Systematic Review of an Emerging Trend in China: Resting-State Functional Connectivity in Major Depressive Disorder
Abstract
Major depressive disorder continues to challenge medical and psychological resources worldwide. A marked surge has occurred recently in China in neuroimaging studies of major depressive disorder. Those studies represent an emerging trend in neuropsychiatry in that such research has previously been extremely rare in China. The present article provides a systematic review of reports published in English by research institutes in China on resting-state functional connectivity studied by MRI in depressed subjects and healthy control subjects. Particular attention is given to whether the information may advance effective diagnosis and treatment options for patients with major depressive disorder.
One interesting feature of brain imaging in China concerns the clinical condition of depressed subjects that participate in research projects. In many cases, they are severely depressed but have never received treatment during their first episode of major depressive disorder that may have lasted up to several months. The fact that such treatment-naïve individuals can be found for research projects in China, despite the adversity of their depressive condition, was accounted for recently by Wang et al.1 They explained that a large number of patients with mental diseases in China remain unidentified until their disorder becomes particularly severe. In fact, 90% of persons with mood disorders requiring treatment never receive any type of professional help before turning up at major mental health centers.2 Those conditions in China have made it possible to perform large studies of first-episode treatment-naive patients with severe mood disorders that have lasted for several months. Such a population of subjects with major depressive disorder is unique in providing favorable conditions for studying possible links between brain function and psychopathology.
China continues to expand in terms of neuroscience research. Of particular interest for this review is a recent surge in the use of MRI with mood disorders in China. That surge is evidenced by the fact that only six studies from China had been published in English on functional connectivity in mood disorders prior to April 2012,3 whereas more than 30 reports are included in the present account (Table 1). My interest in studies carried out on mood disorders in China stems primarily from a recent collaboration that alerted me to uncertainties in such findings.4 The present review concerns studies published in English on functional connectivity in the brain of depressed subjects by groups in China, with particular interest in the potential impact of such research for effective diagnosis and treatment options for patients with neuropsychiatric disorders.
| Data Analysis | MDD>HC | MDD<HC | Reference |
|---|---|---|---|
| First-episode, treatment-naïve depressed subjects | |||
| ReHo | Left middle temporal gyrus genotype- dependent | 92 | |
| ReHo | No differences | No differences | 93 |
| Seed-based anterior cingulate cortex and thalamus | Left parahippocampus, parietal lobe and frontal lobe | Right parietal lobe and cingulate gyrus | 45 |
| Seed-based left posterior cingulate cortex | Bilateral dorsolateral prefrontal cortex and inferior parietal lobe | Medial prefrontal cortex / orbitofrontal cortex | 46 |
| VMHC | Medial prefrontal cortex and posterior cingulate cortex / precuneus | 47 | |
| ALFF | Fusiform gyri and anterior and posterior lobes of the cerebellum | Left inferior temporal gyrus and right inferior parietal lobe | 1 |
| ALFF | Left superior occipital gyrus and cuneus | Right cerebellum posterior lobe, left parahippocampal gyrus and right middle frontal gyrus | 39 |
| ALFF | Right dorsolateral frontal cortex, bilateral inferior frontal gyrus and orbital frontal region | Left insula, bilateral caudate and left hippocampus | 43 |
| Network analysis | Left hippocampus and right parahippocampal gyrus | Left superior orbitofrontal cortex and left medial frontal cortex | 48 |
| Graph analysis | Anterior cingulate cortex, dorsolateral, medial and inferior prefrontal cortex, insula, amgydala and superior and middle temporal gyri | 44 | |
| Depressed outpatients | |||
| ReHo | Insula, cerebellum anterior lobe, middle frontal gyrus, superior temporal gyrus, lentiform nucleus, precentral gyrus and postcentral gyrus | Interior frontal gyrus, inferior parietal lobule and posterior lobe of the cerebellum | 50 |
