Predictors of Major Depression and Posttraumatic Stress Disorder Following Traumatic Brain Injury: A Systematic Review and Meta-Analysis
Abstract
Although major depressive disorder (MDD) and posttraumatic stress disorder (PTSD) are prevalent after traumatic brain injury (TBI), little is known about which patients are at risk for developing them. The authors systematically reviewed the literature on predictors and multivariable models for MDD and PTSD after TBI. The authors included 26 observational studies. MDD was associated with female gender, preinjury depression, postinjury unemployment, and lower brain volume, whereas PTSD was related to shorter posttraumatic amnesia, memory of the traumatic event, and early posttraumatic symptoms. Risk of bias ratings for most studies were acceptable, although studies that developed a multivariable model suffered from methodological shortcomings.
Traumatic brain injury (TBI), which is defined as “an alteration in brain function, or other evidence of brain pathology, caused by an external force,”1 comprises a serious public health concern with 262 per 100,000 patients admitted to the hospital each year.2 A substantial percentage of TBI patients develops psychiatric disorders in the first year postinjury,3,4 among which major depressive disorder (MDD) and posttraumatic stress disorder (PTSD) are the most frequently reported.4–7 MDD and PTSD after TBI are associated with functional impairments3,8,9 and a decrease in health-related quality of life.9 They subsequently interfere with rehabilitative interventions and negatively affect recovery from TBI.3 Moreover, they are associated with high direct and indirect costs,10–12 resulting in a tremendous individual and societal burden.
Although the significance of MDD and PTSD after TBI is well established, the literature yields limited information about which patients are at risk of developing these psychiatric conditions. This knowledge could be used to flag patients who might benefit from additional monitoring or (preventive) therapeutic interventions, which have shown to be effective in people at risk for MDD and PTSD.13–15 Multivariable models, which combine a number of characteristics to predict MDD or PTSD, might be particularly useful for this purpose.
To our knowledge, there is currently one systematic review assessing psychological and psychosocial predictors of PTSD.16 The authors found that comorbid depression and anxiety, acute stress disorder (ASD), psychological processes (coping styles and attribution), and psychosocial variables (role impairment and reintegration) were associated with PTSD post-TBI.16 The authors, however, included all factors associated with PTSD rather than factors predicting PTSD. It is therefore unclear whether these specific factors predicted PTSD or were predicted by PTSD. Moreover, they included self-reported measurements to diagnose PTSD. Self-reported measurements might not be reliable in a TBI population because of overlap between psychiatric symptoms and TBI symptoms (e.g., anxiety, irritability, fatigue), memory deficits, low self-awareness, attention problems, and evidence that TBI patients tend to underestimate their problems.17–22 Structured diagnostic interviews, such as the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders (SCID), constitute a better alternative, because these interviews distinguish psychopathology symptoms from TBI symptoms and are less influenced by TBI-related problems such as memory deficits.18
The objective of this systematic review and meta-analysis was to examine univariable predictors of and multivariable models for MDD and PTSD following TBI using structured diagnostic interviews.
Materials and Methods
Information Sources
We conducted a comprehensive literature search until October 2016. The search strategy was developed in consultation with a search expert using a combination of subheadings and text words (see the data supplement accompanying the online version of this article). The following databases were searched: EMBASE, MEDLINE, Cochrane Central, PubMed, PsycINFO, and Google Scholar. Reference lists and citation indices of included papers and relevant reviews were further inspected to identify any additional publications. The search strategy was restricted to studies published in peer-reviewed English-language journals. We did not use any date restrictions.
Study Selection
We selected studies examining univariable predictors of or multivariable models for MDD and PTSD after TBI. We used the following inclusion and exclusion criteria to determine eligibility of a study.
Participants.
The participants were civilian adults (age ≥16 years) who sustained TBI. TBI was defined as “an alteration in brain function or other evidence of brain pathology, caused by an external force.”1 We included patients with mild, moderate, and severe TBI (as defined by the study authors). We excluded military patients because there are major differences between military and civilian TBI. In the military, approximately 75% of the TBIs involve blast exposures,23 which may have unique injury mechanisms.24 In addition, mental health symptoms are more prevalent in the military than in civilians,25 which might also be due to other causes than the sustained TBI.
Outcome measurement.
MDD and PTSD were diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders (DSM) or International Classification of Diseases (ICD) classification systems. We restricted our inclusion criteria to studies that used a structured diagnostic interview to diagnose MDD and PTSD, because structured diagnostic interviews are regarded as the gold standard in diagnosing psychopathology19 and better distinguish psychiatric symptoms from TBI symptoms. Moreover, structured diagnostic interviews are less influenced by potential memory deficits, low self-awareness, and over- or underestimation by TBI patients. In addition, with respect to PTSD, clinical interviews can be used to specifically anchor the interview to the event during which the patient was injured.26
Predictors.
