Negative Attention Bias and Processing Deficits During the Cognitive Reappraisal of Unpleasant Emotions in HIV+ Women
Abstract
Deficits in emotional processing may be attributed to HIV disease or comorbid psychiatric disorders. Electrocortical markers of emotional attention, i.e., amplitude of the P2 and late positive potential (LPP), were compared between 26 HIV+ women and 25 healthy controls during an emotional regulation paradigm. HIV+ women showed early attention bias to negative stimuli indexed by greater P2 amplitude. In contrast, compared with the passive viewing of unpleasant images, HIV+ women demonstrated attenuation of the early and late LPP during positive reappraisal. This interaction remained significant after adjusting for individual differences in apathy, anxiety, and depression. Post hoc analyses implicated time since HIV diagnosis with LPP attenuation during positive reappraisal. Advancing HIV disease may disrupt neural generators associated with the cognitive reappraisal of emotions independent of psychiatric function.
Antiretroviral therapy has extended life for many infected with HIV, yet emotional deficits remain a stable feature of the disease.1 Emotional deficits in HIV may manifest as primary or secondary psychiatric symptoms. Apathy is a common primary neuropsychiatric symptom of HIV linked to disease progression and neurocognitive dysfunction.2–4 Anxiety and depressive disorders are the most commonly reported secondary psychiatric symptoms that may be prevalent at any stage of the disease.5–7 Increased vulnerability for anxiety may be attributed to greater sensitivity to physical concerns, psychosocial stress, or other catastrophic cognitions brought on by HIV infection.8 Conversely, symptoms of apathy and depression may result from CNS impairment or emotional consequences associated with diagnosis and treatment.9,10
The incidence of psychiatric symptoms in HIV may also reflect lack of cognitive control of emotions or limited ability to use emotion regulation (ER) strategies.11,12 ER refers to the ability to amplify, dampen, or maintain one's emotional, behavioral, or physiological experience to a given stimulus and may predict lower psychiatric disturbance.13,14 Our understanding of how HIV and psychiatric illness relate to emotional processing is quite limited, perhaps because objective markers of emotional responding are so elusive in the HIV/AIDS literature. The event-related potential (ERP) reflects changes in endogenous and exogenous electrocortical activity and because of its temporal sensitivity is used extensively as an index of motivated attention to affective stimuli in emotion regulation paradigms.15,16
Motivationally salient images, whether pleasant or unpleasant, typically demand greater cognitive processing than stimuli devoid of such perceptual properties.15 Amplitude of the P2 (200–300 ms) reflects early attention bias to high-arousal negative stimuli (e.g., fear, disgust, sadness),17–19 whereas the LPP (after 400 ms) indexes activation of motivationally salient circuits in the brain that facilitate the allocation of attention to emotionally salient stimuli.2 The magnitude of emotional processing reflected by the LPP can be modulated by the use antecedent or response-focused emotion regulation strategies.16,20,21 ER-ERP paradigms typically report LPP enhancement following cues to increase unpleasant emotions and attenuations following cues to decrease,16,20 although some have shown enhancement of the LPP amplitude following instructions to decrease unpleasant emotions through positive reappraisal.21,22
Both primary and secondary neuro-psychiatric deficits are linked to modulation of early and late affective ERP components. Persons diagnosed with major depression show reduced LPP amplitude during affective picture processing.23 Attenuated LPPs are also linked to subclinical levels of apathy.24 Conversely, elevated levels of anxiety predict greater P2 amplitudes suggesting heightened vigilance to salient stimuli25 and increased magnitude of the LPP indicating greater sensitivity to threat-related images.26 A basic feature of emotionally salient information is to prime attention to resources to subsequent stimuli. A recent study showed that compared with HIV− controls, high-arousal images failed to elicit enhancement of the LPP in HIV+ women, thus preventing affective priming in a subsequent cognitive task.27 Although self-reported depressive symptomology did not drive this effect, it is not clear whether primary neuropsychiatric impairment or automatic or controlled regulatory processes contributed to attenuation of the LPP in HIV+ women.
The current study aimed to replicate findings of an attenuated LPP effect to unpleasant versus neutral stimuli and further determine whether ER instructions modulate affective ERPs independent of primary and secondary neuro-psychatric symptoms. An ER paradigm adopted from a previous study20 was used to compare magnitude of change in (1) P2 (200–300 ms), (2) early LPP (400–700 ms), and (3) late LPP (701–1500 ms), measured at frontal, central, and parietal scalp locations, as HIV+ and HIV− women viewed neutral and unpleasant images and increased/decreased their negative emotions to those unpleasant images. The electrode locations and ERP windows chosen coincide with the most robust activations found during ER paradigms.15 In line with previous research,16,18 HIV– controls are expected to exhibit valence effects for unpleasant versus neutral pictures for the P2, early and late LPP. Regulatory effects were expected to take place beginning with the early LPP and extending into the late LPP time window, with more robust activations taking place at centralparietal electrode locations. Furthermore, we expect the early and late LPP to be enhanced during the negative reappraisal compared with passive viewing conditions and an omnibus hypothesis for the change in LPP amplitude for the positive reappraisal based on reports of LPP increase16,20 and decrease19,21 for the decrease of unpleasant emotions. We also expected individual differences in anxiety to drive higher P2 and LPP amplitudes, whereas greater levels of apathy and depression were expected to be associated with smaller deflections of the ERP waveforms.