| ReHo and ROI analysis | Bilateral anterior cingulate cortex, medial prefrontal cortex, right insula and right parahippocampalgyrus | Left posterior fusiform gyrus, inferior frontal area, inferior parietal lobule and caudate | 51 |
| ReHo | Right orbitofrontal cortex, fusiform gyrus, ventral anterior cingulate gyrus, posterior cingulate gyrus. Left dorsal anterior cingulated gyrus and lentiform nucleus | 55 | |
| ReHo | Right inferior temporal gyrus | Left posterior lobe of the cerebellum, right fusiform gyrus, left parahippocampal gyrus and right postcentral gyrus | 56 |
| ReHo | Left thalamus, left temporal lobe, bilateral occipital lobe and left posterior lobe of the cerebellum | 94 | |
| Seed-based cerebellar regions | Regions of the cerebellum, left middle frontal cortex and poles of the temporal cortex | Regions of the cerebellum, posterior cingulate cortex, ventromedial prefrontal cortex, superior frontal gyri, left orbitalfrontal gyrus and hippocampus | 12 |
| Seed-based right dorsolateral prefrontal cortex | Right dorsolateral prefrontal cortex, left anterior cingulate cortex, left parahippocampal gyrus, thalamus and precentral gyrus | Right dorsolateral prefrontal cortex and right parietal lobe | 52 |
| Seed-based left and right hippocampus | Left and right hippocampus, middle frontal gyrus, inferior parietal lobe and cerebellum | 53 | |
| Seed-based left and right amygdala | Amygdala and left ventral prefrontal cortex | 54 | |
| Seed-based 16 ROIs | Left thalamus and right cingulate cortex; right insula and precuneus | 57 | |
| ALFF | Right dorsomedial frontal gyrus and right precuneus | Bilateral lingual gyri | 49 |
| Treatment-resistant, depressed subjects | |||
| ReHo | Left superior temporal gyrus, anterior and posterior lobe of the cerebellum, right cerebellar tonsil and bilateral fusiform gyrus | Left insula, superior temporal gyrus, inferior frontal gyrus, lingual gyrus and anterior lobe of the cerebellum | 95 |
| Seed-based 16 ROIs | Precuneus, left middle frontal gyrus and cingulate cortex | 57 | |
| Seed-based gray matter volume of right middle temporal gyrus and right caudate nucleus | Right precuneus, middle temporal gyrus, bilateral superior frontal gyrus and left middle frontal gyrus | Right cuneus | 63 |
| Elderly depressed subjects | |||
| ReHo | Left anterior insula, right posterior insula and right dorsolateral prefrontal cortex | Right orbitofrontal cortex, left middle frontal gyrus, left dorsolateral prefrontal, left inferior temporal gyrus and left postcentral gyrus | 67 |
| ReHo | Superior frontal gyrus, postcentral gyrus and putamen | Superior and middle frontal and temporal gyri, postcentral gyrus, fusiform gyrus and precuneus | 68 |
| Cohe-ReHo | Left posterior lobe of the cerebellum, left superior temporal gyrus, bilateral supplementary motor area and right postcentral gyrus | Left caudate nucleus, right anterior cingulate gyrus, left dorsolateral prefrontal cortex, right angular gyrus, bilateral medial prefrontal cortex and right precuneus | 64 |
| Seed-based posterior cingulate cortex | Posterior cingulate cortex and temporal cortex | Posterior cingulate cortex and frontal cortex | 65 |
| Suicidal, traumatized, or somatically ill depressed subjects | |||
| ALFF | Parahippocampal gyrus, anterior cingular gyrus and angular gyrus of the parietal lobe | 69 | |
| ALFF | Anterior and posterior lobes of the cerebellum | Right dorsolateral prefrontal cortex, ventromedial prefrontal cortex, superior frontal cortex, right middle temporal gyrus and rostral anterior cingulate cortex | 71 |
| Graph analysis | Prefrontal cortex and anterior cingulate gyrus | 70 |
TABLE 1. Summary of Resting-State MRI Studies of Functional Connectivity in Subjects With Major Depressive Disorder and Healthy Control Subjects in Chinaa
Methods
Literature Search
Reports cited in this article were retrieved on June 20, 2013 using PubMed and keywords “resting state fMRI major depression.” Abstracts of the retrieved articles were examined to find studies carried out in China. Reference lists of those articles were searched for further reports of interest. Four criteria were used for inclusion of reports in this review (Figure 1): (1) the research must have been carried out in China alone, (2) the report must contain an adequate description of experimental methods including appropriate diagnostic procedures, (3) the report must provide an adequate presentation of statistical methods and empirical results, and (4) the study must include a nondepressed, healthy control group for comparison with individuals that have a history of major depressive disorder.