We selected studies that examined at least one predictor of or multivariable model for MDD or PTSD after TBI. To be included, studies had to report at least one of the following: (1) baseline differences in predictors between patients diagnosed with MDD or PTSD (MDD+ and PTSD+) and patients not diagnosed with MDD or PTSD (MDD− and PTSD−; i.e., means and standard deviations for continuous predictors and number of patients for categorical predictors); (2) descriptive statistics (e.g., results from t test, chi-square test, p values); or (3) statistics from the multivariable model (e.g., odds ratio, area under the curve [AUC], Nagelkerke R2). To be included as a predictor, these factors must have preceded the diagnosis of MDD or PTSD. Preceding was defined as either (1) being measured earlier than the psychiatric diagnosis (in prospective studies) or (2) obviously preceding the diagnosis of MDD or PTSD such as gender, age, and computed tomography (CT) abnormalities (in retrospective, cross-sectional and case-control studies). Multivariable models were defined as models that combined at least two factors to predict a clinical outcome,27,28 in our case, MDD or PTSD.
Study design.
We included retrospective and prospective cohort studies, cross-sectional studies, and case-control studies.
Data Extraction and Assessment of Risk of Bias
One author (M.C.C. or A.C.S.) screened citations on the title and abstract, and then again on full text, excluding those that did not meet the inclusion criteria. Any doubts were resolved by consulting a senior member of the team (J.H. or S.P.). As an audit of performance, a random 20% of the full-text screening was repeated by the other reviewer (M.C.C. or A.C.S.), and concordance rates were calculated accordingly. The search process was documented according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart.29
We developed a data extraction form on the basis of the Critical Appraisal and Data Extraction for Systematic Reviews of Prediction Modeling Studies checklist30 and subsequently extracted information on type of prediction modeling study, target population, participants, outcome measurements, candidate predictors, sample size, handling of missing values, and model development methods. We additionally extracted baseline information on univariable associations between predictors and outcome by collecting means and standard deviations (SD) for MDD+/PTSD+ and MDD–/PTSD– groups (continuous predictors) or number of patients with and without the predictor in MDD+/PTSD+ and MDD–/PTSD– groups (categorical predictors). We further extracted univariable and multivariable statistics and effect measurements, if available.
Risk of bias, which refers to the risk of systematic errors that may result in the over- or underestimation of effects,31 was assessed using the Quality in Prognostic Studies (QUIPS) risk-of-bias tool. The QUIPS has been recommended by the Cochrane Prognosis Methods Groups and has acceptable interrater reliability.32 We included information on the following domains: study participation, study attrition, prognostic factor measurement, outcome measurement, study confounding, and statistical analysis and presentation. Each domain was subsequently rated as “low,” “moderate,” or “high” risk of bias. A domain obtained the score “low risk” if all individual items of the domain were rated as “low risk.” A domain was rated as “moderate risk” if at least one and a maximum of 50% of the items implied a high risk of bias or an unknown risk of bias, and a study received a score of high risk if >50% of the items implied a high risk of bias or an unknown risk of bias.
We applied a quality threshold for study inclusion in the meta-analyses; that is, studies were omitted from the meta-analyses if they obtained a high score on at least two out of the following QUIPS domains: study participation, study attrition, prognostic factor measurement, outcome measurement, and statistical analysis and presentation. Such a strategy is recommended by Cochrane.33 We did not include study confounding as a criterion because we aimed to perform a meta-analysis with univariable predictors. Studies were additionally excluded from the meta-analyses if they included fewer than 20 patients. The data extraction and risk of bias were done independently by one author (M.C.C.), with the data and decisions checked by a second author (A.C.S.). Any discrepancies were resolved by discussion with a senior member of the team (S.P.).
Data Synthesis
We performed meta-analyses of univariable predictors of MDD and PTSD. Predictors were included in the meta-analysis if univariable data (mean (SD) or numbers in MDD+/PTSD+ and MDD−/PTSD− groups) were reported in two or more studies measuring the same predictor. Studies were excluded from the meta-analyses if they measured the predictor differently from other studies (e.g., age dichotomized into two age groups instead of continuous), if they obtained a high risk of bias on at least two QUIPS domains (excluding confounding) of if they included less than 20 patients. If a study assessed predictors for multiple time points or multiple outcomes (e.g., chronic depression, late onset depression, and recovered depression) scores were combined, or if this was not possible, the time point or outcome that was closest to that in the other studies in the same meta-analysis was chosen. We used Review Manager (Revman, version 5.3)34 to perform the meta-analyses. All tests were two-sided, and a p value of 0.05 was considered statistically significant. We used the Mantel-Haenszel statistic for categorical predictors because this method is recommended by Cochrane31 and the inverse variance to analyze continuous predictors because this is not possible with the Mantal-Haenszel statistic. For all analyses, random effect models were used because we expected heterogeneity in time span and measurements. For dichotomous predictors, we reported the pooled odds ratio (pOR) and confidence interval (CI), and for continuous predictors, we reported the pooled mean difference (pMD) and CI. Heterogeneity was determined using I2 and was defined as high when I2 was ≥50% (substantial heterogeneity according to Cochrane31). In that case, pooled results should not be calculated, or at the very least, should be interpreted with caution.
Because we included studies using the DSM-IV, DSM-III, or ICD-10 criteria, we may have introduced heterogeneity in the association between predictor and the diagnosis of MDD or PTSD. We therefore performed sensitivity analyses in which we excluded studies using criteria other than those of the DSM-IV.
Predictors that were reported in at least two studies, but not included in the meta-analyses, were narratively described. Multivariable models of MDD and PTSD were narratively described by comparing model performance (e.g., AUC/Nagelkerke R2/calibration) and methods (e.g., number of candidate predictors).
