The Neurobiology of Placebo and Nocebo: How Expectations Influence Treatment Outcomes
Abstract
It has been recognized since the very early days of medicine that factors other than active treatment can alter therapeutic efficacy.5–7 Thus, creating positive expectations was an accepted part of medical practice. The effects of a patient’s conscious and unconscious expectations and experiences on their physiological responses can be either positive or negative.4,5,7–12 A placebo response is induced by an expectation of benefit or help; a nocebo response is induced by an expectation of detriment or harm. Thus, receiving a placebo (or nocebo) is actually an active intervention. It is not the same as receiving no treatment. In the context of a clinical trial or research study, the placebo (or nocebo) response is a psychobiological phenomenon occurring in the subject’s brain after the administration of an inert substance or sham physical treatment, often in combination with verbal suggestions of clinical change.4,5,11 Additional factors that may affect outcomes over the course of a clinical trial or research study include spontaneous changes in the disease or condition (natural history), being studied (Hawthorne effect), regression to the mean (a statistical phenomenon due to selection biases), and biases in outcome-reporting.4,11–13 The need for blinding of both research subjects and research personnel attests to the power of beliefs and expectations (conscious and unconscious) of both parties to influence outcomes.12 Perhaps the earliest, and certainly the most well known, blinded trial was the investigation in 1784 evaluating Mesmer’s claims for the curative power of his magnetism-based treatments.5,14 The Royal Commission, led by Benjamin Franklin, fully recognized the likelihood that Mesmer’s success might be due to the power of suggestion to evoke healing. Franklin and Antoine Lavoisier designed a set of trials clearly demonstrating that the patients’ belief that they were being given an effective treatment was the essential component—not the treatment itself. Henry Beecher’s early summary of placebo-controlled trials for a wide range of conditions found that placebos provided a clinically relevant level of benefit for about 35% of patients studied, and influenced objective as well as subjective outcome measures.9,15 Beecher also reported that injured combat soldiers (in World War II) requested less pain medication than civilians after surgery.7,16 He speculated that the key difference was that wounding carried positive meanings for soldiers (e.g., surviving the event, removal from combat, etc.) that were not present for civilians. Although most commonly considered in the context of clinical trials, recent evidence delineating some of the neurobiological changes that occur as a result of inducing a placebo or nocebo response serve as a strong reminder that these effects are also an active part of medical treatment.4–7,9–13
Neurobiology
Subjects’ expectations can alter their interpretation of events, so a major issue in studying the placebo response is the influence of bias on outcome-reporting.5,13,17,18 In many cases, biased outcome-reporting was presumed to underlie claimed improvements after placebo administration, rather than actual biological changes.17,18 In clinical settings, the presence of placebo responses has sometimes been considered evidence of symptom exaggeration, or evidence that symptoms are psychogenic rather than organic, based on the assumption that an inactive treatment cannot cause a true physiological response.6 However, studies in a wide range of animal models demonstrating placebo-induced changes (suppression, enhancement) of immune system functioning after pre-conditioning provide strong evidence that associative learning is one mechanism by which placebo and nocebo responses can induce physiological change.19