Methods
Participants
HIV+ women currently undergoing treatment and HIV− control subjects were recruited by flyer and referral from a woman’s outpatient center located in south Florida. HIV seropositivity for women, 25–45 years of age, was confirmed by medical report, i.e., absolute cluster of differentiation 4 (CD4) (a glycoprotein expressed on the surface of T-helper cells indicating enhanced immune state) and HIV-1 viral load. HIV− serostatus was determined by proof of a negative HIV antibody test taken before study completion. Persons with a prior psychiatric history or indication of gross cognitive impairment, i.e., total score <22 on the Folstein Examination28 were excluded. A total of 59 women met the eligibility criteria. Eight women were excluded from analyses due to the presence of excessive artifacts (e.g., gross eye or face movements) during EEG recordings. The 25 primarily African American HIV+ women included in the analyses were both symptomatic and asymptomatic, with an absolute CD4 count (MCD4(abs)= 409.3) ranging from 6 to 888 and viral load (Mviral-load = 68,619) ranging from 20 to 981,682 copies/mL blood plasma. There were no significant differences in annual income, years of education, and lifetime occurrence of mental health counseling (MHC) for HIV+ and HIV− women (p>0.05). The mean age for HIV+ women (mean=35.6, SD=5.17) was higher than control subjects [mean=31.9, SD=5.67; F(1,49)=5.97, p<0.05, ηp2=0.11]. The mean length of HIV seropositivity was 8.7 years (SD=3.76).
Measures
Apathy.
The Apathy Evaluation Scale29 defines simultaneous deficits in the overt behavioral, cognitive, and emotional concomitants of goal-directed behavior. Subjects were required to respond to their degree of agreement with each question using a Likert scale. Higher total scores reflect greater apathy. The 18-question measure has good validity and reliability.29
Depression.
The Beck’s Depression Inventory-230 is a 21-item inventory used for somatic, affective, behavioral, and cognitive aspects of depression. Good correspondence between Beck’s Depression Inventory scores and clinical judgments of diagnosticians has been documented, suggesting adequate validity of items in this scale which also has good reliability.
Anxiety.
The State-Trait Anxiety Inventory31 is a 40-item questionnaire assessing current state and trait anxiety levels of anxiety. It demonstrates adequate validity, with high intercorrelations with other anxiety checklists and objective corroboration of the presence or absence of stress. This scale has good internal consistency.
Emotional regulation questionnaire.
The Emotional Regulation Questionnaire32 is a 10-item self-report scale and it assesses individual differences corresponding to cognitive reappraisal (six items) and expressive suppression (four items). The scale requires that participants respond, on a Likert-scale, to 10 questions pertaining to how they regulate/manage their emotions in various situations. The Emotional Regulation Questionnaire has high reliability.
Vocabulary.
The Vocabulary subtest of the WAIS33 was used to assess the participant’s verbal knowledge and concept formation and is shown to have high reliability.
Block design.
The Block Design subtest of the WAIS33 was used to assess participant’s spatial problem solving, manipulative abilities, and part-whole organization and is shown to have high reliability.
Digit span.
The Digit Span (forward and backward) subtest of the WAIS33 was used to assess working memory. Higher scores were indicative of a greater working memory capacity, and this test is shown to have high reliability.
Letter number sequencing.
The Letter Number Sequencing subtest of the WAIS33 was used to assess attention and working memory and is shown to have high reliability. These subtests were used descriptively to compare groups.
Procedure
The study took part in two separate experimental sessions using a counterbalanced design. The EEG session took place in a quiet auxiliary room of the outpatient clinic; participants were seated comfortably in front of a 17-inch monitor. After informed consent was obtained, participants were instructed to increase, decease, or maintain emotional responses based on an emotion regulation paradigm adopted for ERPs.20 A total of 60 affectively neutral or unpleasant pictures selected from the International Affective Picture System (IAPS)34 were used across two experimental blocks. [A total of 30 unpleasant IAPS images (see Lange et al.34) were chosen: images of an evolutionary context, mutilations, and depictions of violence/threat. Thirty neutral IAPS images were chosen.] The unpleasant picture set included images of mutilation and threat (human and animal). The neutral picture set included images of household items and neutral faces. Unpleasant and neutral images differed significantly from each other on IAPS normative valence (mean=2.42 and 5.05) and arousal (mean=6.19 and 2.43) ratings for unpleasant and neutral pictures, respectively. The timing for trials within each block was identical. A cue word, indicating regulatory instructions for the upcoming images, was presented for 2 seconds followed by a black screen on which centered a white fixation cross for 0.5 seconds. The IAPS image was subsequently displayed for 4 seconds, followed by a 3-second period where participants used a key-response to rate the valence (positive or negative) of their emotional arousal depicted in a Self-Assessment Manikin.
