Cortical Thickness and Anxiety Symptoms Among Cognitively Normal Elderly Persons: The Mayo Clinic Study of Aging
Abstract
The authors conducted a cross-sectional study to investigate the association between anxiety symptoms and cortical thickness, as well as amygdalar volume. A total of 1,505 cognitively normal participants, aged ≥70 years, were recruited from the Mayo Clinic Study of Aging in Olmsted County, Minnesota, on whom Beck Anxiety Inventory and 3T brain MRI data were available. Even though the effect sizes were small in this community-dwelling group of participants, anxiety symptoms were associated with reduced global cortical thickness and reduced thickness within the frontal and temporal cortex. However, after additionally adjusting for comorbid depressive symptoms, only the association between anxiety symptoms and reduced insular thickness remained significant.
Late-life depression has been extensively investigated; however, less is known about anxiety in old age. Yet, subsyndromal symptoms of anxiety are prevalent in the elderly, with a reported 12-month prevalence rate of 26.2% for subthreshold anxiety, versus 5.6% for DSM-IV anxiety disorders.1 In addition, subsyndromal symptoms of anxiety are associated with poor outcomes such as cognitive decline,2 increased health care cost, and reduced health-related quality of life.3 Experts have recently suggested conceptualizing anxiety in late life as a dimensional rather than categorical construct.4 Based on this concept, it is crucial to investigate symptoms of anxiety rather than anxiety disorders. In addition, a National Institute on Aging and Alzheimer’s Association expert panel has called for characterizing and understanding the presymptomatic phase of Alzheimer’s disease (AD) by using biomarkers such as brain MRI.5 Investigating the association of subsyndromal anxiety with biomarkers in presymptomatic AD will be a value-added approach toward an understanding of presymptomatic AD.
Changes in cortical thickness have been observed in normal aging, mild cognitive impairment (MCI), Alzheimer’s disease dementia,6 and also in psychiatric disorders, including major depression7 and anxiety.8 An automated method has been developed to accurately measure cortical thickness across the entire brain, providing an opportunity to investigate the structural correlates of anxiety.9 Only few studies have examined cortical thickness in participants with anxiety disorders8 or symptoms.3,10 Most reports were based on small sample sizes and involved a wide age range, with mostly middle-aged participants.
The amygdala has been shown to play an important role in anxiety, with reports on reduced volume in panic disorder.11,12 A small number of studies also reported associations between hippocampal volume and anxiety.13 Even though, previous studies showed high co-occurrence of anxiety and depression14–16 most previous imaging studies that investigated anxiety did not adjust for co-occurring depression.
We therefore sought to examine the associations between anxiety symptoms and cortical thickness in elderly community-dwelling participants. In addition, we performed further analyses also adjusting for depressive symptoms. Furthermore, we investigated the association between anxiety symptoms and the amygdalar, as well as hippocampal volume.
Methods
Setting
Participants were recruited from the Mayo Clinic Study of Aging. Details of the study procedures have been previously described.17 Briefly, the Mayo Clinic Study of Aging is an ongoing population-based study in Olmsted County, Minnesota that was designed to study the prevalence, incidence, and risk factors of cognitive aging, mild cognitive impairment, and dementia. Only participants who had undergone both brain MRI imaging and anxiety symptom assessment were included in this study. When we compared Mayo Clinic Study of Aging participants who underwent MRI versus others, we observed the following: compared with the study participants who did not undergo MRI, participants who underwent MRI scans had lower numbers of medical comorbidities (median: 3 versus 4, p<0.01), higher z-scores for global cognition (median: 0.52 versus 0.14, p<0.01), higher scores on the Short Test of Mental Status (median: 35 versus 34, p<0.01), and slightly lower numbers of anxiety (median: 1 versus 2, p<0.01) and depressive (median: 3.5 versus 4, p<0.01) symptoms. This study was approved by the Mayo Clinic and Olmsted Medical Center Institutional Review Boards, and written informed consent was obtained from every participant prior to enrollment in the study.