Multiple Publications
Multiple publications were dealt with by selecting one main study on the basis of the following criteria: (1) the study that uses multivariable analyses; (2) the study with the largest number of patients included; and (3) the study with the largest number of predictors. If a second paper was written on the basis of the same data as the “main study” but mentioned any new predictors, only the information on these new predictors was extracted from the study.
Results
Study Selection
A total of 9,695 citations were identified through the electronic search strategy (Figure 1). After removing duplicates, 6,291 were screened on title and abstract, and 5,966 citations were excluded. We obtained 325 citations in full text, of which 295 were subsequently excluded. The most common reason for exclusion was using self-reported measurements instead of a structured diagnostic interview (N=144). The 20% audit on full-text screening obtained a concordance rate of 100% between two review authors. Five additional citations were found via reference lists and citation indices. We included 26 studies (reported in 36 publications) in the narrative synthesis. Of these, 14 studies were included in the meta-analyses.
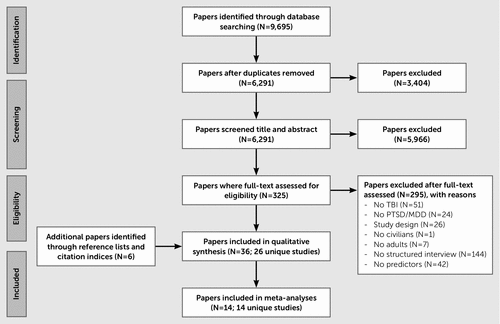
FIGURE 1. PRISMA Flowchart of the Selection Processa
a MDD, major depressive disorder; PTSD, posttraumatic stress disorder; TBI, traumatic brain injury. The figure is adapted with permission from Moher D, Liberati A, Tetzlaff J, et al: Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010; 8:336–341. Copyright © International Journal of Surgery 2010.
Study Characteristics
Of the 26 studies included, the majority (N=17) were prospective cohort studies.19,26,35–49 Four studies used a retrospective cohort design,50–53 three a cross-sectional design,54–56 and two were case-control studies.5,57 Studies were published between 1992 and 2016 and were conducted all over the globe, but mainly in high-income countries such as the United States (N=7) and Australia (N=5). Patients were recruited from general hospitals in the majority of studies (N=9). Other studies included self-identified TBI patients (N=3), patients admitted to a trauma center (N=4) or ICU (N=1), and patients in the postacute phase in a rehabilitation unit (N=3) or neuropsychological/neurocognitive TBI clinic (N=6). The large majority of studies derived their patients from a single center (N=20).
Forty-two percent (N=11) included patients with mild, moderate, and severe TBI. The diagnosis of MDD/PTSD was determined according to the DSM-IV criteria in the large majority of studies (N=20). Five studies used the DSM-III criteria36,38,39,46,51 and one study the ICD-10 criteria of MDD/PTSD.40
Fourteen studies examined predictors of MDD,5,19,36,40–42,44–46,51,52,55–57 nine studies examined predictors of PTSD,35,37–39,47–50,53 and three studies examined both.26,43,54 Nine studies included multiple predictors in a multivariable model to predict MDD (N=5), PTSD (N=3), or both (N=1).
Studies included on average 125 patients (range: 16–404). Studies that assessed predictors of MDD included on average 26 patients (range: 9–65) with MDD (“cases”) and 83 patients without MDD. Studies that assessed predictors of PTSD included on average 32 patients (range: 7–127) with PTSD (“cases”) and 142 patients without PTSD. The majority of studies included predominately male patients with a mean age between 30 and 40 years. Motor vehicle accidents (MVA) were the most reported cause of injury.
Most predictors were measured during emergency department visits or very soon after discharge. Outcome was measured between 1 month and 6 years postinjury with the majority of studies measuring MDD/PTSD between 3 months and 1 year postinjury (Table 1).