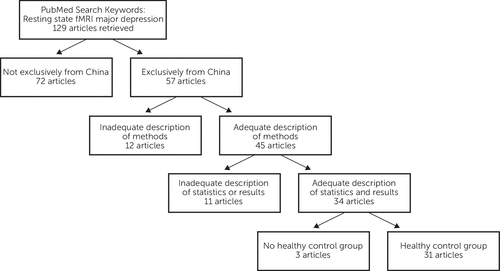
FIGURE 1. Flux Diagram Showing the Criteria for Selection of Articles for Review
Main Features of Resting-State Neuroimaging
Recent advances in the acquisition and analysis of resting-state MRI data have provided a means of assessing functional connectivity in the human brain.5 Originally, Raichle et al. used positron emission tomography (PET) to study oxygen metabolism in the normal adult human brain and noted an unexpected decrease in oxygen metabolism in some brain regions when subjects changed from a resting condition to a more active condition, in which they processed visual stimuli.6 They coined the term “default mode network” to encompass brain regions that had higher levels of oxygen metabolism while at rest than while actively engaged in a cognitive task. They stated that the default mode network, consisting primarily of regions of the medial parietal cortex (i.e., precuneus and posterior cingulate), bilateral inferior parietal cortex, ventromedial frontal cortex, and cerebellum may be intimately involved in cognitive functions.7 Since then, possible relationships between brain function in the resting state and psychic disorders have inspired studies of major depressive disorder, and numerous networks have been described.8–11
Resting-state MRI requires only that subjects remain as motionless as possible while they lay on their back within the confines of the scanner. They are instructed to relax and not to think of anything in particular.12,13 Subjects are told to keep their eyes opened in some studies, whereas in other studies they are instructed to keep their eyes closed. Subjects are fitted with earplugs to reduce the penetrating noise generated by the scanner, and their head is held in place by padding inside a sturdy mask-like head holder that contains signal detection equipment. Although the time needed for resting-state MRI is usually less than 10 minutes, subjects may spend more than 30 minutes in the scanner for the technicians to properly carry out all procedures.
MR scanners use rapid fluctuations in electromagnetic fields to induce changes in the orientation of electrons spinning about atoms in the body. For an MRI scan of the brain, an echo-planar scanning sequence is typically used for recording the temporal level of oxygen in the bloodstream throughout the brain, a parameter known as the blood-oxygen-level-dependent (BOLD) response.14–16 The software provided by the MR scanner records a digital time series of BOLD signals emanating from millions of individual cubic units, known as voxels, in the brain.17,18 Thus, MRI data are time series of the frequency and amplitude of oscillations in the BOLD signal that occurred in the brain of the subject in the MR scanner during the recording session. It is important to keep in mind that the BOLD signal is only an indirect indicator of hemodynamic events in the brain and that the relationship of the BOLD signal to neuronal activity remains unsettled.19 Recent studies show that variables affecting cerebral blood flow, such as respiration rate, caffeine, and psychotropic drugs,20–22 require close attention in comparisons of BOLD signal activations between subjects with neuropsychiatric disorders and healthy control subjects.
Main Features of Resting-State Functional Connectivity MRI
Analysis of data from studies of resting-state functional connectivity MRI (rs-fcMRI) produces a correlation matrix for BOLD signals arising from pairs of individual voxels in the brain.23,24 Voxel pairs that have a relatively high correlation coefficient for the time course of their BOLD signals are said to show functional connectivity.24–26 Correlation coefficients for depressed subjects are compared with those for healthy subjects to determine whether functional connectivity differs markedly between the two groups.
Analysis of resting-state MRI data can be data-driven or model-driven.5,13 Data-driven analyses make few assumptions concerning the characteristics of the BOLD signal and regarding whether they must be similar between brain regions. Data-driven functional MRI (fMRI) analyses can often detect short-lived variations in BOLD signals that may identify unexpected functional links between brain regions.27 In contrast, model-driven analyses assume that the defining characteristics of the BOLD signal are known and that they are identical for all brain regions. For those who are unfamiliar with the methods that are currently used for data analysis of rs-fcMRI recordings, here is a brief account of their main principles.
Main Principles of rs-fcMRI Data Analysis
Regional homogeneity (ReHo) is a data-driven approach that assumes that the time series of BOLD signals for a given voxel is temporally similar to that of its neighbors.13 The outcome of ReHo analysis of fMRI data are an array of ranked correlations describing the synchrony of BOLD signals emanating from adjacent voxels or clusters of voxels. Higher correlations of BOLD signals among adjacent regions in one group of subjects than in another group reflects greater ReHo that is commonly interpreted as an indication of more functional connectivity. One advantage of ReHo for analysis of fMRI recordings is claimed to be disclosure of unexpected regional functional connectivity, because the method has no a priori requirement for intraregional hypotheses.13 ReHo has become increasingly popular in studies of resting-state fMRI in China and elsewhere.28 Recent work in China shows, however, that the outcome of ReHo depends heavily on the selection of experimental procedures.29 Analysis of fMRI data according to ReHo is carried out by a nonparametric statistical procedure known as Kendall’s coefficient of concordance (KCC), which normalizes the data and assigns a value to the time series of an individual voxel with respect to its nearest neighbors ranging from 0 (no similarity) to 1 (identical). Thus, ReHo provides a qualitative, ranked index of the similarity of fluctuations of BOLD signals (i.e., synchrony) among adjacent voxels within the brain. ReHo uses only the correlation between the ranks of BOLD time series to assess functional connectivity.