Cognitive Evaluation
Participants of the Mayo Clinic Study of Aging underwent face-to-face cognitive and risk factor evaluations that are published in detail elsewhere.17 Briefly, the assessment included 1) baseline evaluation (including Clinical Dementia Rating Scale18 and risk factor ascertainment (including Beck Anxiety Inventory) performed by a nurse or study coordinator and 2) neurological evaluation performed by behavioral neurologists. Participants also underwent neuropsychological evaluation of four cognitive domains: memory (delayed recall trials from the Auditory Verbal Learning Test19 and the Wechsler Memory Scale-Revised,20 Logical Memory and Visual Reproduction subtests); language (Boston Naming Test21 and category fluency); visuospatial (Wechsler Adult Intelligence Scale-Revised,22 Picture Completion and Block Design subtests); and executive function (Trail-Making Test, Part B23 and the Wechsler Adult Intelligence Scale-Revised, Digit Symbol subtest). In the Results section, the neuropsychological performance within the separate cognitive domains is expressed as z-score, which reflects the number of standard deviations the performance is above the mean.
An expert consensus panel of physicians, neuropsychologists, and nurses/study coordinators reviewed all the data for each participant and made the diagnosis of normal cognition, MCI, or dementia based on published criteria. Only participants with normal cognition were included in this study.
Measurement of Anxiety Symptoms
Within 150 days of the MRI scan, anxiety symptoms were measured using the Beck Anxiety Inventory (BAI), which is a validated, self-administered questionnaire.24 The BAI is an ordinal measurement consisting of 21 items that are assessed over the last week. The severity of each symptom is rated ranging from 0 to 3, with total scores ranging from 0 to 63.
MRI
Brain MRI scans were performed on 3-T scanners (Signa, GE Healthcare, Little Chalfont, United Kingdom) equipped with an eight-channel phased array coil (GE Healthcare). A three-dimensional magnetization-prepared rapid gradient echo sequence was performed,25,26 and images were corrected for bias field and for distortion due to gradient nonlinearity.27 Cortical thickness and amygdalar and hippocampal volumes were measured with FreeSurfer software, version 5.3.28 The following regions of interest (ROIs) of cortical thickness labeled by FreeSurfer were included in the analysis: frontal, temporal, and parietal thickness, as well as global cortical thickness, which was the mean thickness of all regions measured by FreeSurfer, including frontal, temporal, parietal, and occipital thickness. In addition, we investigated the following subregions within the frontal and temporal cortex: anterior cingulate cortex (ACC) (combined thickness of the rostral, isthmus, and caudal ACC cortex), dorsolateral prefrontal cortex (DLPFC) (combined superior frontal, rostral middle frontal, and caudal middle frontal cortex), ventrolateral prefrontal cortex (combined pars opercularis, pars triangularis, and pars orbitalis), and orbitofrontal cortex (OFC) (combined lateral and medial orbitofrontal cortex) as labeled by FreeSurfer. For a secondary analysis, we calculated amygdalar and hippocampal volumes that were measured by FreeSurfer and were adjusted by total intracranial volume measured by SPM12.29
Participation in imaging is voluntary and is available to all study participants. There are a few exceptions that will not allow a participant to be imaged (e.g., participants with pacemakers). Study participants can decline imaging for any reason. For ethical reasons, if they decline, no further effort is made to make them change their mind or inquire as to why they are unwilling.
The majority of participants underwent MRI within 120 days after the administration of the anxiety assessment. About 5% of our cohort had the MRI after 120 days. There was one subject with a maximum of 150 days between the BAI visit date and the MRI.
Measurement of Covariates
We defined the following variables as covariates: age, sex, education, medical comorbidity, antidepressant medication, and global cognition. Medical comorbidity was measured via the Charlson index, a widely used weighted index that accounts for number and severity of diseases.30 Global cognition was measured as a composite z-score computed from memory, executive function, visuospatial, and language domain scores from an extensive neuropsychological test battery described in detail elsewhere.17 Antidepressant medication use was self-reported and included use of selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, citalopram, and tetracyclic or tricyclic antidepressants.
