Refractory OCD Due to Thalamic Infarct With Response to Dronabinol
Abstract
Structural brain lesions can be a rare cause of refractory psychiatric symptoms. The analysis of such cases may lead to insights into psychiatric neurobiology. Here we present a case of a dronabinol-responsive obsessive-compulsive syndrome after thalamic infarct.
Case Report
A 24-year-old male presented to our clinic in November 2013 with a 10-year history of insulin dependent diabetes and bipolar I disorder since age 16 (2005), which was stable on ziprasidone. At age 19, in July 2009, he suffered a lacunar infarct of the left thalamus in the paramedian artery territory (Figure 1). A patent foramen ovale was discovered and repaired, but no certain etiology of his stroke was uncovered. Shortly thereafter, the patient began to experience persistent, repetitive, unwanted thoughts primarily related to doing unintentional harm or engaging in sexual acts with others. The related compulsions were to seek reassurance from his mother or engage in strenuous exercise. Symptoms escalated and in response to these internal thoughts he would yell in public and seek reassurance so frequently he could not function outside of the home. Neuropsychological testing demonstrated a dysexecutive and mild amnestic syndrome, consistent with thalamic injury to the mediodorsal nucleus (MDN) and part of the anterior nucleus, respectively. His symptoms remained mostly refractory to numerous trials of high dose medication and augmentation strategies including combinations of fluvoxamine, clomipramine, mirtazapine, risperidone, olanzapine, clozapine, ziprasidone, haloperidol, quetiapine, memantine, ondansetron, intravenous ketamine, N-acetylcysteine, gabapentin, clonazepam, plus traditional mood stabilizing agents for his bipolar disorder. The best combinations of the above took the edge off his internal agitation, but his intrusive thoughts remained essentially unchanged; Yale-Brown Obsessive Compulsive Scale (Y-BOCS)1 score was 39. The patient had researched refractory obsessive-compulsive disorder (OCD) and requested evaluation for deep brain stimulator placement. Dronabinol augmentation was initiated and titrated up to a total of 20 mg daily. Benefit was noted within 2 weeks and Y-BOCS score continued to decline over months to a nadir of 10, correlated with a significant improvement in quality of life. He has subsequently been able to tolerate and make use of cognitive behavioral therapy (CBT). Previous attempts at CBT were unsuccessful because the patient found CBT techniques intolerable and unhelpful and felt a medication or surgical solution was necessary.
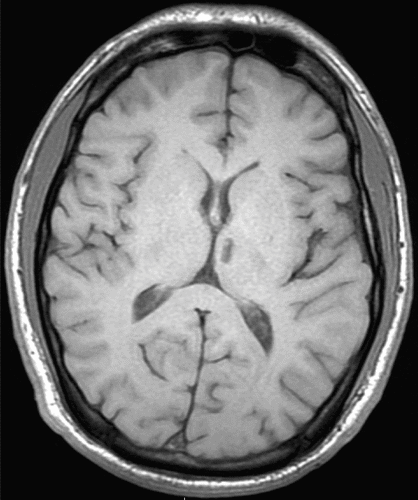
FIGURE 1. T1-Weighted MRI of the Left Thalamic Lacunar Infarct
Discussion
The neurobiology of OCD involves aberration in the circuitry of the orbitofrontal cortex, basal ganglia, anterior cingulate cortex, and thalamus, specifically the MDN.2 These corticostriatal-thalamocortical circuits are thought to underlie the pathophysiology of OCD.2 Our case may be considered analogous to how in Wernicke-Korsakoff syndrome a hypothalamaic-thalamic lesion can cause an anterograde amnesia similar to a hippocampal disorder, due to a disruption of the same circuit, in a different location. Since there have been case reports of OCD following orbitofrontal3,4 and basal ganglia5,6 lesions, a MDN lesion recapitulating a similar syndrome, such as our case, is a logical possibility.
The striatum functions to initiate behavior patterns, and the role of the MDN may be to pause these behavioral patterns and allow for modulation.7 This role, described as “pausing to regroup,”7 may be essential to shift from one behavioral pattern to another. OCD pathology can be conceptualized as a difficulty terminating one behavioral pattern and shifting to the next one,2 and this has been fairly consistently supported by studies finding deficits in set shifting/cognitive flexibility in individuals with OCD.8 MDN damage might damage this capacity to shift, leading to unrelenting behavioral patterns of OCD.
The endocannabinoid system has been implicated in the balance between direct and indirect striatal pathways, which are also essential in the starting and stopping of behavioral patterns.9 To our knowledge, this is the first case report of an OCD-like syndrome following a focal lacunar infarct of the MDN. We speculate that this patient’s capacities to pause behavior and thought patterns were disrupted by this lesion and partially restored by treatment with dronabinol.
1 : The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry 1989; 46:1006–1011Crossref, Medline, Google Scholar
2 : A psychological and neuroanatomical model of obsessive-compulsive disorder. J Neuropsychiatry Clin Neurosci 2008; 20:390–408Link, Google Scholar
3 : A right orbitofrontal region and OCD symptoms: a case report. Acta Psychiatr Scand 2005; 111:74–76, discussion 76–77Crossref, Medline, Google Scholar
4 : Obsessive-compulsive disorder associated with a left orbitofrontal infarct. J Neuropsychiatry Clin Neurosci 2002; 14:88–89Link, Google Scholar
5 : Obsessive compulsive disorder in a woman with left basal ganglia infarct: A case report. Behav Neurol 1997; 10:101–103Crossref, Medline, Google Scholar
6 : Treatment of late-onset OCD following basal ganglia infarct. Depress Anxiety 2002; 15:87–90Crossref, Medline, Google Scholar
7 : Pausing to regroup: thalamic gating of cortico-basal ganglia networks. Neuron 2010; 67:175–178Crossref, Medline, Google Scholar
8 : Impaired cognitive flexibility and motor inhibition in unaffected first-degree relatives of patients with obsessive-compulsive disorder. Am J Psychiatry 2007; 164:335–338Crossref, Medline, Google Scholar
9 : Direct and indirect pathways of basal ganglia: a critical reappraisal. Nat Neurosci 2014; 17:1022–1030Crossref, Medline, Google Scholar