Prior to the increase and decrease experiment, participants were instructed on how to passively view 30 neutral and 30 unpleasant images and cognitively reappraise 30 unpleasant images using instructions adopted from a previous study. Images were presented in a semirandomized fashion. Each neutral IAPS image was presented at least once in both the increase and decrease experimental blocks. The 30 available IAPS images were randomly presented such that six IAPS images were presented twice within the view, increase, or decrease trials. The total time for both increase and decrease experiments was approximately 90 minutes.
Upon completion of the two experimental blocks, electrodes were removed, and participants filled out a postexperiment emotion regulation questionnaire as an experimental manipulation check. A self-report neuro-psychiatric and intelligence measure was assessed during a second session lasting approximately 90 minutes. The order of sessions 1 and 2 was counterbalanced across all participants.
EEG data acquisition.
Continuous EEGs were recorded using Psychlab (Contact Precision Instrument, Cambridge, MA) EEG amplifying and recording equipment. Electrodes were attached at Fz, Cz, Pz, OZ, C3, and C4 in accordance with the International 10-20 System.35 Rigorous procedures for infection control were followed for attaching and removing electrodes.36 Scalp signals were referenced to linked electrodes attached to earlobes. Face and eye movements were recorded and were referenced from electrodes placed 1 cm above and to the right of the right eye. The EEG amplifier was set at a gain of 30,000, and the sampling rate of the EEG was 500 Hz. Electrode impedance was maintained under 5 kΩ. High-pass filters were calibrated to 0.1 Hz and low pass to 40 Hz. A notch filter was also active at 60 Hz. The EEG signal was recorded for 1600 ms in each trial (100 ms prior to and 1500 ms after picture onset). Three ERP windows, P2 (200–300 ms), early LPP (400–700 ms), and late LPP (701–1500 ms), were analyzed offline with Psylab8 software (Contact Precision Instruments, Cambridge, MA). Trials with physiological artifacts, identified by a voltage deflection >65.0 µV, were excluded from analysis. A 2 (serostatus) × 3 (instruction) × 2 (block) repeated-measures analysis of variance (ANOVA) was conducted to determine whether the total number of artifact-free trials were significantly different across the increase and decrease blocks.3 The entire waveform was baseline adjusted to the average EEG activity that occurred 100 ms prior to picture onset.
Statistical analyses.
The data were analyzed in a 2 (serostatus: HIV+, HIV−) × 2 (block: increase, decrease) × 3 (scalp site: Fz, Cz, Pz) × 3 (instructions: view-neutral, view-unpleasant, reappraise-unpleasant) repeated-measures ANOVA for each of the three ERP components (P2, early LPP, and late LPP). The scalp locations and time windows chosen for this study are shown to yield the most consistent and robust positive waveforms within emotion regulation paradigms.16,19,20 When applicable, interactive effects were decomposed by simple group comparisons tested using Bonferroni adjustment. Significant main effects and interactions involving the serostatus factor were reexamined with an analysis of covariance (ANCOVA) controlling for individual differences in apathy, anxiety, and depression. A three-way repeated-measures ANOVA was also conducted on Self-Assessment Manikin ratings with HIV serostatus as the between-subject factor and experimental block and task instructions as the within-subject factors. A final ANOVA was used to compare apathy, depression, anxiety, and trait regulatory strategy (suppress versus reappraise) between groups. In all cases, effects were statistically evaluated at p<0.05 using SPSS (Version 21.0, IBM, Armonk, N.Y.) General Linear Model software.
Results
Table 1 shows that group differences were not evident in levels of apathy, anxiety, or depression, nor were any differences found in the frequency of use in cognitive reappraisal and emotional suppression (p>0.05). HIV+ women were older and performed worse on the Letter Number Sequencing subscale of the WAIS. A summary of the results of the repeated-measures ANOVA and ANCOVA are found in Table 2. Figures 1 and 2 depict results for the grand-averaged waveforms from the increase and decrease experiment. The visual ERP waveform, for HIV+ and HIV− women, reveals modulation of P2 and LPP amplitudes separated by electrode location and regulatory instructions.