Depressive and anxiety symptoms often co-occur in late life.31–33 Therefore, when investigating the association of anxiety with biomarkers such as cortical thickness, it is critical to adjust for depressive symptoms in order to make sure that the observed association between anxiety and cortical thickness is not confounded by the comorbid depressive symptoms. It is for this reason that we adjusted for depressive symptoms as measured by Beck Depression Inventory-II (BDI-II).30 Similar to the BAI, the BDI-II is also an ordinal measurement consisting of 21 items. Depressive symptoms are assessed over the last 2 weeks and are rated in severity on a 4-point scale ranging from 0 to 3, with a total score ranging from 0 to 63.
Statistical Analysis
We conducted a cross-sectional study involving cognitively normal elderly individuals with available MRI and anxiety data. To test for pairwise differences in demographic characteristics, Wilcoxon two-sided rank sum tests were calculated for participants with BAI scores ≥1 versus participants without anxiety symptoms. When we examined the association between anxiety symptoms and MRI measures, we treated anxiety symptoms, measured by BAI, as a continuous variable, in order to avoid arbitrary categorical cut-offs. Therefore, we calculated Spearman rank-order correlations between MRI measures (cortical thickness by ROI, amygdalar, and hippocampal volumes) and BAI (as continuous variables) after adjusting for age, sex, education, medical comorbidity, global cognition, and antidepressant medication. We ran an additional analysis in which we also adjusted for depressive symptoms as measured by BDI-II.30 We computed estimates of associations using SAS System, version 9.3 software (SAS Institute, Cary, N.C.) and visually displayed the data as forest plots using R statistical software, version 3.0.2 (R Foundation for Statistical Computing, Vienna).
Results
The complete demographic characteristics are presented in Table 1. Only a small percentage of this population-based study met the threshold for clinical anxiety (BAI >10; 5.3%) and depression (BDI >13; 5.6%). Participants with anxiety symptoms were significantly older, had fewer years of education, lower scores on the Short Test of Mental Status,34 lower z-scores for global cognition, higher number of depressive symptoms, and higher use of antidepressant medication compared with participants without anxiety symptoms.
| Characteristic | All Study Participants (N=1,505) | No Anxiety Symptoms (N=612)b | ≥1 Anxiety Symptoms (N=893)c | p |
|---|---|---|---|---|
| Males, N (%) | 767 (51) | 345 (56) | 422 (47) | <0.01 |
| APOE ε4 carriers, N (%) | 380 (25) | 146 (24) | 234 (26) | 0.31 |
| Age (years), median (IQR) | 77 (74 to 82) | 77 (73 to 81) | 78 (74 to 82) | <0.01 |
| Education (years), median (IQR) | 13 (12 to 16) | 14 (12 to 16) | 13 (12 to 16) | <0.01 |
| Short Test, median (IQR) | 35 (33 to 36) | 35 (33 to 37) | 35 (33 to 36) | <0.01 |
| Z scores of cognitive domains | ||||
| Memory, median (IQR) | 0.50 (–0.21 to 1.17) | 0.57 (–0.17 to 1.23) | 0.43 (–0.23 to 1.11) | 0.03 |
| Language, median (IQR) | 0.37 (–0.17 to 0.95) | 0.39 (–0.09 to 0.96) | 0.36 (–0.24 to 0.93) | 0.17 |
