. There is evidence that OT increases emotional empathy (affective sharing), but not cognitive empathy (mentalizing), indicating improved recognition of feelings, but perhaps not greater understanding of problems. OT can also increase behaviors that might be considered negative, such as envy and social aversion, possibly a reflection of enhanced noncooperation with individuals considered to be rivals (outgroup members). Variability in results across studies is likely partially due to the absence of standardization in key experimental methods related to OT delivery (e.g., drug formulation, amount and method of administration, and time between administration and behavioral measures).60 Also, there is growing evidence that both situational factors (e.g., task difficulty, social context) and individual factors (e.g., gender, social proficiency, emotional sensitivity, coping style, personality traits, genetics) can influence the effect of OT administration.25,49,58,61–64 The complex interplay between other hormonal and modulatory neurotransmitter systems and OT also complicates interpretation of findings.
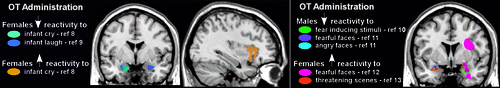
Functional neuroimaging (primarily fMRI) has begun to shed light on the brain areas affected by intranasal administration of OT.24,57,59 Altered amygdala reactivity to emotional stimuli has been the most common finding. For example, OT decreased amygdala reactivity in women exposed to either infant laughter or crying (Figure 2) and in men exposed to negative emotional stimuli (e.g., fearful or angry faces, threatening scenes; Figure 2).8–11 These findings are consistent with proposed anxiolytic effects of OT. However, some of OT’s modulatory effects appear to be gender-specific, as amygdala activation was increased in most studies utilizing similar negative emotional stimuli in women (Figure 2).12,13,57,65 Several studies have also reported alterations in functional coupling between areas of the brain implicated in emotional processing (e.g., amygdala) and other brain regions after OT administration, although the specifics vary.57,59,66,67
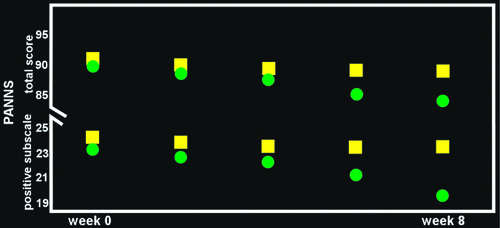
Intranasal administration of OT has been considered as a potential treatment for several neuropsychiatric disorders, with indications of benefit for some conditions, although few randomized control trials (RCTs) have been reported.19,25 Several studies in patients (mostly adult) with ASD indicate that OT administration improved measures related to social behavior (e.g., emotion recognition, gaze time on eye region, trust) and beneficial therapeutic effects have been noted in a few case reports.19,24,25,48 Studies in patients with schizophrenia also have shown positive effects on measures related to common symptoms, including cognition, emotion recognition, and social perception.68–70 Several recent RCTs in patients with schizophrenia on stable doses of antipsychotic medication have reported that addition of OT had beneficial effects (e.g., reduced psychotic symptoms, improved social cognition; Figure 3), although contrary results have also been reported.14,47,69–76 The few studies in patients with borderline personality disorder (BPD) have been mixed, with one reporting a beneficial effect on emotional regulation and the other reporting reduction in trust and cooperation.25 Findings in anxiety disorders have also been mixed. Although administration of OT has been shown to decrease fear-related amygdala activity in patients with generalized anxiety disorder, an RCT in patients with SAD found no main effect of OT administered before Sessions 2–5 of exposure therapy on objective outcome measures, although patients rated their appearance and performance as improved.25 Two studies have reported symptom reduction in patients with posttraumatic stress disorder (PTSD) after a single dose of OT (e.g., diminished combat imagery-evoked physiological response, decreased intensity of anxiety) and one reported improved mood.25 Two recent RCTs reported that the addition of 3,4-methylenedioxymethamphetamine (MDMA) to psychotherapy was associated with better outcomes (statistically significant in one) for patients with chronic, treatment-resistant PTSD, and MDMA-induced OT release has been implicated as an important mechanism of action.77–79 It has also been suggested that administration of OT before psychotherapy may enable the patient to build trust in the therapist and strengthen engagement, thus facilitating the therapeutic process.80,81
Conclusions
Although still in the early stages of development as a treatment, OT’s ability to increase trust and enhance emotional empathy suggests considerable potential in neuropsychiatry, either as an addition to other medications or in combination with psychotherapy. At present, there are multiple theories for OT’s effect (e.g., anxiety-reduction hypothesis, affiliative-motivation hypothesis, social-salience hypothesis), with varying implications for use of OT as a therapeutic agent.49 For example, OT might be beneficial by increasing attention to social information in patients with deficits in this domain (e.g., ASD), yet be detrimental in patients already hypersensitive to social cues (e.g., BPD).49 More studies are required to more clearly delineate the impact of both context and individual differences to OT’s effects.