Coherence-based regional homogeneity takes the frequency distribution of BOLD signals into account for estimating the degree of similarity between voxels.30
Seed-based analysis of resting-state fMRI data is a model-driven approach that requires selection of a region of interest in the brain as a starting point for generating correlation matrices based on BOLD signals in the region of interest compared with other regions.7,31
Voxel-mirrored homotopic connectivity (homotopic means “same place”) is a computer-based procedure that uses data from resting-state fMRI to explore correlations between BOLD signals from identical sites on opposite sides of the brain.32–34 Because ReHo and seed-based analyses of resting-state fMRI recordings fail to indicate the direction and the mutual throughput of functional connectivity, voxel-mirrored homotopic connectivity was developed to address these issues.23,35
Amplitude of low frequency fluctuations (ALFF) takes the amplitude of brain activity as measured by BOLD signals in resting-state fMRI into account.36,37 In ALFF, the power spectrum of BOLD signals in the low-frequency range is used for calculating correlations to estimate the degree of functional connectivity among voxels.
Fractional ALFF is the procedure in which ALFF values for each voxel are divided by the global ALFF mean to normalize the data prior to statistical comparisons.38,39
Network analysis is a computational procedure that views the brain as a whole in search of dynamic, functional networks.40
Graph analysis, on the other hand, is a computational procedure that views the human brain as consisting mainly of dense clusters of local connectivity with relatively few long-range connections between any pair of neurons or brain regions.41 In graph theory, the likelihood that a particular region is a node in a network is defined by the number of nodes with strong correlations with the target node.42 Graph analysis uses correlation coefficients to determine the degree of connectedness of nodes identified on the basis of whole-brain BOLD recordings.
Results
First-Episode, Treatment-Naïve Depressed Subjects
Ten studies were found that have been carried out in China and published in English on rs-fcMRI in first-episode, treatment-naïve depressed subjects (Table 1). These studies are unique in providing information on potential disturbances in pristine cases of severe and untreated major depressive disorder. If consistent differences were to be found by rs-fcMRI between such subjects and healthy control subjects, then the methodology would provide invaluable information on cerebral function of great clinical utility. Sadly, these studies failed to provide consistent findings. Thus, four studies noted greater functional connectivity in regions of the frontal cortex of first-episode, treatment-naïve depressed subjects than healthy control subjects,43–46 whereas three other studies found lesser functional connectivity in frontal regions of depressed subjects than in healthy control subjects.39,47,48
The regions of the parietal and temporal lobes, as well as the hippocampus, have also received marked attention in studies carried out in China on first-episode, treatment-naïve depressed subjects and healthy control subjects, but they too have failed to reveal consistent patterns of differences in rs-fcMRI findings between the two groups (Table 1). For example, greater functional connectivity was noted in the brain of first-episode, treatment-naïve depressed subjects than in healthy control subjects in parietal and temporal regions in five studies,39,44–46,48 but three other studies obtained opposite results.1,43,47
Depressed Outpatients
Eleven studies were identified that used rs-MRI to study the functional connection in the brain of depressed subjects that were outpatients at psychiatric facilities in China (Table 1). Some of these outpatients may have been both in their first episode and treatment naïve, but no information was provided on that in the published reports. Five studies noted greater functional connectivity in frontal and prefrontal regions of depressed subjects than in healthy control subjects,12,49–52 but opposite findings were described in five other reports.12,52–55 Regions of the posterior cingulate, precureus, and temporal cortex were found to have greater functional connectivity in depressed subjects than in healthy control subjects in four studies,12,49,50,56 but the situation was reversed in six other reports.51–53,55,57,58 Functional connectivity in subcortical regions such as the thalamus, caudate, insula, and hippocampus also failed to differentiate consistently between depressed outpatients and healthy control subjects in this series of studies.
Treatment-Resistant Depressed Subjects
Three studies were noted on rs-fcMRI in treatment-resistant depression, which is currently a major challenge in clinical practice (Table 1).59,60 Over the years, much attention has been given to whether consistent disturbances in brain function characterize treatment-resistant depression, in hope of finding effective cures.61,62 Of the studies on rs-fcMRI that have been carried out in China in treatment-resistant depressed subjects and healthy control subjects, no consistent findings have been obtained. For instance, functional connectivity was greater in the precuneus and middle frontal region of treatment-resistant depressed subjects than in healthy control subjects in one study,63 whereas opposite findings were noted in another report.57 It is, however, too soon to say whether further studies can disclose reliable features of functional connectivity that govern treatment-resistant depression.
Elderly Depressed Subjects
Four studies were found that compared functional connectivity in the brain of elderly depressed subjects with that in age-matched healthy control subjects (Table 1). Two of the studies noted greater functional activity in temporal cortex of elderly depressed subjects than in elderly healthy control subjects,64,65 whereas opposite findings were reported in the other two studies.66–68 Thus, no general conclusion can be drawn on the direction of differences in functional connectivity that may characterize brain function in elderly depressed subjects versus age-matched healthy control subjects.