| Attention, median (IQR) | 0.46 (–0.08 to 0.98) | 0.52 (0.00 to 1.05) | 0.42 (–0.16 to 0.95) | <0.01 |
| Visuospatial, median (IQR) | 0.37 (–0.26 to 0.97) | 0.44 (–0.13 to 1.01) | 0.31 (–0.31 to 0.90) | <0.01 |
| Global, median (IQR) | 0.52 (–0.08 to 1.06) | 0.62 (0.03 to 1.12) | 0.43 (–0.12 to 1.01) | <0.01 |
| Beck Anxiety Inventory, median (IQR) | 1 (0 to 3) | 0 (0 to 0) | 3 (1 to 5) | <0.01 |
| Beck Depression Inventory, median (IQR) | 3.5 (1 to 7) | 1 (0 to 4) | 5 (2 to 8) | <0.01 |
| Charlson Index, median (IQR) | 3 (2 to 5) | 3 (1 to 5) | 3 (2 to 5) | 0.02 |
| Medications | ||||
| Selective serotonin reuptake inhibitor/serotonin-norepinephrine reuptake inhibitors, N (%) | 150 (10) | 28 (5) | 122 (14) | <0.01 |
| Citalopram, N (%) | 56 (4) | 12 (2) | 44 (5) | <0.01 |
| Tetracyclic, N (%) | 13 (1) | 5 (1) | 8 (1) | 0.87 |
| Tricyclic, N (%) | 38 (3) | 13 (2) | 25 (3) | 0.41 |
| Any, N (%) | 195 (13) | 45 (7) | 150 (17) | <0.01 |
TABLE 1. Demographic Characteristics of Study Participantsa
When examining anxiety symptoms as a continuous variable, we observed associations with reduced thickness in most ROIs in unadjusted models (Table 2). In multivariate analyses, the higher the BAI scores the lower the cortical thickness of the ROI, noted as follows: global/composite thickness (r=−0.06, p=0.02), frontal (r=−0.07, p=0.01), and temporal (r=−0.07, p<0.01), DLPFC (r=−0.07, p=0.01), insula (r=−0.09, p<0.01), parahippocampal (r=−0.06, p=0.04), and ACC (r=−0.05, p=0.04), after adjusting for age, sex, education, medical comorbidity, antidepressant medication, and global cognition. When we additionally adjusted for depressive symptoms (as measured by BDI-II), only the associations between anxiety symptoms and reduced insular cortical thickness remained significant (r=−0.07, p<0.01). We further observed a correlation between OFC with anxiety that nearly reached statistical significance (p=0.06) after correction for depression. A secondary analysis did not show associations between anxiety symptoms and amygdalar or hippocampal volume, after adjusting for age, sex, education, medical comorbidity, global cognition, and antidepressant medication use (Table 2). Figure 1 shows forest plots of the partial Spearman rank-order correlations, and Figure 2 shows a boxplot of the BAI scores by sex and age groups.
| Region | BAI Unadjusted r (95% CI)a | p | BAI r (95% CI)b | p | Additional Adjustment for the Beck Depression Inventory r (95% CI)b | p |
|---|---|---|---|---|---|---|
| Composite | –0.10 (–0.15 to –0.05) | <0.01 | –0.06 (–0.11 to –0.01) | 0.02 | –0.02 (–0.08 to 0.03) | 0.39 |
| Frontal | –0.09 (–0.14 to –0.04) | <0.01 | –0.07 (–0.12 to –0.01) | 0.01 | –0.03 (–0.08 to 0.02) | 0.27 |
| Temporal | –0.11 (–0.16 to –0.06) | <0.01 | –0.07 (–0.12 to –0.02) | <0.01 | –0.03 (–0.08 to 0.02) | 0.30 |
| Parietal | –0.06 (–0.11 to –0.01) | 0.03 | –0.02 (–0.07 to 0.03) | 0.43 | 0.00 (–0.05 to 0.06) | 0.91 |
| Ventrolateral prefrontal cortex | –0.07 (–0.12 to –0.02) | 0.01 | –0.04 (–0.09 to 0.01) | 0.11 | –0.00 (–0.05 to 0.05) | 0.94 |
| Dorsolateral prefrontal cortex | –0.10 (–0.15, –0.05) | <0.01 | –0.07 (–0.12 to –0.01) | 0.01 | –0.03 (–0.08 to 0.02) | 0.29 |