| Variable | HIV+, N=26 [Mean (SD)] | HIV−, N=25 [Mean (SD)] | F | ηp2 |
|---|---|---|---|---|
| Age | 35.6 (5.17) | 31.9 (5.67) | 5.97b | 0.11 |
| Years of education | 11.5 (1.95) | 12.5 (2.40) | 2.38 | 0.05 |
| Annual income | <15,000b | <15,000c | 0.57 | 0.01 |
| Apathy (AES) | 26.3 (3.79) | 25.5 (4.5) | 0.26 | 0.01 |
| Depression (BDI-2) | 11.9 (10.4) | 9.5 (7.6) | 0.87 | 0.02 |
| Trait anxiety (TAI) | 41.7 (13.2) | 39.8 (10.6) | 0.25 | 0.01 |
| WAIS Vocabulary | 17.4 (9.7) | 25.9 (10.2) | 0.59 | 0.16 |
| WAIS Spatial Reasoning | 21.4 (9.4) | 25.8 (10.4) | 2.61 | 0.05 |
| WAIS Digit Span | 15.4 (3.9) | 15.3 (3.4) | 0.01 | 0.01 |
| WAIS Letter-Number | 8.8 (3.1) | 10.7 (2.6) | 6.42b | 0.10 |
TABLE 1. Participant Characteristicsa
| Factor | P2 | Early LPP | Late LPP | |||
|---|---|---|---|---|---|---|
| ANOVA | ANCOVA | ANOVA | ANCOVA | ANOVA | ANCOVA | |
| Block | F1,49 = 3.40b | F1,46 = 0.17 | F1,49 = 2.00 | F1,46 = 0.21 | F1,49 = 0.01 | F1,46 = 0.45 |
| HIV serostatus | F1,49 = 3.89b | F1,46 = 4.21b | F1,49 = 0.46 | F1,46 = 1.75 | F1,49 = 1.27 | F1,46 = 0.86 |
| Instructions | F2,98 = 1.62 | F2,92 = 0.33 | F2,98 = 63.67c | F2,92 = 1.31 | F2,98 = 37.77c | F2,92 = 1.69 |
| Electrodes | F2,98 = 0.44 | F2,92 = 0.38 | F2,98 = 17.10c | F2,92 = 1.31 | F2,98 = 26.50c | F2,92 = 0.59 |
| HIV × instructions | F2,98 = 0.11 | F2,92 = 0.23 | F2,98 = 6.12d | F2,92 = 5.38d | F2,98 = 4.97d | F2,92 = 4.22d |
TABLE 2. Summary of F Ratios for Repeated-Measures Analysis of Variance (ANOVA) and Analysis of Covariance (ANCOVA)a
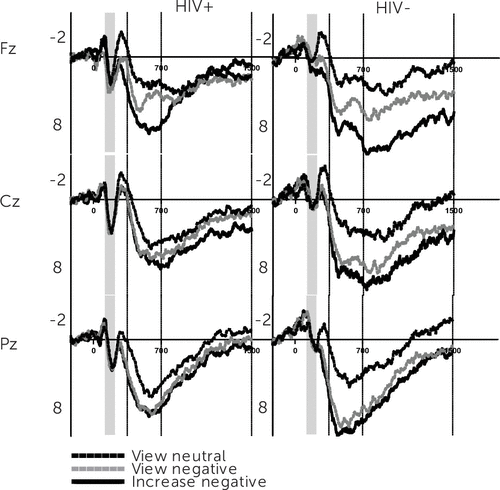
FIGURE 1. Event-Related Potential Amplitude (µv) on y-Axis Plotted Against Time (ms) on x-Axis for Increase Block
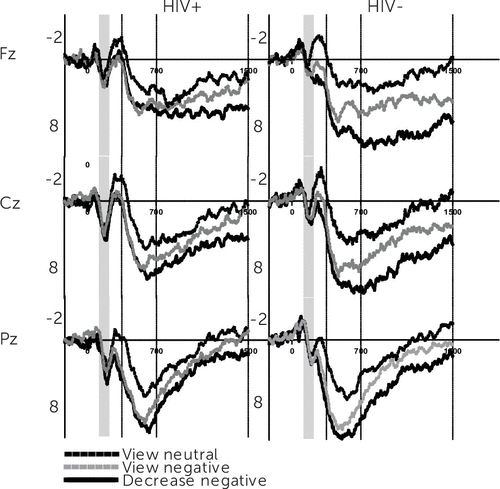
FIGURE 2. Event-Related Potential Amplitude (µv) on y-Axis Plotted Against Time (ms) on x-Axis for Decrease Block
P2
Defined by a positive deflection in the 200- to 300-ms range, the 2×2×3×3 repeated-measures ANOVA for P2 resulted in unexpected effects for HIV serostatus and experimental block (Table 1). On reexamination of these effects, controlling for individual differences in apathy and anxiety, only the main effect for HIV serostatus was found to prevail, whereby HIV+ women exhibited a larger P2 (mean=1.76, SE=0.52) compared with controls (mean=0.32, SE=0.51). Because the P2 is thought to index initial higher-order perceptual processing, these findings suggest an early automatic attention bias to negative stimuli for HIV+ women.