1 : The oxytocin receptor system: structure, function, and regulation. Physiol Rev 2001; 81:629–683Crossref, Medline, Google Scholar
2 : Oxytocin, a neuropeptide for affiliation: evidence from behavioral, receptor autoradiographic, and comparative studies. Psychoneuroendocrinology 1992; 17:3–35Crossref, Medline, Google Scholar
3 : Localization of oxytocin binding sites in the human brainstem and upper spinal cord: an autoradiographic study. Brain Res 1989; 500:223–230Crossref, Medline, Google Scholar
4 : Localization of high-affinity binding sites for oxytocin and vasopressin in the human brain: an autoradiographic study. Brain Res 1991; 555:220–232Crossref, Medline, Google Scholar
5 : Projections from vasopressin, oxytocin, and neurophysin neurons to neural targets in the rat and human. J Histochem Cytochem 1980; 28:475–478Crossref, Medline, Google Scholar
6 : Immunohistochemistry of vasopressin, oxytocin, and neurophysin in the hypothalamus and extrahypothalamic regions of the human and primate brain. Acta Histochem Suppl 1981; 24:79–95Medline, Google Scholar
7 : Extrahypothalamic vasopressin and oxytocin in the human brain; presence of vasopressin cells in the bed nucleus of the stria terminalis. Brain Res 1986; 375:363–367Crossref, Medline, Google Scholar
8 : Oxytocin modulates amygdala, insula, and inferior frontal gyrus responses to infant crying: a randomized, controlled trial. Biol Psychiatry 2011; 70:291–297Crossref, Medline, Google Scholar
9 : No laughing matter: intranasal oxytocin administration changes functional brain connectivity during exposure to infant laughter. Neuropsychopharmacology 2012; 37:1257–1266Crossref, Medline, Google Scholar
10 : Oxytocin modulates neural circuitry for social cognition and fear in humans. J Neurosci 2005; 25:11489–11493Crossref, Medline, Google Scholar
11 : Oxytocin attenuates amygdala responses to emotional faces regardless of valence. Biol Psychiatry 2007; 62:1187–1190Crossref, Medline, Google Scholar
12 : Effects of intranasal oxytocin on emotional face-processing in women. Psychoneuroendocrinology 2010; 35:83–93Crossref, Medline, Google Scholar
13 : Oxytocin increases amygdala reactivity to threatening scenes in females. Psychoneuroendocrinology 2012; 37:1431–1438Crossref, Medline, Google Scholar
14 : Intranasal oxytocin as an adjunct to risperidone in patients with schizophrenia: an 8-week, randomized, double-blind, placebo-controlled study. CNS Drugs 2013; 27:57–65Crossref, Medline, Google Scholar
15 : Editorial comment: oxytocin, vasopressin, and social behavior. Horm Behav 2012; 61:227–229Crossref, Medline, Google Scholar
16 : Oxytocin, vasopressin, and human social behavior. Front Neuroendocrinol 2009; 30:548–557Crossref, Medline, Google Scholar
17 : Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front Neuroendocrinol 2009; 30:534–547Crossref, Medline, Google Scholar
18 : The challenge of translation in social neuroscience: a review of oxytocin, vasopressin, and affiliative behavior. Neuron 2010; 65:768–779Crossref, Medline, Google Scholar
19 : Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat Rev Neurosci 2011; 12:524–538Crossref, Medline, Google Scholar
20 : Modulating social behavior with oxytocin: how does it work? What does it mean? Horm Behav 2012; 61:392–399Crossref, Medline, Google Scholar
21 : Oxytocin modulates cooperation within and competition between groups: an integrative review and research agenda. Horm Behav 2012; 61:419–428Crossref, Medline, Google Scholar