| Study | Study Design, Setting | Study Population | Inclusion and Exclusion Criteria | Patient Characteristicsb | No. of Predictors | Disorder and No. of Patients With Disorder | Interview | Timing Outcome | Assessment |
|---|---|---|---|---|---|---|---|---|---|
| Alway et al.37 | Pros cohort, Australia | Consecutive moderate and severe TBI admitted to hospital (N=203) | PTA >24 h; age 16–80 y; no prior TBI/neurological disorder; residence in Australia, sufficient English language | Age: 34 y ±16 y | 5 | PTSD (N=27)c | SCID (DSM-IV) | 3 m to 5 y | Face-to-face interview at initial assessment; telephone interview at follow-up |
| 78% male | |||||||||
| Related: Alway et al.62 | GCS: 9.3±4.3 | ||||||||
| 80% MVA | |||||||||
| Ashman et al.54 | Cross-sectional, longitudinal, and cross-sequential, United States | Self-identified mild to severe TBI from community (N=188) | US residents in the community 3 m to 4 y postinjury; age 18–87; capable of giving informed consent; no acquired brain injury/ neurocognitive disorder/psychotic disorder | Age: 40 y ±15 y | 3 MDD /3 PTSD | MDD (N=66)d and PTSD (N=56)d | SCID (DSM-IV) | 1–6 y | Interview by clinician with ≥3 y experience |
| Related: Hibbard et al.59 | 53% male | ||||||||
| GCS: 13–15, 29%; 3–12, 62%; unknown, 9% | |||||||||
| Barker-Collo et al.48 | Pros and retro cohort, New Zealand | Mild to severe TBI from a large incidence and outcome study or self-referred (N=296) | Age ≥16 | Age: 37 y ±18 y | 17 | PTSD (N=53) | PDS (DSM-IV) | 1 y | Interview by trained researchers |
| 60% male | |||||||||
| Worst GCS: 14.1 ±2.3 | |||||||||
| 30% falls, 24% assault, 17% traffic | |||||||||
| Bryant and Harvey38 | Pros cohort, Australia | Consecutive MVA victims admitted to trauma hospital (N=63) | Exclusion: inability to be interviewed with aid of an interpreter; not medically fit; taking narcotic analgesia 4weeks after trauma; PTA >24 h | Age: 29 y ±13 ye | 25 | PTSD (N=15) | CIDI (DSM-III) | 6 m | Interview by clinical psychologist blinded for ASD status |
| Related: Harvey and Bryant58 | 70% malee | ||||||||
| Bryant et al.39 | Pros cohort, Australia | Severe TBI admitted to rehabilitation unit (N=96) | Exclusion: inability to be interviewed with aid of an interpreter; insufficient cognitive abilities | Age: 34 y ±13 y | 5 | PTSD (N=26) | PTSD-I (DSM-III) | 6 m | Interview by rehabilitation consultant |
| 80% male | |||||||||
| Caspi et al.50 | Retro cohort, Israel | Mild to moderate TBI admitted to neurocognitive clinic (N=120) | Age: 18–50 y, fluent in Hebrew; no active chronic medical condition; no preinjury psychiatric illness, substance abuse, cognitive deficits, or brain damage | Age: 36 y ±6 y | 4 | PTSD (N=22) | SCID-I (DSM-IV) | 3 y | Interview |
| 59% male | |||||||||
| 84% car accident | |||||||||
| Deb and Burns40 | Pros cohort, United Kingdom | Minor to severe TBI admitted to hospital (N=165) | Any of the following: unconsciousness; evidence of skull fracture on x-rays; contusion/hemorrhage on CT or MRI; focal neurological signs; GCS <15 | Age: young group: 36; elderly group: 79 | 1 | MDD (N=24) | SCAN (ICD–10) | 1 y | Interview by two trained psychiatrists |
| 67% male | |||||||||
| 82% mild, 13% moderate, 5% severe TBI | |||||||||
| Diaz et al.41 | Pros cohort, Brazil | Consecutive severe TBI admitted to ICU (N=33) | GCS ≤8 within 48 h; age ≥18 y; resident of the Florianopolis metropolitan area; no gunshot injury | Age: 31 y ±11 y | 7 | MDD (N=10) | SCID (DSM-IV) | 18 m | Interview by two board-certified psychiatrists, blinded for hospital data |
| 88% male | |||||||||
| GCS: 7–8, 46%; 5–6, 30%; 3–4, 24% | |||||||||
| Fedoroff et al.36 | Pros cohort, United States | Consecutive mild to severe TBI admitted to shock trauma center (N=64) | Acute closed HI, no open HI, no spinal cord injury, no multiple system injury, no decreased consciousness or aphasia | Age: MDD 27 y ±6 y; no MDD: 30 y ±11 y | 25 | MDD (N=17) | PSE (DSM-III) | 1 m | Interview by trained research psychiatrist |
| Related: Jorge et al.79, Jorge et al.80b | 86% male | ||||||||
| GCS: 12–15, 17%; 8–15 & intracranial surgery or focal lesions >35 cc, 58%; 3–7, 15% | |||||||||
| Gil et al.35 | Pros cohort, Israel | Mild TBI admitted to surgical ward (N=120) | Age 18–50 y; fluent in Hebrew | Age: 31 y ±3 y | 16 | PTSD (N=17) | SCID (DSM-IV) | 6 m | Interview by trained clinician |
| 58% male | |||||||||
| Exclusion: psychiatric care at time of injury; prior HI; cognitive deficits; substance abuse; major untreated medical condition | 90% traffic accident | ||||||||
| GCS: 13–15 100% | |||||||||
| Gould et al.42 | Pros cohort, Australia | Consecutive TBI admissions to a rehabilitation hospital (N=122) | Mild, moderate, or severe TBI; age 16–80; no previous TBI/ neurological disorder; residence in Australia; sufficient cognitive and English ability | Age: 35; 16 y | 7 | MDD (N=40) | SCID (DSM-IV) | 12 m | Interview |