Early LPP
Table 2 summarizes the 2×2×3×3 repeated-measures ANOVA for the early LPP wherein significant main effects for electrode location and ER instructions emerged. Meaning, across participants and experimental blocks the early LPP increased from the passive viewing of neutral images to the cognitive reappraisal of unpleasant ones, with greater amplitudes observed in central-parietal than frontal electrode locations. In line with our hypothesis there was also an interaction between ER instructions and HIV status. The interaction was decomposed separately for each block. ER instructions to view neutral [F(1,49)=0.04, p>0.05, η2=0.001], view unpleasant [F(1,49)=1.64, p>0.05, η2=0.03], and negatively reappraise unpleasant stimuli [F(1,49)=2.79, p>0.05, η2=0.05] modulated the early LPP similarly across groups for the increase condition. A similar pattern for view neutral [F(1,49)=0.49, p>0.05, η2=0.001] and view negative conditions [F(1,49)=1.63, p>0.05, η2=0.03] was observed during the decrease experiment; however, instructions to positively reappraise unpleasant images yielded a significantly lower LPP amplitude for HIV+ compared with healthy control subjects [F(1,49)=6.85, p<0.01, η2=0.12]. These interactions prevailed after adjusting for apathy, anxiety, and depression, suggesting that for HIV+ women, the use of positive reappraisal to decrease unpleasant emotions does not necessitate additional emotional circuitry beyond that which is required to passively view affective stimuli. Because HIV, independent of psychiatric symptoms, was associated with early LPP attenuation during positive reappraisal, we conducted a post hoc ANCOVA to determine whether the duration of HIV infection predicted this deficit. Longer duration of HIV infection was inversely associated with amplitude changes in the 400- to 700-millsecond window of the LPP [F(2,46)=5.42, p<0.01, η2=0.19).
Late LPP
Table 2 also shows results from analysis of the late LPP (701–1500 ms). A main effect for electrode location and regulatory instructions was present along with an interaction for HIV serostatus × ER instructions. Decomposition of this interaction for the increase condition revealed late LPP amplitudes to neutral images were not different between groups [F(1,49)=0.05, p>0.05, η2=0.001]; however, for HIV+ women, the late LPP was marginally [F(1,49)=2.90, p<0.10, η2=0.05] and significantly lower [F(1,49)=4.07, p<0.05, η2=0.08] for the passive viewing and negative reappraisal of unpleasant images, respectively. During the decrease experiment, there was no difference in the late LPP for passively viewing neutral [F(1,49)=0.28, p>0.05, η2=0.006] and unpleasant [F(1,49)=0.08, p>0.05, η2=0.002] images, suggesting an LPP effect for both groups. However, positive reappraisal resulted in a marginally lower late LPP for HIV+ women [F(1,49)=3.50, p<0.10, η2=0.07] compared with controls. These interactions remained robust after controlling for individual differences in apathy, anxiety, and depression (Table 2). Again, a post hoc ANCOVA revealed an instruction × disease duration interaction whereby longer time since HIV diagnosis was associated with an attenuated late LPP across ER conditions of the decrease block [F(2,46)=3.99, p<0.05, η2=0.15].
Self-Assessment Manikin Ratings
To confirm that appropriate manipulation of emotions took place during the experiments, subjective valence ratings based on a Self-Assessment Manikin were compared using a 2×2×3 repeated-measures ANOVA. There was no effect for serostatus, suggesting HIV+ and HIV− women experience similar range of emotions during regulation despite demonstrating different levels of electrocortical activity. The main effect for experimental block was expected [F(1,31)=76.66, p<0.001, ηp2=0.71], whereby less negative emotions were evident during the decrease (mean=5.19, SE=0.20) compared with the increase experiment (mean=3.22, SE=0.11). As expected, Self-Assessment Manikin ratings also varied as a function of ER task instructions [F(2,62)=45.97, p<0.001, ηp2=0.60], whereby exposure to neutral images (mean=5.38, SE=0.15) resulted in a less negative experience than was associated with the passive viewing (mean=3.27, SE=0.17) or cognitive reappraisal of unpleasant images (mean=3.96, SE=0.19).
Discussion
This study adopted an emotion regulation paradigm20 to determine whether the P2 (200–300 ms), early LPP (400–700 ms), and late LPP (701–1500 ms) would vary in amplitude as a function of emotion regulation instructions independent of apathy, anxiety, and depressive symptomology. Unexpectedly, HIV+ women showed greater P2 relative to control subjects, suggesting an early attention bias to negative valence stimuli. In contrast, there was an HIV-related deficit in LPP amplitude during the decrease experiment that occurred independent of apathy, anxiety, and depressive symptoms.