| Orbitofrontal | –0.08 (–0.13 to –0.03) | <0.01 | –0.07 (–0.12 to –0.02) | <0.01 | –0.05 (–0.10 to 0.00) | 0.06 |
| Anterior cingulate cortex | –0.02 (–0.07 to 0.03) | 0.42 | –0.05 (–0.11 to –0.00) | 0.04 | –0.04 (–0.09 to 0.01) | 0.15 |
| Insula | –0.11 (–0.16 to –0.06) | <0.01 | –0.09 (–0.14 to –0.04) | <0.01 | –0.07 (–0.12 to –0.02) | <0.01 |
| Parahippocampal | –0.07 (–0.12 to –0.02) | <0.01 | –0.06 (–0.11 to –0.00) | 0.04 | –0.04 (–0.09, 0.01) | 0.11 |
| Entorhinal | –0.09 (–0.14 to –0.04) | <0.01 | –0.05 (–0.10 to 0.00) | 0.06 | –0.01 (–0.07 to 0.04) | 0.58 |
| Hippocampus volume | –0.11 (–0.16 to –0.06) | <0.01 | –0.03 (–0.08 to 0.02) | 0.24 | –0.02 (–0.07, 0.04) | 0.57 |
| Amygdala volume | –0.11 (–0.16 to –0.06) | <0.01 | –0.03 (–0.08 to 0.02) | 0.27 | –0.01 (–0.06 to 0.04) | 0.65 |
TABLE 2. Spearman Rank-Order Adjusted Correlations (p value) Between Anxiety Symptoms as Measured by the Beck Anxiety Inventory (BAI) and Cortical Thickness
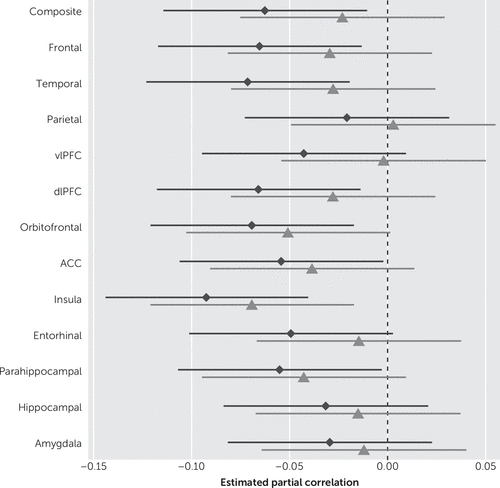
FIGURE 1. Forest Plots of the Spearman Rank-Order Correlations Between Beck Anxiety Inventory and MRI Measuresa
a Diamonds: Adjusting for age, sex, education, antidepressant medication, medical comorbidities, and global cognition; triangles: Additionally adjusting for depressive symptoms. Hippocampal and amygdala volumes were additionally adjusted for total intracranial volume. Abbreviations: dlPFC=dorsolateral prefrontal cortex; vlPFC=ventrolateral prefrontal cortex; ACC=anterior cingulate cortex.
FIGURE 2. Box Plot of Beck Anxiety Inventory (BAI) Scores by Sex and Age Groupsa
a y-axis: BAI score.
Discussion
Herein we report the associations between cortical thickness and anxiety symptoms in a large sample of cognitively normal elderly participants enrolled in the population-based Mayo Clinic Study of Aging. In previous studies, we and others have shown that anxiety symptoms increase the risk of developing mild cognitive impairment in cognitively normal elderly participants. This may indicate that anxiety symptoms could be a marker of preclinical AD. Thus, we looked at elderly participants who were still free of MCI and dementia. We then sought to examine whether there was already a structural correlate to anxiety symptoms. However, since these are cross-sectional data, whatever is reported about structural biomarker relationships is not yet answering this question of preclinical AD indicators.
We observed associations between anxiety symptoms and lower global thickness, as well as lower thickness in frontal and temporal cortical regions, after adjusting for age, sex, education, medical comorbidity, antidepressant medication, and global cognition. The results changed markedly after we additionally adjusted for depressive symptoms. Furthermore, as expected, the effect sizes were very small. In contrast to patients from clinical settings, we randomly sampled and recruited participants from the general population with the result that participants had very mild symptoms of anxiety. Only a small percentage of this population-based study met the threshold for clinical anxiety (BAI >10; 5.3%).