| Related: Gould et al.60 and Schonberger et al.81 | GCS: 9.15 ±4.3 | ||||||||
| Hibbard et al.43 | Pros cohort, United States | Mild to severe TBI randomly selected for quality of life survey (N=100) | TBI ≤1 y prior to interview; age 18–65; resident of New York state; living in the community; no nontraumatic brain injury | Agef:40 y ±10 y | 5 MDD /1 PTSD | MDD (N=48) and PTSD (N=17) | SCID (DSM-IV) | 8 y | Interview by licensed psychologist with background in clinical neuropsychology and brain injury |
| 53% male | |||||||||
| 62% MVA | |||||||||
| Jorge et al.57 | Pros case-control, United States | Consecutive mild to severe TBI admitted to hospital (N=91) | Exclude: penetrating HI; spinal cord injury; severe comprehension deficits | Age: 36 y ±16 y | 32 | MDD (N=30) | PSE and SCID-I (DSM-IV) | 9 m | Interview by psychiatrist |
| 59% male | |||||||||
| Related: Jorge et al.61 | 44% mild, 33% moderate, 23% severe TBI | ||||||||
| 75% MVA | |||||||||
| Kennedy et al.19 | Pros cohort, United States | Mild to mod TBI admitted to neuropsychiatric clinic (N=78) | 3 m postinjury; age≥ 18 | Age: 38 y ±12 y | 10 | MDD (N=23) | SCID (DSM-IV) | 76 m | Interview by three trained research team members |
| 69% male | |||||||||
| Mean GCS: 9.3 ±4.8 | |||||||||
| 77% MVA | |||||||||
| Koponen et al.51 | Retro cohort, Finland | Mild to severe TBI seen for neuropsychological evaluation (N=60) | TBI causing neurological symptoms ≥1 week; one of the following: 1) LOC ≥1 min; 2) PTA ≥30 min; 3) neurological symptoms during the first 3 d; 4) neuroradiological findings suggesting TBI. No nontraumatic neurological illness | Age: 29 y ±11 y | 2 | MDD (N=16) | SCAN (DSM-III) | 31 y | Interview by trained research psychiatrist |
| Related: Koponen et al.82 | 68% male | ||||||||
| Levin et al.44 | Pros cohort, United States | Consecutive mild TBI admitted to level I trauma hospital (N=129) | Hospital arrival ≤24 h; BAL ≤200 mg/dl; age ≥16 y; fluent in English or Spanish; resident in catchment area | Age: 32±13 y | 8 | MDD (N=15) | SCID (DSM-IV) | 3 m | Interview |
| 67% male | |||||||||
| Exclusion: undocumented alien; incarcerated; homeless; active military service; spinal cord injury; previous TBI requiring hospitalization; preinjury substance dependence, mental retardation, psychiatric disorders or other central nervous system disturbances; no preexisting condition preventing outcome measurement | GCS: 14.8±0.5 | ||||||||
| 67% MVA | |||||||||
| Li et al.49 | Pros cohort, China | Consecutive mild TBI patients at the ED of three hospitals (N=43) | LOC <20 min, PTA <24 h, GCS 13–15, no abnormal CT/MRI findings | Age: PTSD 35.8 y ±7.6; no PTSD 36.7 y ±7.1 | 9 | PTSD (N=21)— | CAPS | 6 m | Interview |
| 49% male | |||||||||
| Mauri et al.5 | Pros case-control, Italy | Consecutive closed HI admitted to neurosurgery (N=16) | LOC ≥1m; PTA ≥30 min; neuroradiological evidence of TBI; no preinjury neurological/cardiorespiratory/psychiatric conditions; no substance abuse | Age: 40 y ±14 y | 4 | MDD (N=10) | SCID (DSM-IV) | 1 m | Interview by expert clinician |
| 63% male | |||||||||
| GCS 10.6±4.4 | |||||||||
| 81% MVA | |||||||||
| O’Donnell et al.26 | Pros cohort, Australia | Randomly selected mild TBI patients at four level I trauma centers (N=404) | Age 18–70 y; English proficiency, hospitalized≥24 h, LOC ≤30 min, GCS 13–15, PTA ≤24 h, not currently psychotic or suicidal | Age: 37.9 y ±14 y | 2 MDD /2 PTSD | MDD (N=65) and PTSD (N=32) | MINI (MDD, DSM-IV); CAPS (PTSD, DSM-IV) | 12 m | Telephone interview |
| 72% male | |||||||||
| 62% transport accidents, 17% falls | |||||||||
| Rao et al.55 | Cross-sectional, United States | Closed HI recruited by advertisements in local newspapers (N=17) | Age ≥18 y; TBI 3–60 m prior to evaluation; no history of diagnosable mood disorder; MMSE >18, stable medical history; sufficient cognitive capacity | Age: MDD, 53; no MDD, 27 | 38 | MDD (N=10) | SCID (DSM-IV) | 3–60 m | Interview |
| Rapoport et al.17 | Pros cohort, Canada | Consecutive mild TBI with appointment at TBI clinic (N=210) | Nonpenetrating mild TBI | Age: 47 y ±20 y | 10 | MDD (N=35) | SCID (DSM-IV) | 49 d | Interview by psychiatrist |
| Related: Rapoport et al.45 | Exclusion: preinjury focal brain disease; serious acute medical illness; schizophrenia; bipolar disorder; dementia | 60% male | |||||||
| 61% MVA | |||||||||
| Rapoport et al.56 | Cross-sectional, Canada | Mild and mod TBI attending a TBI clinic (N=74) | Exclusion: premorbid focal brain disease; serious medical illness; schizophrenia; bipolar disorder; dementia | Age: 35y; ±13y | 16 | MDD (N=21) | SCID (DSM-IV) | 200 d | Interview |
| van Reekum et al.46 | Pros cohort, Canada | Mild to severe TBI admitted to TBI rehabilitation program. Patients were contacted with a female:male ratio of 3:1 (N=18) | TBI due to MVA ≥2 y prior to the study; age <50 y; sufficient language, motor, and perceptual skills to permit testing; no preinjury psychiatric disorder; living in the community | Age: 31 y ±9 y | 4 | MDD (N=9) | SADS-L (DSM-III) | 5 y | Interview by experienced registered psychiatric nurse |