Success in using this type of placebo response to enhance outcomes in animal models of autoimmune disease and organ transplant support clinical relevance, as do the few studies in human patients.19 A study in patients with psoriasis found that after 3 weeks of daily active topical treatment (pre-conditioning), full clinical response was maintained during an 8-week trial in which the daily treatment contained the active drug only 25% or 50% of the time.19,20 A study in patients with allergic rhinitis demonstrated that 5 days of daily treatment with a histamine receptor-antagonist (pre-conditioning) is sufficient for a robust placebo-induced antihistamine response after a 9-day washout.19,21 Thus, unconscious physiological processes, such as immune functions, appear to be powerfully modulated by conditioned responses.4 It has been proposed that the autonomic nervous system may provide an important path for placebo and nocebo interventions to affect peripheral functioning.17
Another line of evidence supporting neurobiological mechanisms for placebo and nocebo responses is the ability of systemically-administered neurotransmitter agonists or antagonists to block responses. The hypothesis that placebo analgesic interventions activate descending modulation of pain by the endogenous opioid (OP) system was first supported by the demonstration that a mu-OP antagonist, naloxone, blocked placebo analgesic responses in post-surgical patients.4,22 Changes in other neurotransmitter systems have also been implicated in specific aspects of placebo or nocebo pain responses. Placebo analgesia pre-conditioned by exposure to a nonsteroidal anti-inflammatory drug is OP-insensitive, but can be blocked by rimonabant, a cannabinoid (CB) receptor-antagonist.4,23 Both the OP and CB systems were activated when pain tolerance was increased by a verbal manipulation equating induced pain with improved muscle functioning, a positive outcome.24 Differences in relative activation of the OP and CB systems were substantial across the group, indicating that individuals predominately activated one system or the other. A series of studies have also demonstrated a role for the neurotransmitter cholecystokinin (CCK) in nocebo hyperalgesia responses.4 In post-surgical patients, administration of proglumide, a CCK antagonist, resulted in a dose-dependent decrease in anxiety-induced hyperalgesia. The action of proglumide was OP-independent, as it was not blocked by naloxone. Anxiety-induced hyperalgesia in healthy subjects was associated with hyperactivity of the hypothalamic-pituitary-adrenal (HPA) axis.4 Both the hyperalgesia and HPA hyperactivity were blocked by administration of an anxiolytic (diazepam), suggesting a role for anxiety in the nocebo response. However, proglumide prevented nocebo-induced hyperalgesia, but not the HPA hyperactivity.
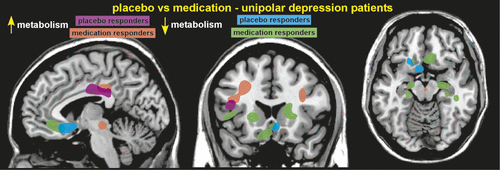
Functional brain imaging (hemodynamic, metabolic) has been used to identify areas with altered activity after administration of a placebo or nocebo. A study of patients with depression comparing changes in cerebral metabolic rate (positron emission tomography [PET]) between placebo responders and fluoxetine responders (with 6 weeks of treatment) reported considerable overlap in altered brain areas. These included cortical increases (dorsolateral prefrontal, inferior parietal, posterior insula, and posterior cingulate) and limbic–paralimbic decreases (subgenual cingulate cortex, hypothalamus, thalamus, sensory insula, parahippocampus) in metabolic activity (Figure 1).1,4 Responders to fluoxetine had changes in additional areas, as well. Placebo analgesia-related changes in functional activations (on functional magnetic resonance imaging [fMRI]) have been demonstrated at multiple levels of the pain system, including cortical (dorsolateral prefrontal, rostral anterior cingulate [rACC]), subcortical (amygdala, hypothalamus, brainstem) and spinal cord levels.9,10,25,26 Most were modulated by administration of naloxone, indicating participation of the OP system.25 This study also found that strength of placebo responses (as measured by pain ratings) correlated with increased connectivity between the rACC and the periaqueductal gray (PAG) areas.25 A later study found that rACC activation (fMRI) correlated with strength of placebo responses.27 A recent metaanalysis of hemodynamic studies of placebo analgesia found that increased activations were consistently identified in areas implicated in emotion-regulation and in descending modulation of pain, whereas decreased activations were identified in the pain system.28 Although nocebo hyperalgesia is much less studied, increased pain-related activation (fMRI) in the spinal cord have been reported after verbal suggestion.29