Several studies have examined associations between brain volumes and disorders of anxiety, based on categorical classifications, for example, generalized anxiety disorder or panic disorder. They mainly reported altered volumes of the amygdala,11,12,35 insular cortex,13,35–37 and ACC.10,36,37 Few studies also reported altered volumes in the prefrontal cortex3,10,12 and temporal regions.12,38,39 However, little is known about the association between cortical thickness and anxiety symptoms in the elderly. Moreover, most previous studies that examined anxiety did not specifically adjust for comorbid depression. Yet, our study findings changed markedly after additionally adjusting for depressive symptoms. While the associations with various regions remained in the same direction, only the association between anxiety symptoms and reduced insula thickness remained statistically significant and the association between OFC with anxiety remained marginally significant after additionally adjusting for depressive symptoms. This finding suggests that many regions that appear to be associated with anxiety could be rather confounded by the presence of co-occurring depressive symptoms. Indeed, our data suggest that only insular thickness may specifically be associated with anxiety symptoms. This is in line with previous studies that have shown an association between reduced insular volume and disorders of anxiety.13,35–37 The role of reduced insular thickness in anxiety symptoms may be explained by the insula’s high interconnections with other cortical and subcortical regions. Moreover, the insula has been shown to be involved in emotional response, and its size and reactivity have been linked to awareness of bodily responses, and especially anxiety.40
We observed no relationship between anxiety symptoms and hippocampal or parahippocampal MRI measures. This may indicate that anxiety may not be a valuable predictor for AD risk in the subclinical stages of AD. The amygdala is probably the most widely investigated region in anxiety,3,11,35 and several studies have reported associations between reduced amygdalar volume and anxiety disorders.11,12 We only observed significant associations in our unadjusted results. However, similar to other studies that investigated mild anxiety symptoms,2,10 we also did not observe significant associations between amygdalar volume and very mild anxiety symptoms in community-dwelling participants after adjusting for multiple possible confounders. This may indicate that in contrast to studies that reported significant associations with clinical anxiety disorders, mild symptoms in community-dwelling individuals may not be severe enough to manifest associations with lower amygdalar volume.
Additionally, we conducted further analyses in order to explore correlations among the components of neuronal circuitry related to anxiety. Indeed, we observed significant correlations between the insular cortex and orbitofrontal cortex (r=0.54, p<0.01), as well as between the insular cortex and amygdala (r=0.08, p<0.01). This finding is consistent with previous reports.10
Investigators from Italy examined associations between anxiety symptoms, as measured by the Hamilton Anxiety Rating Scale, and amygdalar and hippocampal volume, as well as cortical thickness, in the OFC and ACC in 121 participants with a median age of 36 years. While, similar to our findings, they did not observe reduced amygdalar or hippocampal volume; they also reported associations between anxiety scores and increased ACC thickness.10 Whereas they and others reported increased thickness,2,9,36 other studies have reported reduced ACC volume in individuals with panic disorder.36,37 We observed weak associations between reduced ACC thickness and anxiety symptoms, as measured by BAI, after adjusting for age, sex, education, medical comorbidity, antidepressant medication, and global cognition. Discrepancies across studies may be attributable to methodological issues, including differences in anxiety assessment and participant age groups. Since age is associated with reduced cortical thickness,41 it is possible that studies involving younger individuals may show different results and that associations between anxiety symptoms and lower cortical thickness do not manifest until old age. While the mechanisms that may underlie reduced cortical thickness are not fully understood, it has been reported that the neuronal number is not reduced in age-related cortical atrophy.42 Thus, cortical thinning may be rather associated with neuronal shrinkage or degeneration of synapses. While our cross-sectional study does not provide information about the causal relationship between cortical thickness and anxiety symptoms, we hypothesize that underlying pathological neurodegeneration may result in reduction of synapses in cortical circuits involving the insula. This in turn may be seen on MRI as cortical thinning and lead to anxiety symptoms. Alternatively, it is possible that an additional risk factor may synergistically interact with the cortical thinning pathway and lead to anxiety symptoms. It also seems plausible that a confounder such as age may lead to both cortical thinning and anxiety symptoms.