| 44% male | |||||||||
| GCS: 13–15, 28%; 9–12, 17%; 3–8, 56% | |||||||||
| Roitman et al.47 | Pros cohort, Israel | Consecutive mild TBI attended ED (N=402) | MVA survivors | Age: 37 y ±13 y | 1 | PTSD (N=127) | PSS (DSM-IV) | 8 m | Telephone interview |
| Exclusion: arrived to the hospital in coma; LOC >30 min; admitted to the hospital >7 days | 52% male | ||||||||
| Turnbull et al.53 | Retro cohort, Scotland | Mild to severe TBI attended ED who respond to a postal questionnaire (N=53) | Age: 16–65; evidence of TBI; no chronic alcohol abuse | Age: 35 y ±11 y | 1 | PTSD (N=11) | CAPS (DSM-IV) | 6 m | Telephone interview by postgraduate psychologist |
| 87% male | |||||||||
| 32% traffic; 60% assault | |||||||||
| Whelan-Goodinson et al.52 | Retro cross-sectional, Australia | Mild to severe TBI admitted to rehabilitation unit (N=100) | GCS <15; cognitive capable; reliable historians according to treating doctor/neuropsychologist, sufficiently proficient in English; no previous TBI/neurological disorder | Age: 37 y ±14 y | 13 | MDD (N=46) | SCID (DSM-IV) | 0.5–5.5 y | Face-to-face or telephone interview |
| 71% male | |||||||||
| GCS: 9.1; 4.1 | |||||||||
| 86% MVA |
TABLE 1. Study Characteristics of 26 Studies Examining Predictors of or Multivariable Models for MDD and PTSD After TBIa
Risk of Bias of the Studies
The majority of studies (N=18)5,19,26,36,38,40,41,43,46–49,51,53,55–58 were scored as high risk of bias for study confounding because they assessed only the effect of predictors in univariable analyses. It is therefore unknown whether the effect of the predictor is independent of other factors. Because we sought to perform a meta-analysis with univariable data, we did not exclude any studies on the basis of a high risk of study confounding from the meta-analysis.
Except for the high risk of study confounding, methodological quality of the included studies was acceptable (Table 2). Study participation19,43,55 and attrition40,46,53 were rated at high risk of bias in three studies. Additionally, one study was judged at high risk of bias for prognostic factor measurement5 and outcome measurement,53 and six studies were rated at high risk of bias on statistical analysis and reporting.5,42,47,49,50,53 Three studies5,49,53 were rated at high risk on two out of five (excluding study confounding) domains and were therefore omitted from the meta-analyses. Two other studies46,55 included fewer than 20 patients and were therefore also excluded from the meta-analyses.
| Study | Study Participation | Study Attrition | Prognostic Factor Measurement | Outcome Measurement | Study Confounding | Statistical Analyses and Presentation |
|---|---|---|---|---|---|---|
| Alway et al.37 | Moderate | Moderate | Low | Low | Low | Low |
| Ashman et al.54 | Moderate | Moderate | Moderate | Low | Low | Moderate |
| Barker-Collo et al.48 | Moderate | Moderate | Low | Low | High | Low |
| Bryant and Harvey38 | Low | Low | Low | Low | High | Low |
| Bryant et al.39 | Low | Moderate | Low | Low | High | Low |
| Caspi et al.50 | Low | Moderate | Low | Low | Low | High |
| Deb and Burns40 | Low | High | Low | Moderate | High | Low |
| Diaz et al.41 | Low | Low | Low | Low | High | Low |
| Federoff et al.83 | Low | Low | Low | Low | High | Low |
| Gil et al.35 | Low | Moderate | Low | Low | Low | Low |
| Gould et al.42 | Low | Moderate | Low | Low | Low | High |
| Hibbard et al.43 | High | Moderate | Moderate | Low | High | Moderate |
| Jorge et al.57 | Low | Low | Low | Low | High | Low |
| Kennedy et al.19 | High | Moderate | Low | Low | High | Low |
| Koponen et al.51 | Moderate | Moderate | Moderate | Low | High | Low |
| Levin et al.44 | Low | Moderate | Low | Low | Low | Low |
| Li et al.49 | Moderate | Low | Moderate | Low | High | High |
| Mauri et al.5 | Moderate | Low | High | Low | High | High |
| O’Donnell et al.26 | Low | Low | Low | Low | High | Low |
| Rao et al.55 | High | Low | Low | Low | High | Moderate |
| Rapoport et al.45 | Moderate | Low | Moderate | Low | Low | Low |
| Rapoport et al.56 | Moderate | Low | Moderate | Low | High | Low |
| van Reekum et al.46 | Moderate | High | Low | Low | High | Low |
| Roitman et al.47 | Moderate | Low | Moderate | Low | High | High |
| Turnbull et al.53 | Moderate | High | Moderate | High | High | High |
| Whelan-Goodinson et al.52 | Moderate | Low | Low | Low | Moderate | Moderate |
TABLE 2. Risk of Bias Assessmenta
Meta-Analyses of Univariable Predictors
The included studies examined a total of 112 predictors of MDD and 59 predictors of PTSD (Figure 2). Age and gender were most often assessed. The majority of predictors were assessed in only one study. Consequently, only 18 and six predictors were included in the meta-analyses for MDD and PTSD, respectively (Table 3; also see the online data supplement).