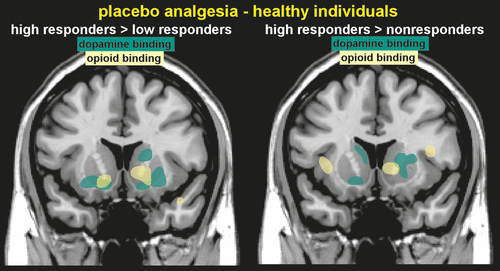
Molecular imaging (PET) has been used to identify changes in neurotransmitter release (indicated by changes in binding potential) after placebo administration. Areas in which placebo analgesia-induced increases in OP neurotransmission (indicated by reductions in binding potential) have been identified included the anterior cingulate, orbitofrontal and insular cortices, ventral striatum, amygdala, and the PAG (Figure 2).2 This study also assessed dopaminergic (DA) neurotransmission, and identified increases in the ventral striatum, caudate, and putamen. Higher placebo analgesia responses were associated with greater DA and OP activity in the ventral striatum. Release of DA in the ventral striatum is associated with reward, so activation of this system in the placebo response may be due to the expectation of positive change, a type of reward-anticipation.2 Several subjects in this study actually reported increased pain during the placebo administration (labeled in the study as a nocebo response), which was associated with deactivation of both DA and OP release.2 Thus, decreases and increases in reported pain after the placebo administration were associated with opposite responses of endogenous DA and OP neurotransmission. The placebo response induced in patients with Parkinson’s disease (PD) by expectation of receiving an active agent (apomorphine, levodopa), is associated with a substantial release of endogenous DA in the striatum (indicated by decreased binding potential for raclopride).11,30,31 Placebo-induced DA release was modulated by the probability (greatest at 75%) of receiving active agent.31 DA release in the dorsal striatum correlated with the magnitude of change in DA release after administration of an active agent (levodopa), suggesting a conditioned response. In contrast, placebo-induced DA release in the ventral striatum correlated with the probability of receiving active agent, and so may represent reward-expectation.31 Sham repetitive transcranial magnetic stimulation (rTMS) also evoked striatal DA release in PD patients who expected therapeutic benefit from rTMS.32 These findings indicated that the placebo response in PD is likely mediated in part through activation of the nigrostriatal DA system.
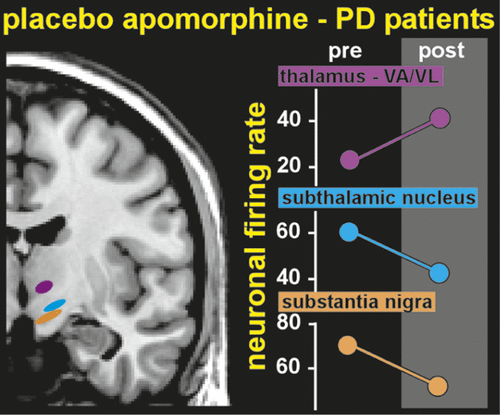
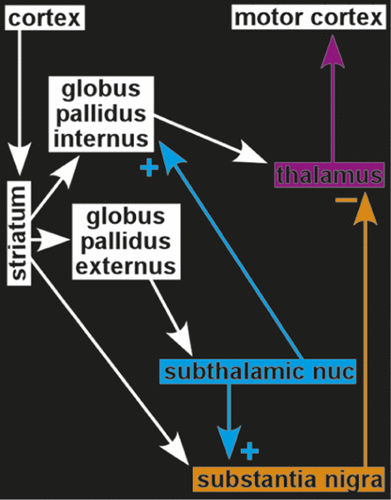
One group has even demonstrated placebo-induced changes in the firing rates of single neurons (Figure 3). Patients with PD undergoing implantation of electrodes for deep brain stimulation were pre-conditioned by exposure to apomorphine for 3 days before surgery.3,4 During surgery, patients were administered a placebo injection and verbal instructions designed to enhance expectation of benefit. Both subjective (patient report) and objective (clinician-assessed muscle rigidity) clinical measures were obtained. Patients who responded to the placebo, based on improved clinical measures, also had significant changes in the firing rates of neurons in relevant areas of the brain. Rates were decreased in the subthalamic nucleus and substantia nigra, and increased in the thalamic motor nuclei (ventral anterior, anterior ventral lateral)—changes consistent with placebo-induced normalization of functioning within this circuit.3,4
Factors Contributing to Placebo and Nocebo Responses
The primary mechanisms for a placebo (or nocebo) response are the individual’s expectation of change in symptoms and conditioned learning from previous experiences.4–9,11–13,18,33 Thus, placebo responding is likely to be stronger when the individual believes the provider’s assurance that the treatment will have a substantial beneficial effect (immediate positive expectation) and also has had a previous experience in which the treatment had a beneficial effect (conditioned learning).