Greater P2 amplitudes index automatic, stimulus driven allocation of attention to negative visual stimuli.18 The effect for greater P2 amplitudes to pictures in the increase block mirror previous reports19,20; however, our ANCOVA findings suggest this effect may be attributed to individual differences in apathy and anxiety. The reason why HIV+ women exhibited larger P2 is not fully understood, given the hypothesis of an HIV-related emotional deficit.1 Alternatively, trait differences in psychosocial function have been implicated in such attention biases. For example, greater levels of intolerance for uncertain and unpleasant events have been linked with P2 enhancement to affective stimuli in adult women.37 Uncertainty surrounding psychosocial stressors and changes in health status are common in persons living with HIV spectrum disease.38 Hence, the heightened vigilance to unpleasant stimuli at the earliest stages of attention may reflect a more automatic/conditioned response to threat-related stimuli.
A basic LPP effect for unpleasant versus neutral images was observed for both groups during the early LPP window. For both groups, the effect for ER instructions carried over to negative reappraisal condition; a finding that mimics the vast majority of ERP studies examining the increase of unpleasant emotions.16,20 In contrast, our finding of an increased LPP amplitude following instructions to decrease unpleasant emotions replicates a minority report among affective ERP studies.21,22 One explanation is whereas the aforementioned studies exclusively featured positive reappraisal as an emotion regulation strategy, the vast majority of ERP paradigms incorporating less restriction of ER strategy also report LPP attenuation following instructions to decrease unpleasant emotions.39,40 This latitude in methodology may allow for less cognitively demanding ER strategies, such as suppression, to be used for the decrease of unpleasant emotions. Furthermore, neuroimaging reports indicate increased hemodynamic response in a number of cortical structures, e.g., bilateral prefrontal areas, for positive reappraisal versus suppression of unpleasant emotions.41,42 In light of the cognitively demanding nature of the positive reappraisal task, our findings suggest less activation of emotional circuits underlying the positive reframing of unpleasant picture stimuli for HIV+ women. Several studies have implicated scarcity in cognitive resource allocation with HIV-related deficits in both cognitive43–45 and affective27 ERP paradigms. Thus, we propose that the LPP attenuation found in HIV+ women may reflect limited allocation of attention during appraisal and reappraisal of unpleasant emotions, which in turn is associated with longer HIV disease duration.
As it pertains to the lack of association between CD4 T-cell count and viral load on the LPP, others have shown that CD4 count and duration of infection better predicted reductions in cortical volume within the parietal, temporal, and frontal lobes.46 HIV disease history may indeed be a better proxy of neurological impairment as several have observed cortical/subcortical atrophy and neurocognitive dysfunction to persist despite low or reduced plasma viral load.47–53 Although the structural and functional etiology of HIV on emotional brain circuitry is still being unraveled, evidence of neuro-inflammatory changes in affective structures such as the insula and anterior cingulate cortex54,55 are rapidly emerging. The integrity of these structures and adjacent circuits could possibly be implicated in our ER findings as structures of the salience network and temporal-parietal-occipital junction may contribute to generation of the scalp-recorded LPP.56–58
Limitations
Sample characteristics, i.e., cognitively intact adult women with subclinical levels of apathy, anxiety, and depression, make it difficult to extrapolate these findings to men, children, or those showing more severe signs of neuropsychiatric or neurocognitive impairment. It should also be noted that despite explicit training in the use of cognitive reappraisal, it is impossible to control intrapersonal variability in emotion regulation strategies that may be covertly used to increase, decrease, or maintain negative emotions. Despite this, participants reported similar use in ER strategy and modulation of emotional arousal throughout the experiments. Nonetheless, absence of a simple picture valence effect for HIV+ women during late LPP may limit interpretation of the interaction between HIV serostatus and emotion regulation at later epochs.
Conclusions
This study revealed an HIV-related deficit in the early LPP, during the positive reappraisal of emotions, which worsened as a function of longer disease duration. Deficits in the cognitive regulation of emotions are a grave concern as use of more avoidance (e.g., suppression) and less approach-oriented coping (e.g., positive reappraisal) contribute to greater levels of psychological distress, particularly in women living with HIV/AIDS.59 In the absence of a cure for HIV, the dysregulation of emotions may create an added barrier for disease management, medication adherence, and psychosocial functioning, as well as provide an avenue for greater psychosocial stress, immune compromise, and mortality.60 This study provides electrophysiological evidence that the cognitive regulation of emotions is impaired in HIV and much like other neurological complaints corresponds with disease history.46,61,62 Future studies will need to investigate whether particular domains of HIV-associated neurocognitive dysfunction underlie deficits in the LPP and further elucidate the functional significance of early attention bias to negative stimuli in persons advancing with HIV disease.