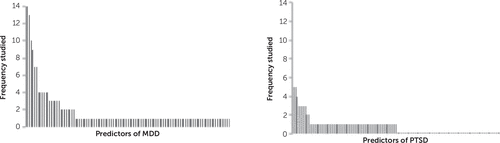
FIGURE 2. Frequency of Predictors of Major Depressive Disorder (MDD) and Posttraumatic Stress Disorder (PTSD) Following Traumatic Brain Injury (TBI)a
a The figure shows how frequent predictors were studied across the included studies. For example, for MDD, one predictor (age) is studied in 14 studies, and one predictor (gender) is studied in 13 studies. The majority of predictors (e.g., MRI abnormalities) were assessed in one study.
| Predictor | No. of Participants (No. of Studies) | Pooled Effect Size Meta-Analysis Odds Ratio (95% CI)b | Heterogeneity (I2) |
|---|---|---|---|
| MDD | |||
| Age (years; MD [95% CI]) | 611 (7) | 1.20 (–1.96 to 4.36) | 49% |
| Female gender | 768 (8) | 1.72 (1.19 to 2.48) | 10% |
| Education (years; MD [95% CI]) | 271 (4) | –0.50 (–1.37 to 0.37) | 43% |
| Caucasian race | 341 (3) | 1.04 (0.61 to 1.75) | 0% |
| Marital statusc | 610 (6) | 1.20 (0.82 to 1.75) | 0% |
| Socioeconomic statusd | 140 (2) | 0.69 (0.33 to 1.43) | 0% |
| Preinjury depression | 470 (5) | 3.86 (2.26 to 6.59) | 0% |
| Preinjury psychiatric disorders | 426 (4) | 1.58 (0.42 to 5.99) | 87% |
| Preinjury alcohol abuse | 244 (2) | 1.49 (0.61 to 3.69) | 0% |
| Preinjury substance abuse | 244 (2) | 2.02 (0.75 to 5.42) | 0% |
| Preinjury unemployment | 244 (2) | 3.80 (0.34 to 42.09) | 77% |
| Family history of psychiatric disorders | 234 (2) | 1.06 (0.52 to 2.14) | 0% |
| Admission GCS (MD [95% CI]) | 151 (2) | 0.49 (0.02 to 0.97) | 0% |
| 24-hour GCS (MD [95% CI]) | 138 (2) | 0.13 (–1.29 to 1.56) | 42% |
| CT abnormalities | 259 (3) | 0.70 (0.35 to 1.43) | 0% |
| Brain contusion | 101 (2) | 1.78 (0.73 to 4.34) | 0% |
| Postinjury unemployment | 211 (3) | 2.04 (1.10 to 3.79) | 9% |
| Postinjury litigation situation | 203 (2) | 0.64 (0.16 to 2.53) | 0% |
| PTSD | |||
| Age (years; MD [95% CI]) | 717 (5) | 1.02 (–1.46 to 3.49) | 75% |
| Female gender | 621 (4) | 1.27 (0.83 to 1.96) | 0% |
| Education (years; MD [95% CI]) | 301 (3) | 0.15 (–0.61 to 0.92) | 11% |
| Preinjury psychiatric disorder | 425 (4) | 1.32 (0.63 to 2.77) | 49% |
| PTA (MD [95% CI]) | 477 (3) | –8.07 (–15.46 to –0.69) | 33% |
| Memory of the traumatic event | 240 (2) | 5.15 (2.37 to 11.21) | 0% |
TABLE 3. Meta-Analyses of Univariable Predictors of Major Depressive Disorder (MDD) and Posttraumatic Stress Disorder (PTSD) Following Traumatic Brain Injurya
We found a significant association between the development of MDD and female gender (pOR 1.72, 95% CI=1.19 to 2.48, I2=10%; eight studies). Additionally, patients with a preinjury depression had higher odds on developing MDD postinjury than did patients without a history of depression (pOR 3.86, 95% CI=2.26 to 6.59, I2=0%; five studies). Also, patients who were unemployed after sustaining TBI had higher odds on developing MDD later on than did the employed patients (pOR 2.04, 95% CI=1.10 to 3.79, I2=9%; three studies). We further found that patients with a higher admission Glasgow Coma Scale (GCS), which refers roughly to moderate TBI versus severe TBI in these studies, had a higher risk on developing MDD (pMD=0.49, 95% CI=0.02 to 0.97, I2=0%). This was, however, only assessed in two studies, and we did not find a significant association between GCS after 24 hours and MDD (pMD=0.13, 95%C=–1.29 to 1.56, I2=42%; two studies). The association between the other predictors and MDD were all nonsignificant.
PTSD was significantly associated with a shorter posttraumatic amnesia ([PTA]; pMD=–8.07, 95% CI=–15.46 to –0.69, I2=33%; three studies) and a memory of the traumatic event (pOR 5.15, 95% CI=2.37 to 11.21, I2=0%; two studies). We did not find a significant association between the remainder of predictors and PTSD. Sensitivity analyses with only those studies using the DSM-IV criteria did not result in any differences (see the online data supplement).