Conditioned placebo responses have been demonstrated to both explicit and implicit cues.34 The context of treatment delivery can have a powerful effect, as shown by a study reporting that patients who knew that pain medication had been received (open administration by a provider) required significantly less medication than patients who did not know when medication was delivered (pre-programmed infusion pump).13 Many types of indirect experiences (social learning) can also enhance responses. Examples include speaking to someone who has had a good response to a similar treatment, observing someone who appears to be responding to the treatment, or viewing a commercial suggesting benefit.8 The converse also occurs. For example, one study demonstrated induction of nocebo responses (as indicated by report of symptoms after a sham exposure) after viewing a media report suggesting that such exposure could cause harm.35 Another found that viewing actors simulating pain induced nocebo hyperalgesia in the observers that was greater when the actor was male.36 Also, both personality and genetic traits may contribute to individual differences in responses.7,9,33,37,38
Conclusions
Induction of placebo (or nocebo) responses have been studied in many conditions, including pain, PD, depression, and immune functioning. The neuroimaging (hemodynamic, metabolic, molecular) and electrophysiological techniques used for the studies summarized above provide objective evidence of neurobiological changes at multiple levels and in different neuroanatomical pathways and circuits. These were specific to the conditions studied and interventions utilized, supporting their physiological relevance. The diversity of factors, past and present, that can influence the patient’s immediate expectations are a reminder of the dynamic and ever-changing nature of the personal meaning that individuals make of the totality of their experiences. Given the evidence that interactions with the clinician can strengthen a patient’s positive expectation and increase the likelihood of improved health, it is entirely consistent with good clinical practices to optimize the therapeutic environment in a manner that promotes healing.6,12
1 : The functional neuroanatomy of the placebo effect. Am J Psychiatry 2002; 159:728–737Crossref, Medline, Google Scholar
2 : Placebo and nocebo effects are defined by opposite opioid and dopaminergic responses. Arch Gen Psychiatry 2008; 65:220–231Crossref, Medline, Google Scholar
3 : Electrophysiological properties of thalamic, subthalamic, and nigral neurons during the anti-parkinsonian placebo response. J Physiol 2009; 587:3869–3883Crossref, Medline, Google Scholar
4 : How placebos change the patient’s brain. Neuropsychopharmacology 2011; 36:339–354Crossref, Medline, Google Scholar
5 : Placebo controls: historical, methodological, and general aspects. Philos Trans R Soc Lond B Biol Sci 2011; 366:1870–1878Crossref, Medline, Google Scholar
6 : Unpacking the placebo response: insight from ethnographic studies of healing. J Mind Body Reg 2011; 1:112–124Google Scholar
7 : Introduction to placebo effects in medicine: mechanisms and clinical implications. Philos Trans R Soc Lond B Biol Sci 2011; 366:1783–1789Crossref, Medline, Google Scholar
8 : How placebo responses are formed: a learning perspective. Philos Trans R Soc Lond B Biol Sci 2011; 366:1859–1869Crossref, Medline, Google Scholar
9 : How expectations shape pain. Neurosci Lett 2012; 520:140–148Crossref, Medline, Google Scholar
10 : Neuroimaging as a tool to investigate how cognitive factors influence analgesic drug outcomes. Neurosci Lett 2012; 520:149–155Crossref, Medline, Google Scholar
11 : Mechanisms and therapeutic implications of the placebo effect in neurological and psychiatric conditions. Pharmacol Ther 2013; [E-pub ahead of print]Crossref, Medline, Google Scholar
12 : Placebo and nocebo effects in randomized controlled trials: the implications for research and practice. J Pain Symptom Manage 2013; [E-pub ahead of print]Crossref, Medline, Google Scholar