1 : Emotional deficit as a neuropsychopathological disturbance in HIV infection. J Int Neuropsychol Soc 2001; 7:775–777Crossref, Medline, Google Scholar
2 : Neuropsychiatric disturbance is associated with executive dysfunction in HIV-1 infection. J Int Neuropsychol Soc 2000; 6:336–347Crossref, Medline, Google Scholar
3 : Relationship between psychiatric status and frontal-subcortical systems in HIV-infected individuals. J Int Neuropsychol Soc 2007; 13:549–554Crossref, Medline, Google Scholar
4 : Apathy is associated with volume of the nucleus accumbens in patients infected with HIV. J Neuropsychiatry Clin Neurosci 2005; 17:167–171Link, Google Scholar
5 : Meta-analysis of the relationship between HIV infection and risk for depressive disorders. Am J Psychiatry 2001; 158:725–730Crossref, Medline, Google Scholar
6 : HIV infection and psychiatric illness. Afr J Psychiatry (Johannesbg) 2009; 12:115–128Medline, Google Scholar
7 : Stability of anxiety and depression in a national sample of adults with human immunodeficiency virus. J Nerv Ment Dis 2004; 192:111–118Crossref, Medline, Google Scholar
8 : Exploration of the relevance of anxiety sensitivity among adults living with HIV/AIDS for understanding anxiety vulnerability. J Health Psychol 2010; 15:138–146Crossref, Medline, Google Scholar
9 : Neuropsychiatric aspects of HIV infection among older adults. J Clin Epidemiol 2001; 54(Suppl 1):S44–S52Crossref, Medline, Google Scholar
10 : Underdiagnosis of depression in HIV: who are we missing? J Gen Intern Med 2003; 18:450–460Crossref, Medline, Google Scholar
11 : The relation between emotional dysregulation and anxiety and depressive symptoms, pain-related anxiety, and HIV-symptom distress among adults with HIV/AIDS. J Psychopathol Behav Assess 2013; 35:1–8Crossref, Google Scholar
12 : Main and interactive effects of emotion dysregulation and breath-holding duration in relation to panic-relevant fear and expectancies about anxiety-related sensations among adult daily smokers. J Anxiety Disord 2012; 26:173–181Crossref, Medline, Google Scholar
13 : Emotion regulation and mental health. Clin Psychol Sci Pract 1995; 2:151–164Crossref, Google Scholar
14 Gross JJ: The emerging field of emotion regulation: An integrative review. Rev Gen Psych 1998; 2:271–299Google Scholar
15 : Affective picture processing: an integrative review of ERP findings. Biol Psychol 2008; 77:247–265Crossref, Medline, Google Scholar
16 : Event-related potentials, emotion, and emotion regulation: an integrative review. Dev Neuropsychol 2010; 35:129–155Crossref, Medline, Google Scholar
17 : Emotion and attention interaction studied through event-related potentials. J Cogn Neurosci 2001; 13:1109–1128Crossref, Medline, Google Scholar
18 : Emotion, attention, and the ‘negativity bias’, studied through event-related potentials. Int J Psychophysiol 2001; 41:75–85Crossref, Medline, Google Scholar
19 : The time course of the influence of valence and arousal on the implicit processing of affective pictures. PLoS ONE 2012; 7:e29668Crossref, Medline, Google Scholar
20 : Electrophysiological correlates of decreasing and increasing emotional responses to unpleasant pictures. Psychophysiology 2009; 46:17–27Crossref, Medline, Google Scholar
21 : Effects of instructed emotion regulation on valence, arousal, and attentional measures of affective processing. Dev Neuropsychol 2011; 36:493–518Crossref, Medline, Google Scholar
22 : Differentiating electrophysiological response to decrease and increase negative emotion regulation. Chin Sci Bull 2013; 58:1543–1550Crossref, Google Scholar
23 : Reduced electrocortical response to threatening faces in major depressive disorder. Depress Anxiety 2010; 27:813–820Crossref, Medline, Google Scholar
24 : The late positive potential, emotion and apathy in Parkinson’s disease. Neuropsychologia 2013; 51:960–966Crossref, Medline, Google Scholar
25 : Enhanced neural reactivity and selective attention to threat in anxiety. Biol Psychol 2010; 85:252–257Crossref, Medline, Google Scholar
26 : Distinct electrocortical and behavioral evidence for increased attention to threat in generalized anxiety disorder. Depress Anxiety 2010; 27:234–243Crossref, Medline, Google Scholar
27 Tartar JL, McIntosh RC, Rosselli, et al: HIV-positive females show blunted neurophysiological responses in an emotion-attention dual task paradigm. Clin Neurophys 2014; 125:1164–1173Google Scholar
28 : “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189–198Crossref, Medline, Google Scholar
29 : Reliability and validity of the Apathy Evaluation Scale. Psychiatry Res 1991; 38:143–162Crossref, Medline, Google Scholar
30 : Internal consistencies of the original and revised Beck Depression Inventory. J Clin Psychol 1984; 40:1365–1367Crossref, Medline, Google Scholar
31 Spielberger CD: Manual for the State-Trait Anxiety Inventory STAI (Form Y)(“ Self-Evaluation Questionnaire”). 1983.Google Scholar