Our study should be interpreted in light of its strengths and limitations. An important strength is that participants were from a large, well-described population-based study, thereby reducing selection bias as would occur with a clinic-based setting. The comprehensive clinical evaluation and access to medical record information from the Rochester Epidemiology Project provided the opportunity to adjust for multiple possible confounders, including age, sex, education, antidepressant medication use, medical comorbidities, and cognition. In contrast to most previous studies, we also adjusted for depressive symptoms. The comorbidity between anxiety and depression is very high; therefore, we additionally adjusted for depressive symptoms. The observed association between anxiety and cortical thickness remained the same, indicating that the association is over and above what can be explained by comorbid depressive symptoms. Furthermore, the study sample allowed us to investigate associations of anxiety with imaging variables (i.e., cortical thickness and amygdalar and hippocampal volume) in more than 1,500 participants. Additionally, we examined associations between anxiety symptoms and cortical thickness as continuous variables, whereas most previous studies chose categorical classifications.
This study also has potential limitations. The BAI is a self-reported questionnaire and may therefore be subject to recall bias. However, the BAI is a well-validated and widely used scale.24 Additionally, our study is limited by its cross-sectional design, which does not allow for interpretations of the direction of causality. Therefore, longitudinal studies are needed to replicate our findings. Furthermore, even though the Mayo Clinic Study of Aging extensively evaluates cognition and we were able to exclude participants with MCI or dementia from this study, we could not account for subjective cognitive impairment, which can also be accompanied by symptoms of anxiety.
Our findings indicate that a higher level of anxiety symptoms was associated with lower thickness in various cortical regions after adjusting for age, sex, education, medical comorbidity, antidepressant medication, and global cognition. This cross-sectional association is informative in that a dimensional approach has raised an important hypothesis that needs to be tested in a future longitudinal cohort study to investigate as to who will eventually develop further neurodegeneration leading to a phenotype of cognitive impairment. However, after additionally adjusting for depressive symptoms, the findings changed markedly, and only the association between anxiety symptoms and reduced insular thickness remained significant. Therefore, this finding may provide insight into the specific structural correlates of anxiety symptoms after adjusting for possible confounding effects of co-occurring depression.
1 : The impact of DSM-IV symptom and clinical significance criteria on the prevalence estimates of subthreshold and threshold anxiety in the older adult population. Am J Geriatr Psychiatry 2011; 19:316–326Crossref, Medline, Google Scholar
2 : Baseline neuropsychiatric symptoms and the risk of incident mild cognitive impairment: a population-based study. Am J Psychiatry 2014; 171:572–581Crossref, Medline, Google Scholar
3 : Depression and anxiety impair health-related quality of life and are associated with increased costs in general medical inpatients. Psychosomatics 2002; 43:302–309Crossref, Medline, Google Scholar
4 : Threshold and subthreshold generalized anxiety disorder in later life. Am J Geriatr Psychiatry 2015; 23:633–641Crossref, Medline, Google Scholar
5 : Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011; 7:280–292Crossref, Medline, Google Scholar
6 : Spatial patterns of cortical thinning in mild cognitive impairment and Alzheimer’s disease. Brain 2006; 129:2885–2893Crossref, Medline, Google Scholar
7 : Widespread reductions in gray matter volume in depression. Neuroimage Clin 2013; 3:332–339Crossref, Medline, Google Scholar
8 : Cortical thickness alterations in social anxiety disorder. Neurosci Lett 2013; 536:52–55Crossref, Medline, Google Scholar
9 : Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA 2000; 97:11050–11055Crossref, Medline, Google Scholar
10 : The neuroanatomical correlates of anxiety in a healthy population: differences between the State-Trait Anxiety Inventory and the Hamilton Anxiety Rating Scale. Brain Behav 2014; 4:504–514Crossref, Medline, Google Scholar