22 : Oxytocin and social affiliation in humans. Horm Behav 2012; 61:380–391Crossref, Medline, Google Scholar
23 : Neuromodulation by oxytocin and vasopressin. Neuron 2012; 76:142–159Crossref, Medline, Google Scholar
24 : Integrative approaches utilizing oxytocin to enhance prosocial behavior: from animal and human social behavior to autistic social dysfunction. J Neurosci 2012; 32:14109–14117Crossref, Medline, Google Scholar
25 : Prosocial effects of oxytocin and clinical evidence for its therapeutic potential. Front Neuroendocrinol 2011; 32:426–450Crossref, Medline, Google Scholar
26 : Predictors of sustained therapeutic change. Psychother Res 2010; 20:37–54Crossref, Medline, Google Scholar
27 : Deconstructing sociality, social evolution, and relevant nonapeptide functions. Psychoneuroendocrinology 2013; 38:465–478Crossref, Medline, Google Scholar
28 : Oxytocin-messages via the cerebrospinal fluid: behavioral effects; a review. Physiol Behav 2010; 101:193–210Crossref, Medline, Google Scholar
29 : On the role of volume transmission and receptor–receptor interactions in social behaviour: focus on central catecholamine and oxytocin neurons. Brain Res 2012; 1476:119–131Crossref, Medline, Google Scholar
30 : Maternal and paternal plasma, salivary, and urinary oxytocin and parent–infant synchrony: considering stress and affiliation components of human bonding. Dev Sci 2011; 14:752–761Crossref, Medline, Google Scholar
31 : Plasma, salivary, and urinary oxytocin in anorexia nervosa: a pilot study. Eat Behav 2012; 13:256–259Crossref, Medline, Google Scholar
32 : Oxytocin: behavioral associations and potential as a salivary biomarker. Ann N Y Acad Sci 2007; 1098:312–322Crossref, Medline, Google Scholar
33 : Plasma oxytocin increases in the human sexual response. J Clin Endocrinol Metab 1987; 64:27–31Crossref, Medline, Google Scholar
34 : Plasma oxytocin concentration during pregnancy is associated with development of postpartum depression. Neuropsychopharmacology 2011; 36:1886–1893Crossref, Medline, Google Scholar
35 : Adult attachment predicts maternal brain and oxytocin response to infant cues. Neuropsychopharmacology 2009; 34:2655–2666Crossref, Medline, Google Scholar
36 : Maternal oxytocin response during mother–infant interaction: associations with adult temperament. Horm Behav 2012; 61:429–435Crossref, Medline, Google Scholar
37 : Oxytocin administration to parent enhances infant physiological and behavioral readiness for social engagement. Biol Psychiatry 2012; 72:982–989Crossref, Medline, Google Scholar
38 : Early experience in humans is associated with changes in neuropeptides critical for regulating social behavior. Proc Natl Acad Sci U S A 2005; 102:17237–17240Crossref, Medline, Google Scholar
39 : Oxytocin's fingerprint in personality traits and regional brain volume. Cereb Cortex 2012; [Epub ahead of print]Medline, Google Scholar
40 : Effects of partner support on resting oxytocin, cortisol, norepinephrine, and blood pressure before and after warm partner contact. Psychosom Med 2005; 67:531–538Crossref, Medline, Google Scholar
41 : Romantic love and sexual desire in close relationships. Emotion 2006; 6:163–179Crossref, Medline, Google Scholar
42 : Oxytocin during the initial stages of romantic attachment: relations to couples’ interactive reciprocity. Psychoneuroendocrinology 2012; 37:1277–1285Crossref, Medline, Google Scholar