32 : Individual differences in two emotion regulation processes: implications for affect, relationships, and well-being. J Pers Soc Psychol 2003; 85:348–362Crossref, Medline, Google Scholar
33 Wechsler D: Adult Intelligence Scale–Third Edition (WAIS-III), San Antonio, TX: Psychological Corporation, 1997Google Scholar
34 : International Affective Picture System (IAPS): Instruction Manual and Affective Ratings, Center for Research in Psychophysiology, University of Florida, 1999Google Scholar
35 : The ten twenty electrode system of the international federation. Electroencephalogr Clin Neurophysiol 1958; 10:371–375Google Scholar
36 : Guidelines for reducing the risk of disease transmission in the psychophysiology laboratory. Psychophysiology 1992; 29:127–141Crossref, Medline, Google Scholar
37 : Event-related potentials during exposure to aversion and its anticipation: the moderating effect of intolerance of uncertainty. Neurosci Lett 2012; 507:112–117Crossref, Medline, Google Scholar
38 : Social support and the management of uncertainty for people living with HIV or AIDS. Health Commun 2004; 16:305–331Crossref, Medline, Google Scholar
39 : Increasing negative emotions by reappraisal enhances subsequent cognitive control: a combined behavioral and electrophysiological study. Cogn Affect Behav Neurosci 2010; 10:195–207Crossref, Medline, Google Scholar
40 : Previously reappraised: the lasting effect of description type on picture-elicited electrocortical activity. Soc Cogn Affect Neurosci 2011; 6:348–358Crossref, Medline, Google Scholar
41 : The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biol Psychiatry 2008; 63:577–586Crossref, Medline, Google Scholar
42 : Neural correlates of positive and negative emotion regulation. J Cogn Neurosci 2007; 19:776–798Crossref, Medline, Google Scholar
43 : ERP evidence of impaired central nervous system function in virally suppressed HIV patients on antiretroviral therapy. Clin Neurophysiol 2004; 115:1583–1591Crossref, Medline, Google Scholar
44 : Neuroelectric assessment of HIV: EEG, ERP, and viral load. Int J Psychophysiol 2000; 38:97–108Crossref, Medline, Google Scholar
45 : ERPs differ from neurometric tests in assessing HIV-associated cognitive deficit. Neuroreport 2004; 15:1675–1678Crossref, Medline, Google Scholar
46 : Effects of nadir CD4 count and duration of human immunodeficiency virus infection on brain volumes in the highly active antiretroviral therapy era. J Neurovirol 2010; 16:25–32Crossref, Medline, Google Scholar
47 : Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS 2010; 24:1243–1250Medline, Google Scholar
48 : HIV dementia: an evolving disease. J Neuroimmunol 2004; 157:3–10Crossref, Medline, Google Scholar
49 : HIV-associated cognitive impairment before and after the advent of combination therapy. J Neurovirol 2002; 8:136–142Crossref, Medline, Google Scholar
50 : Persistence of HIV-associated cognitive impairment, inflammation, and neuronal injury in era of highly active antiretroviral treatment. AIDS 2011; 25:625–633Crossref, Medline, Google Scholar
51 : Subcortical brain atrophy persists even in HAART-regulated HIV disease. Brain Imaging Behav 2011; 5:77–85Crossref, Medline, Google Scholar
52 : Accelerated Aging of Selective Brain Structures in HIV Infection: A Controlled, Longitudinal MRI Study. Neurobiol Aging 2014Crossref, Medline, Google Scholar
53 : Lowest ever CD4 lymphocyte count (CD4 nadir) as a predictor of current cognitive and neurological status in human immunodeficiency virus type 1 infection—The Hawaii Aging with HIV Cohort. J Neurovirol 2006; 12:387–391Crossref, Medline, Google Scholar
54 : Regional cortical thinning associated with detectable levels of HIV DNA. Cereb Cortex 2012; 22:2065–2075Crossref, Medline, Google Scholar
55 : Regional brain structural dysmorphology in human immunodeficiency virus infection: effects of acquired immune deficiency syndrome, alcoholism, and age. Biol Psychiatry 2012; 72:361–370Crossref, Medline, Google Scholar
56 : Emotional perception: correspondence of early and late event-related potentials with cortical and subcortical functional MRI. Biol Psychol 2013; 92:513–519Crossref, Medline, Google Scholar
57 : Neural substrate of the late positive potential in emotional processing. J Neurosci 2012; 32:14563–14572Crossref, Medline, Google Scholar
58 : Where and when the anterior cingulate cortex modulates attentional response: combined fMRI and ERP evidence. J Cogn Neurosci 2006; 18:766–780Crossref, Medline, Google Scholar
59 : Stress and coping in women living with HIV: a meta-analytic review. AIDS Behav 2012; 16:2144–2159Crossref, Medline, Google Scholar
60 : Stress management and psychoneuroimmunology in HIV infection. CNS Spectrums 2003; 8:40–51Crossref, Google Scholar
61 : Neurological complications of HIV infection. Lancet Neurol 2005; 4:543–555Crossref, Medline, Google Scholar
62 : HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 2010; 75:2087–2096Crossref, Medline, Google Scholar