13 : Placebo analgesia: psychological and neurobiological mechanisms. Pain 2013; 154:511–514Crossref, Medline, Google Scholar
14 : Franklin, Lavoisier, and Mesmer: origin of the controlled clinical trial. Urol Oncol 2005; 23:346–351Crossref, Medline, Google Scholar
15 : The powerful placebo. JAMA 1955; 159:1602–1606Crossref, Medline, Google Scholar
16 : Relationship of significance of wound to pain experienced. JAMA 1956; 161:1609–1613Crossref, Medline, Google Scholar
17 : The placebo effect and the autonomic nervous system: evidence for an intimate relationship. Philos Trans R Soc Lond B Biol Sci 2011; 366:1808–1817Crossref, Medline, Google Scholar
18 : The placebo effect: advances from different methodological approaches. J Neurosci 2011; 31:16117–16124Crossref, Medline, Google Scholar
19 : Behavioural conditioning as the mediator of placebo responses in the immune system. Philos Trans R Soc Lond B Biol Sci 2011; 366:1799–1807Crossref, Medline, Google Scholar
20 : Conditioned pharmacotherapeutic effects: a preliminary study. Psychosom Med 2010; 72:192–197Crossref, Medline, Google Scholar
21 : Behavioral conditioning of antihistamine effects in patients with allergic rhinitis. Psychother Psychosom 2008; 77:227–234Crossref, Medline, Google Scholar
22 : The mechanism of placebo analgesia. Lancet 1978; 2:654–657Crossref, Medline, Google Scholar
23 : Non-opioid placebo analgesia is mediated by CB1 cannabinoid receptors. Nat Med 2011; 17:1228–1230Crossref, Medline, Google Scholar
24 : Pain as a reward: changing the meaning of pain from negative to positive co-activates opioid and cannabinoid systems. Pain 2013; 154:361–367Crossref, Medline, Google Scholar
25 : Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron 2009; 63:533–543Crossref, Medline, Google Scholar
26 : Direct evidence for spinal cord involvement in placebo analgesia. Science 2009; 326:404–404Crossref, Medline, Google Scholar
27 : Cortical and subcortical responses to high and low effective placebo treatments. Neuroimage 2013; 67:227–236Crossref, Medline, Google Scholar
28 : Activation likelihood estimation meta-analysis of brain correlates of placebo analgesia in human experimental pain. Hum Brain Mapp 2013; 34:738–752Medline, Google Scholar
29 : Facilitation of pain in the human spinal cord by nocebo treatment. J Neurosci 2013; 33:13784–13790Crossref, Medline, Google Scholar
30 : Expectation and dopamine release: mechanism of the placebo effect in Parkinson’s disease. Science 2001; 293:1164–1166Crossref, Medline, Google Scholar
31 : Effects of expectation on placebo-induced dopamine release in Parkinson disease. Arch Gen Psychiatry 2010; 67:857–865Crossref, Medline, Google Scholar
32 : Therapeutic application of transcranial magnetic stimulation in Parkinson’s disease: the contribution of expectation. Neuroimage 2006; 31:1666–1672Crossref, Medline, Google Scholar
33 : Placebo analgesia: cognitive influences on therapeutic outcome. Arthritis Res Ther 2012; 14:206Crossref, Medline, Google Scholar
34 : Nonconscious activation of placebo and nocebo pain responses. Proc Natl Acad Sci U S A 2012; 109:15959–15964Crossref, Medline, Google Scholar
35 : Are media warnings about the adverse health effects of modern life self-fulfilling? an experimental study on idiopathic environmental intolerance attributed to electromagnetic fields (IEI-EMF). J Psychosom Res 2013; 74:206–212Crossref, Medline, Google Scholar
36 : The effect of the sex of a model on nocebo hyperalgesia induced by social observational learning. Pain 2013; 154:1312–1317Crossref, Medline, Google Scholar
37 : The anatomy of the mesolimbic reward system: a link between personality and the placebo analgesic response. J Neurosci 2009; 29:4882–4887Crossref, Medline, Google Scholar
38 : Does personality play a relevant role in the placebo effect? Psychiatr Danub 2013; 25:17–23Medline, Google Scholar