43 : A relationship between oxytocin and anxiety of romantic attachment. Clin Pract Epidemol Ment Health 2006; 2:28Crossref, Medline, Google Scholar
44 : Are plasma oxytocin in women and plasma vasopressin in men biomarkers of distressed pair-bond relationships? Psychol Sci 2010; 21:3–7Crossref, Medline, Google Scholar
45 : Oxytocin indexes relational distress following interpersonal harms in women. Psychoneuroendocrinology 2011; 36:115–122Crossref, Medline, Google Scholar
46 : Plasma oxytocin distributions in a large cohort of women and men: their gender-specific associations with anxiety. Psychoneuroendocrinology 2013; [E-pub ahead of print]Crossref, Google Scholar
47 : Oxytocin in schizophrenia: a review of evidence for its therapeutic effects. Acta Neuropsychiatr 2012; 24:130–146Crossref, Medline, Google Scholar
48 : Research review: social motivation and oxytocin in autism: implications for joint attention development and intervention. J Child Psychol Psychiatry 2013; [E-pub ahead of print]Crossref, Medline, Google Scholar
49 : Social effects of oxytocin in humans: context and person matter. Trends Cogn Sci 2011; 15:301–309Medline, Google Scholar
50 : Sniffing neuropeptides: a transnasal approach to the human brain. Nat Neurosci 2002; 5:514–516Crossref, Medline, Google Scholar
51 : Brain potential changes after intranasal vs. intravenous administration of vasopressin: evidence for a direct nose–brain pathway for peptide effects in humans. Biol Psychiatry 1996; 39:332–340Crossref, Medline, Google Scholar
52 : Intranasal oxytocin administration is reflected in human saliva. Psychoneuroendocrinology 2012; 37:1582–1586Crossref, Medline, Google Scholar
53 : Elevated salivary levels of oxytocin persist more than 7 hrs. after intranasal administration. Front Neurosci 2012; 6:174Crossref, Medline, Google Scholar
54 : Inhaled oxytocin amplifies both vicarious reinforcement and self-reinforcement in rhesus macaques (Macaca mulatta). Proc Natl Acad Sci U S A 2012; 109:959–964Crossref, Medline, Google Scholar
55 : A critical review of the influence of oxytocin nasal spray on social cognition in humans: evidence and future directions. Horm Behav 2012; 61:410–418Crossref, Medline, Google Scholar
56 : Acute effects of steroid hormones and neuropeptides on human social-emotional behavior: a review of single-administration studies. Front Neuroendocrinol 2012; 33:17–35Crossref, Medline, Google Scholar
57 : Human neuroimaging of oxytocin and vasopressin in social cognition. Horm Behav 2012; 61:400–409Crossref, Medline, Google Scholar
58 : Sex, receptors, and attachment: a review of individual factors influencing response to oxytocin. Front Neurosci 2012; 6:194Medline, Google Scholar
59 : Oxytocin, brain physiology, and functional connectivity: a review of intranasal oxytocin fMRI studies. Psychoneuroendocrinology 2012; [E-pub ahead of print]Medline, Google Scholar
60 , Hickie I, McGuinness M, et al: Recommendations for the standardisation of oxytocin nasal administration and guidelines for its reporting in human research. Psychoneuroendocrinology 2013; E-pub ahead of printCrossref, Google Scholar
61 : Oxytocin and the social brain: beware the complexity. Neuropsychopharmacology 2012; 37:1795–1796Crossref, Medline, Google Scholar
62 : Coping style moderates the effect of intranasal oxytocin on the mood response to interpersonal stress. Exp Clin Psychopharmacol 2012; 20:84–91Crossref, Medline, Google Scholar