Suicidal, Traumatized, or Somatically Ill Depressed Subjects
A few recent studies have been carried out in China on functional connectivity in the brain of healthy control subjects versus depressed patients who were suicidal, traumatized in childhood, or afflicted by Parkinson’s disease (Table 1). Greater functional connectivity was noted in the anterior cingulate cortex of depressed suicidal subjects than in healthy control subjects,69 whereas lesser functional connectivity was found in the anterior cingulate cortex of depressed subjects traumatized in childhood and in depressed subjects with Parkinson’s disease than in healthy control subjects.70,71
Discussion
The main conclusion to be drawn from studies reviewed here on rs-fcMRI in depressed subjects and healthy control subjects in China is that consistent differences failed to appear for functional connectivity between the two groups. This conclusion coincides with the failure of other neuroimaging modalities to identify biomarkers that are specific for major depression disorder.72–74
One of the factors that may contribute to the failure of traditional research designs to identify reliable biomarkers of major depressive disorder concerns the heterogeneity of the disease.75,76 Future studies of major depressive disorder may, therefore, require stratifying the disorder by longitudinal designs that assess brain function in relation to temporal variations in symptoms.73 Another factor that may contribute to the failure of rs-fMRI to show consistent differences between depressed subjects and health control subjects relates to the diversity of techniques used for processing the BOLD signal.77–81 Most data-processing procedures used for rs-fcMRI rest on correlation analysis, which is notorious for both false-positive findings and failing to demonstrate causal relationships.82,83 Novel research designs in which rs-fcMRI recordings are carried out repeatedly to estimate test–retest reliability84 in individual subjects in varying mood states may aid in identifying reliable relationships between mood state and functional connectivity in the brain. A third factor that may affect the outcome of studies on rs-fMRI in major depressive disorder relates to confirmation bias.85–87 Over the years, neuroscience research has become an increasingly competitive field with an engrained “publish or perish” atmosphere. That situation may tend to cause researchers to use ever-increasing computing power plus a never-ending array of procedures for data analysis to achieve statistically significant findings that correspond with current notions on relationships between resting-state brain activity and psychic depression. Conformation bias may be limited by requiring preregistration of research project protocols and electronic submission of empirical data at public websites,87 so that others can determine whether their procedures can replicate the outcome of the original study.88,89
For those interested in converting results from brain imaging studies in major depressive disorder into useful clinical guidelines, there is still a long way to go for rs-fcMRI. The failure of traditional research on rs-fcMRI to disclose reliable links in depressed subjects between symptoms and brain function has stimulated interest in alternative research strategies.73,90 Detailed clinical assessment and neuroimaging of individual depressed subjects over time may serve to stratify subjects with major depressive disorder into evidence-based categories on the basis of syndrome subtypes. Neuroimaging studies may then aid in the selection of effective antidepressant treatment regimens.91 Although longitudinal studies may require more subjects and more staff than are typically included in traditional cross-sectional research projects on rs-fcMRI, the use of mobile MRI units with highly trained technicians in major cities may facilitate the task. Such revisions of experimental designs in resting-state studies of functional connectivity in major depressive disorder in China as elsewhere may eventually provide information of clinical utility.
1 : Amplitude of low-frequency oscillations in first-episode, treatment-naive patients with major depressive disorder: a resting-state functional MRI study. PLoS ONE 2012; 7:e48658Crossref, Medline, Google Scholar
2 : Prevalence, treatment, and associated disability of mental disorders in four provinces in China during 2001-05: an epidemiological survey. Lancet 2009; 373:2041–2053Crossref, Medline, Google Scholar
3 Wang L, Hermens DF, Hickie IB: A systematic review of resting-state functional-MRI studies in major depression. J Affect Disord 2012; 142:6–12Crossref, Medline, Google Scholar
4 : Replication attempt to estimate depression severity by fuzzy logic analysis of emotion-focused fMRI. J Magn Reson Imaging 2013; 37:498–499Crossref, Medline, Google Scholar