11 : Smaller amygdala is associated with anxiety in patients with panic disorder. Psychiatry Clin Neurosci 2009; 63:266–276Crossref, Medline, Google Scholar
12 : Amygdalar atrophy in panic disorder patients detected by volumetric magnetic resonance imaging. Neuroimage 2003; 19:80–90Crossref, Medline, Google Scholar
13 : Whole-brain gray matter volume abnormalities in patients with generalized anxiety disorder: voxel-based morphometry. Neuroreport 2014; 25:184–189Crossref, Medline, Google Scholar
14 : Anxiety and depression in later life: co-occurrence and communality of risk factors. Am J Psychiatry 2000; 157:89–95Crossref, Medline, Google Scholar
15 : Epidemiology and comorbidity of anxiety disorders in the elderly. Am J Psychiatry 1994; 151:640–649Crossref, Medline, Google Scholar
16 : Age differences in the prevalence and co-morbidity of DSM-IV major depressive episodes: results from the WHO World Mental Health Survey Initiative. Depress Anxiety 2010; 27:351–364Crossref, Medline, Google Scholar
17 : The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology 2008; 30:58–69Crossref, Medline, Google Scholar
18 : The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993; 43:2412–2414Crossref, Medline, Google Scholar
19 : L’examen clinique en psychologie. Paris, Presses Universitaires de France, 1964Google Scholar
20 : Wechsler Memory Scale-Revised. New York, Psychological Corporation, 1987Google Scholar
21 : Boston Naming Test. Philadelphia, Lea & Febiger, 1983Google Scholar
22 : Wechsler Adult Intelligence Scale-Revised. New York, Psychological Corporation, 1981Google Scholar
23 : Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills 1958; 8:271–276Crossref, Google Scholar
24 : An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol 1988; 56:893–897Crossref, Medline, Google Scholar
25 : Update on the magnetic resonance imaging core of the Alzheimer’s Disease Neuroimaging Initiative. Alzheimers Dement 2010; 6:212–220Crossref, Medline, Google Scholar
26 : 11C PiB and structural MRI provide complementary information in imaging of Alzheimer’s disease and amnestic mild cognitive impairment. Brain 2008; 131:665–680Crossref, Medline, Google Scholar
27 : Measurement of MRI scanner performance with the ADNI phantom. Med Phys 2009; 36:2193–2205Crossref, Medline, Google Scholar
28 : Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 2002; 33:341–355Crossref, Medline, Google Scholar
29 : Anterior temporal lobes and hippocampal formations: normative volumetric measurements from MR images in young adults. Radiology 1989; 172:549–554Crossref, Medline, Google Scholar
30 : BDI-II, Beck Depression Inventory: Manual. San Antonio, Tex, Psychological Corporation, 1996Google Scholar
31 : Comorbidity and risk-patterns of depression, generalised anxiety disorder and mixed anxiety-depression in later life: results from the AMSTEL study. Int J Geriatr Psychiatry 2003; 18:994–1001Crossref, Medline, Google Scholar
32 : Sex and depression in the National Comorbidity Survey, II: cohort effects. J Affect Disord 1994; 30:15–26Crossref, Medline, Google Scholar
33 : Prevalence of neuropsychiatric symptoms in mild cognitive impairment and normal cognitive aging: population-based study. Arch Gen Psychiatry 2008; 65:1193–1198Crossref, Medline, Google Scholar
34 : A short test of mental status: description and preliminary results. Mayo Clin Proc 1987; 62:281–288Crossref, Medline, Google Scholar
35 : Regional brain volume in depression and anxiety disorders. Arch Gen Psychiatry 2010; 67:1002–1011Crossref, Medline, Google Scholar
36 : Regional gray matter abnormalities in panic disorder: a voxel-based morphometry study. Psychiatry Res 2008; 163:21–29Crossref, Medline, Google Scholar
37 : Anterior cingulate cortex volume reduction in patients with panic disorder. Psychiatry Clin Neurosci 2008; 62:322–330Crossref, Medline, Google Scholar
38 : Putaminal gray matter volume decrease in panic disorder: an optimized voxel-based morphometry study. Eur J Neurosci 2005; 22:2089–2094Crossref, Medline, Google Scholar
39 : Temporal lobe volume in panic disorder: a quantitative magnetic resonance imaging study. Psychiatry Res 2000; 99:75–82Crossref, Medline, Google Scholar
40 : A common role of insula in feelings, empathy and uncertainty. Trends Cogn Sci 2009; 13:334–340Crossref, Medline, Google Scholar
41 : Thinning of the cerebral cortex in aging. Cereb Cortex 2004; 14:721–730Crossref, Medline, Google Scholar
42 : Preservation of neuronal number despite age-related cortical brain atrophy in elderly subjects without Alzheimer disease. J Neuropathol Exp Neurol 2008; 67:1205–1212Crossref, Medline, Google Scholar