63 : Oxytocin attenuates feelings of hostility depending on emotional context and individuals’ characteristics. Sci Rep 2012; 2:384Crossref, Medline, Google Scholar
64 : Oxytocin enhances pupil dilation and sensitivity to “hidden” emotional expressions. Soc Cogn Affect Neurosci 2012; [E-pub ahead of print]Medline, Google Scholar
65 : Amygdala response to negative images in postpartum vs nulliparous women and intranasal oxytocin. Soc Cogn Affect Neurosci 2013; [E-pub ahead of print]Google Scholar
66 : Oxytocin enhances resting-state connectivity between amygdala and medial frontal cortex. Int J Neuropsychopharmacol 2013; 16:255–260Crossref, Medline, Google Scholar
67 : Oxytocin effects on complex brain networks are moderated by experiences of maternal love withdrawal. Eur Neuropsychopharmacol 2013; [E-pub ahead of print]Crossref, Medline, Google Scholar
68 : Emotion recognition and oxytocin in patients with schizophrenia. Psychol Med 2011; 42:1–8Medline, Google Scholar
69 : Adjunctive intranasal oxytocin improves verbal memory in people with schizophrenia. Schizophr Res 2012; 139:207–210Crossref, Medline, Google Scholar
70 : Improving social perception in schizophrenia: the role of oxytocin. Schizophr Res 2013; [E-pub ahead of print]Crossref, Medline, Google Scholar
71 : Adjunctive intranasal oxytocin reduces symptoms in schizophrenia patients. Biol Psychiatry 2010; 68:678–680Crossref, Medline, Google Scholar
72 : Intranasal oxytocin reduces psychotic symptoms and improves Theory of Mind and social perception in schizophrenia. Schizophr Res 2011; 132:50–53Crossref, Medline, Google Scholar
73 : Intranasal oxytocin reduces core symptoms of schizophrenia. Schizophr Res 2012; 136:S69–S70Crossref, Google Scholar
74 : Oxytocin treatment improves social cognition, PANSS social items scores, and verbal learning in schizophrenia. Schizophr Res 2012; 136:S70Crossref, Medline, Google Scholar
75 : Intranasal oxytocin effects on social anxiety and depression in schizophrenia: results from a double-blind, placebo-controlled trial. Schizophr Res 2012; 136:S360Crossref, Google Scholar
76 : Effects of adjunctive intranasal oxytocin on olfactory identification and clinical symptoms in schizophrenia: results from a randomized, double-blind, placebo controlled pilot study. Schizophr Res 2013; 145:110–115Crossref, Medline, Google Scholar
77 : The safety and efficacy of +/-3,4-methylenedioxymethamphetamine-assisted psychotherapy in subjects with chronic, treatment-resistant posttraumatic stress disorder: the first randomized, controlled pilot study. J Psychopharmacol 2011; 25:439–452Crossref, Medline, Google Scholar
78 : Durability of improvement in post-traumatic stress disorder symptoms and absence of harmful effects or drug dependency after 3, 4-methylenedioxymethamphetamine: a prospective, long-term follow-up study. J Psychopharmacol 2013; 27:28–39Crossref, Medline, Google Scholar
79 : A randomized, controlled pilot study of MDMA (± 3,4-methylenedioxymethamphetamine)-assisted psychotherapy for treatment of resistant, chronic post-traumatic stress disorder (PTSD). J Psychopharmacol 2013; 27:40–52Crossref, Medline, Google Scholar
80 : The peptide that binds: a systematic review of oxytocin and its prosocial effects in humans. Harv Rev Psychiatry 2010; 18:1–21Crossref, Medline, Google Scholar
81 : A psychobiological rationale for oxytocin in the treatment of posttraumatic stress disorder. CNS Spectr 2010; 15:522–530Crossref, Medline, Google Scholar