5 : Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur Neuropsychopharmacol 2010; 20:519–534Crossref, Medline, Google Scholar
6 : A default mode of brain function: a brief history of an evolving idea. Neuroimage 2007; 37:1083–1090, discussion 1097–1099Crossref, Medline, Google Scholar
7 : Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 2007; 8:700–711Crossref, Medline, Google Scholar
8 : Integrative deficits in depression and in negative mood states as a result of fronto-parietal network dysfunctions. Acta Neurobiol Exp (Warsz) 2013; 73:313–325Medline, Google Scholar
9 : Prefrontal cortical networks related to visceral function and mood. Ann N Y Acad Sci 1999; 877:383–396Crossref, Medline, Google Scholar
10 : Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nat Neurosci 2007; 10:1116–1124Crossref, Medline, Google Scholar
11 : Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct 2008; 213:93–118Crossref, Medline, Google Scholar
12 : Altered cerebellar functional connectivity with intrinsic connectivity networks in adults with major depressive disorder. PLoS ONE 2012; 7:e39516Crossref, Medline, Google Scholar
13 : Regional homogeneity approach to fMRI data analysis. Neuroimage 2004; 22:394–400Crossref, Medline, Google Scholar
14 : Echo-planar imaging (EPI) and functional MRI, in Functional MRI. Edited by Moonen CTW, Bandettini PA. Berlin, Germany, Springer, 1998, pp 137–148Google Scholar
15 : The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA 2005; 102:9673–9678Crossref, Medline, Google Scholar
16 Nierhaus T, Margulies DS, Long X, et al: fMRI for the Assessment of Functional Connectivity, in Neuroimaging Methods. Edited by Bright P. Rijeka, Croatia, University Campus STeP Ri; 2012; pp. 29-46Google Scholar
17 : Beyond mind-reading: multi-voxel pattern analysis of fMRI data. Trends Cogn Sci 2006; 10:424–430Crossref, Medline, Google Scholar
18 : The problem of low variance voxels in statistical parametric mapping; a new hat avoids a ‘haircut’. Neuroimage 2012; 59:2131–2141Crossref, Medline, Google Scholar
19 : Neuronal inhibition and excitation, and the dichotomic control of brain hemodynamic and oxygen responses. Neuroimage 2012; 62:1040–1050Crossref, Medline, Google Scholar
20 : Effects of medication on neuroimaging findings in bipolar disorder: an updated review. Bipolar Disord 2012; 14:375–410Crossref, Medline, Google Scholar
21 : Caffeine alters resting-state functional connectivity measured by blood oxygenation level-dependent MRI. NMR Biomed 2014; 27:444–452Crossref, Google Scholar
22 : An analysis of the use of hyperoxia for measuring venous cerebral blood volume: comparison of the existing method with a new analysis approach. Neuroimage 2013; 72:33–40Crossref, Medline, Google Scholar
23 : Identifying neural drivers with functional MRI: an electrophysiological validation. PLoS Biol 2008; 6:2683–2697Crossref, Medline, Google Scholar
24 : Functional and effective connectivity: a review. Brain Connect 2011; 1:13–36Crossref, Medline, Google Scholar
25 : REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS ONE 2011; 6:e25031Crossref, Medline, Google Scholar
26 : Granger causality analysis implementation on MATLAB: a graphic user interface toolkit for fMRI data processing. J Neurosci Methods 2012; 203:418–426Crossref, Medline, Google Scholar
27 : Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry 2007; 62:429–437Crossref, Medline, Google Scholar
28 : A systematic literature review of resting state network—functional MRI in bipolar disorder. J Affect Disord 2013; 150:727–735Crossref, Medline, Google Scholar
29 : Toward reliable characterization of functional homogeneity in the human brain: preprocessing, scan duration, imaging resolution and computational space. Neuroimage 2013; 65:374–386Crossref, Medline, Google Scholar
30 : Using coherence to measure regional homogeneity of resting-state FMRI signal. Front Syst Neurosci 2010; 4:24Medline, Google Scholar
31 : Functional disintegration in paranoid schizophrenia using resting-state fMRI. Schizophr Res 2007; 97:194–205Crossref, Medline, Google Scholar
32 : A graphical approach for evaluating effective connectivity in neural systems. Philos Trans R Soc Lond B Biol Sci 2005; 360:953–967Crossref, Medline, Google Scholar
33 : Undirected graphs of frequency-dependent functional connectivity in whole brain networks. Philos Trans R Soc Lond B Biol Sci 2005; 360:937–946Crossref, Medline, Google Scholar
34 : Growing together and growing apart: regional and sex differences in the lifespan developmental trajectories of functional homotopy. J Neurosci 2010; 30:15034–15043Crossref, Medline, Google Scholar
35 : Is Granger causality a viable technique for analyzing fMRI data? PLoS ONE 2013; 8:e67428Crossref, Medline, Google Scholar
36 : Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev 2007; 29:83–91Crossref, Medline, Google Scholar
37 : An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. J Neurosci Methods 2008; 172:137–141Crossref, Medline, Google Scholar
38 : Resting-state abnormal baseline brain activity in unipolar and bipolar depression. Neurosci Lett 2012; 516:202–206Crossref, Medline, Google Scholar
39 : Abnormal amplitude low-frequency oscillations in medication-naive, first-episode patients with major depressive disorder: a resting-state fMRI study. J Affect Disord 2013; 146:401–406Crossref, Medline, Google Scholar
40 : Metabolic brain networks in neurodegenerative disorders: a functional imaging approach. Trends Neurosci 2009; 32:548–557Crossref, Medline, Google Scholar
41 : Efficiency and cost of economical brain functional networks. PLOS Comput Biol 2007; 3:e17Crossref, Medline, Google Scholar
42 : Dysfunctional connectivity patterns in chronic heroin users: an fMRI study. Neurosci Lett 2009; 460:72–77Crossref, Medline, Google Scholar
43 : Increased activity imbalance in fronto-subcortical circuits in adolescents with major depression. PLoS ONE 2011; 6:e25159Crossref, Medline, Google Scholar
44 : A preliminary study of the dysregulation of the resting networks in first-episode medication-naive adolescent depression. Neurosci Lett 2011; 503:105–109Crossref, Medline, Google Scholar
45 : Abnormal functional connectivity with mood regulating circuit in unmedicated individual with major depression: a resting-state functional magnetic resonance study. Chin Med J (Engl) 2012; 125:3701–3706Medline, Google Scholar
46 : Increased neural resources recruitment in the intrinsic organization in major depression. J Affect Disord 2010; 121:220–230Crossref, Medline, Google Scholar
47 : Decreased interhemispheric resting-state functional connectivity in first-episode, drug-naive major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry 2013; 41:24–29Crossref, Medline, Google Scholar
48 : Depression uncouples brain hate circuit. Mol Psychiatry 2013; 18:101–111Crossref, Medline, Google Scholar
49 : Resting-state brain activity in major depressive disorder patients and their siblings. J Affect Disord 2013; 149:299–306Crossref, Medline, Google Scholar
50 : Decreased regional homogeneity in insula and cerebellum: a resting-state fMRI study in patients with major depression and subjects at high risk for major depression. Psychiatry Res 2010; 182:211–215Crossref, Medline, Google Scholar
51 : Abnormal regional spontaneous neural activity in treatment-refractory depression revealed by resting-state fMRI. Hum Brain Mapp 2011; 32:1290–1299Crossref, Medline, Google Scholar
52 : Altered functional connectivity of the dorsolateral prefrontal cortex in first-episode patients with major depressive disorder. Eur J Radiol 2012; 81:4035–4040Crossref, Medline, Google Scholar
53 : Disrupted resting-state functional connectivity of the hippocampus in medication-naïve patients with major depressive disorder. J Affect Disord 2012; 141:194–203Crossref, Medline, Google Scholar
54 : Decreased functional connectivity between the amygdala and the left ventral prefrontal cortex in treatment-naive patients with major depressive disorder: a resting-state functional magnetic resonance imaging study. Psychol Med 2013; 43:1921–1927Crossref, Medline, Google Scholar
55 : Regional homogeneity in depression and its relationship with separate depressive symptom clusters: a resting-state fMRI study. J Affect Disord 2009; 115:430–438Crossref, Medline, Google Scholar
56 : Abnormal neural activities in first-episode, treatment-naïve, short-illness-duration, and treatment-response patients with major depressive disorder: a resting-state fMRI study. J Affect Disord 2011; 135:326–331Crossref, Medline, Google Scholar
57 : Resting-state functional connectivity in treatment-resistant depression. Am J Psychiatry 2011; 168:642–648Crossref, Medline, Google Scholar
58 : Cerebral and cerebellar gray matter reduction in first-episode patients with major depressive disorder: a voxel-based morphometry study. Eur J Radiol 2011; 80:395–399Crossref, Medline, Google Scholar
59 : Prevalence and management of treatment-resistant depression. J Clin Psychiatry 2007; 68(Suppl 8):17–25Crossref, Medline, Google Scholar
60 : Staging methods for treatment resistant depression. A systematic review. J Affect Disord 2012; 137:35–45Crossref, Medline, Google Scholar
61 : Treatment resistant depression as a failure of brain homeostatic mechanisms: implications for deep brain stimulation. Exp Neurol 2009; 219:44–52Crossref, Medline, Google Scholar
62 : The hidden third: improving outcome in treatment-resistant depression. J Psychopharmacol 2012; 26:587–602Crossref, Medline, Google Scholar
63 : Resting-state functional connectivity bias of middle temporal gyrus and caudate with altered gray matter volume in major depression. PLoS ONE 2012; 7:e45263Crossref, Medline, Google Scholar
64 : Abnormal regional spontaneous neural activity in first-episode, treatment-naive patients with late-life depression: a resting-state fMRI study. Prog Neuropsychopharmacol Biol Psychiatry 2012; 39:326–331Crossref, Medline, Google Scholar
65 : Abnormal default-mode network in angiotensin converting enzyme D allele carriers with remitted geriatric depression. Behav Brain Res 2012; 230:325–332Crossref, Medline, Google Scholar
66 : A treatment-resistant default mode subnetwork in major depression. Biol Psychiatry 2013; 74:48–54Crossref, Medline, Google Scholar
67 : Alterations in regional homogeneity of spontaneous brain activity in late-life subthreshold depression. PLoS ONE 2013; 8:e53148Crossref, Medline, Google Scholar
68 : Abnormal neural activity in the patients with remitted geriatric depression: a resting-state functional magnetic resonance imaging study. J Affect Disord 2008; 111:145–152Crossref, Medline, Google Scholar
69 : Abnormal baseline brain activity in suicidal and non-suicidal patients with major depressive disorder. Neurosci Lett 2013; 534:35–40Crossref, Medline, Google Scholar
70 : Overlapping and segregated resting-state functional connectivity in patients with major depressive disorder with and without childhood neglect. Hum Brain Mapp 2013; 35:1154–1166Google Scholar
71 : Abnormal baseline brain activity in non-depressed Parkinson’s disease and depressed Parkinson’s disease: a resting-state functional magnetic resonance imaging study. PLoS ONE 2013; 8:e63691Crossref, Medline, Google Scholar
72 : Structural and functional neuroimaging studies of the suicidal brain. Prog Neuropsychopharmacol Biol Psychiatry 2011; 35:796–808Crossref, Medline, Google Scholar
73 : Why has it taken so long for biological psychiatry to develop clinical tests and what to do about it? Mol Psychiatry 2012; 17:1174–1179Crossref, Medline, Google Scholar
74 : Molecular neurobiology of depression: PET findings on the elusive correlation with symptom severity. Front Psychiatry 2013; 4:8Crossref, Medline, Google Scholar
75 : Bipolar and major depressive disorder: neuroimaging the developmental-degenerative divide. Neurosci Biobehav Rev 2009; 33:699–771Crossref, Medline, Google Scholar
76 : Normal prefrontal gamma-aminobutyric acid levels in remitted depressed subjects determined by proton magnetic resonance spectroscopy. Biol Psychiatry 2005; 58:969–973Crossref, Medline, Google Scholar
77 : Advances and pitfalls in the analysis and interpretation of resting-state FMRI data. Front Syst Neurosci 2010; 4:8Medline, Google Scholar
78 : Modes or models: a critique on independent component analysis for fMRI. Trends Cogn Sci 1998; 2:373–375Crossref, Medline, Google Scholar
79 : Bayesian network models in brain functional connectivity analysis. Int J Approx Reason 2014; 56:56Google Scholar
80 : Causal modelling and brain connectivity in functional magnetic resonance imaging. PLoS Biol 2009; 7:e33Crossref, Medline, Google Scholar
81 : Modalities, modes, and models in functional neuroimaging. Science 2009; 326:399–403Crossref, Medline, Google Scholar
82 : Puzzlingly high correlations in fMRI studies of emotion, personality, and social cognition. Perspect Psychol Sci 2009; 4:274–290Crossref, Medline, Google Scholar
83 Bowell T, Kemp G: Critical Thinking. A Concise Guide, 2nd ed. New York, Routledge, 2005Google Scholar
84 : Estimating test-retest reliability in functional MR imaging. I: Statistical methodology. Magn Reson Med 1997; 38:497–507Crossref, Medline, Google Scholar
85 : Confirmation Bias: a ubiquitous phenomenon in many guises. Rev Gen Psychol 1998; 2:175–220Crossref, Google Scholar
86 : How We Know What Isn't So. New York, The Free Press, 1993Google Scholar
87 : Cognitive brain mapping for better or worse. Perspect Biol Med 2010; 53:321–329Crossref, Medline, Google Scholar
88 : Confidence intervals and replication: where will the next mean fall? Psychol Methods 2006; 11:217–227Crossref, Medline, Google Scholar
89 : Most published research findings are false-but a little replication goes a long way. PLoS Med 2007; 4:e28Crossref, Medline, Google Scholar
90 : Experimental design in brain activation MRI: cautionary tales. Brain Res Bull 2005; 67:361–367Crossref, Medline, Google Scholar
91 : Depressive subtypes and efficacy of antidepressive pharmacotherapy. World J Biol Psychiatry 2005; 6(Suppl 2):31–37Crossref, Medline, Google Scholar
92 : Genotypic association of the DAOA gene with resting-state brain activity in major depression. Mol Neurobiol 2012; 46:361–373Crossref, Medline, Google Scholar
93 : Comparative study of regional homogeneity in schizophrenia and major depressive disorder. Am J Med Genet B Neuropsychiatr Genet 2013; 162B:36–43Crossref, Medline, Google Scholar
94 : Decreased regional homogeneity in major depression as revealed by resting-state functional magnetic resonance imaging. Chin Med J (Engl) 2011; 124:369–373Medline, Google Scholar
95 : Disrupted regional homogeneity in treatment-resistant depression: a resting-state fMRI study. Prog Neuropsychopharmacol Biol Psychiatry 2011; 35:1297–1302Crossref, Medline, Google Scholar