Narrative Synthesis of Univariable Predictors
For MDD, five out of six studies in the narrative synthesis did not find an association between the development of MDD and age5,40,43,46,52,55 and none of the studies reported a significant association with any other demographic factors and MDD (gender, education, marital status, income [also see the online data supplement]).5,19,43,52,55–57,59 For preinjury variables, patients with a history of psychiatric disorders had a significantly higher risk of developing MDD.42,57,60 We did not find an association between preinjury substance and alcohol abuse,36,42,56 preinjury unemployment,52,56 family history of psychiatric disorders,56,57 preinjury TBI,17,56 or mechanism of injury and MDD.19,45,56 For clinical variables, we did not find an association among GCS,19,36,43,46,57 PTA,51,52,56 and MDD. Bodily injuries were associated with MDD in one out of three studies.42,52,56
Three studies analyzed the association between imaging variables and MDD.55,57,61 Jorge et al.57 found that the percentage of gray matter in the left lateral frontal cortex and the percentage of gray matter at the left inferior frontal gyrus on magnetic resonance imaging were higher in patients who developed MDD. The influence of brain volume was assessed in two studies that consistently found that a lower brain volume was associated with the development of MDD.55,61 Early postinjury anxiety and depression were assessed in two studies.26,42 One study found that early postinjury depression, measured with the SCID, was associated with postinjury MDD and did not found an association between early postinjury anxiety and MDD.42 Another study reported that the Hospital Anxiety and Depression Survey was significantly associated with MDD (AUC 0.72, p<0.01).26 This study additionally developed a screening instrument based on preinjury factors and postinjury irritability and concentration problems, which was also significantly related to MDD (AUC 0.77, p<0.01).
For PTSD, demographic variables were not associated with PTSD in the studies in the narrative synthesis, except for one study54 that found that PTSD was more common among women. PTSD was not associated with injury mechanism in three studies48–50 (see the online data supplement). Also, GCS was not associated with the development of PTSD.39,48,62 One study reported that patients with loss of consciousness (LOC) had higher odds on PTSD,47 whereas two other studies did not find statistical differences.48,49 One-month PTSD symptoms or symptoms of ASD were significantly associated with PTSD in four studies.26,35,38,49 Bryant et al.38 studied individual ASD symptoms and reported that the following symptoms were associated with 6-month PTSD: helplessness, numbing, depersonalization, recurrent images and thoughts, avoidance of thoughts or talk, avoidance of places and people, insomnia, irritability, and motor restlessness. Postinjury anxiety and depression were related to 6-month PTSD in one study.35 Another study developed a screening instrument for PTSD on the basis of preinjury, peri-injury, and postinjury factors and reported an AUC of 0.91 (p<0.001).26
Narrative Synthesis of Multivariable Models
Six studies used a multivariable model to predict MDD (Table 4). On average, models included 6.3 cases (range: 1.2–22) for every predictor in the model. None of the studies described whether there were missing values in predictors and if so, how they were handled. Nagelkerke R2 was calculated in three models42,45,52 and ranged from 0.18 to 0.35. The AUC was calculated in one study44 and indicated good discriminative ability (AUC=0.86). This model included age, depressive symptoms after one week postinjury, and computerized tomography results.
| Study | Timing Model Use | Number of Patients | Number of Casesb | Number of Candidate Predictors | Selection Procedure of Predictors | Statistical Model | Outcome Measurement and Timing | Summary Statistics | Final Predictors in Model |
|---|---|---|---|---|---|---|---|---|---|
| MDD | |||||||||
| Ashman et al.54 | Unknown | 188 | 35; 24; 21c | 3 | Not reported | Linear random effects longitudinal model | SCID-I at 3 m to 4 y | Not reported | Age (OR: 1.00; p=0.77), time postinjury (OR: 0.88, p=0.23) and time of enrollment in the study (OR: 0.59, p<0.001) |
| Federoff et al.83 | ED | 64 | 17 | 14 | All CT lesion location variables measured | Logistic regression model with backward selection (p>0.05) | PSE at 1 m | χ2(6)=31.39, p=0.0001 | Left hemisphere (b: –2.84, p=0.04); |
| right hemisphere (b: 2.40, p=0.03); | |||||||||
| cortical (b:–3.67, p=0.01); | |||||||||
| frontal (b:–3.58, p=0.01); | |||||||||
| left anterior (b: 5.90, p=0.0003); | |||||||||
| parietal-occipital (b: 3.75, p=0.009) | |||||||||
| Gould et al.42 | At discharge | 122 | 40 | 7 | Not reported | Two logistic regression models—(1) preinjury variables; (2) injury-related variables. Significant variables were entered into a final regression model. | SCID-I at 12 m | Nagelkerke R2=0.20; correct classification rate: 70.7% | Preinjury counseling (OR: 2.34, p=0.073); |
| limb injury (OR: 4.07, p=0.009); | |||||||||
| depressive disorder at initial assessment (OR: 6.04, p=0.039) | |||||||||
| Levin et al.44 | 1 wk | 129 | 15 | 8 | Not reported | Logistic regression with backward selection (p>0.05) | SCID-I at 3 m | AUC=0.86 | Age (OR: 1.05; 95% CI: 1.00 to 1.1); |
| CES-D score at 1 wk (OR: 1.11; 95% CI: 1.04 to 1.17); abnormal CT scan (OR: 7.68; 95% CI: 1.36 to 43.48) | |||||||||
| Rapoport et al.45 | ED | 210 | 35 | 11 | Significant differences in univariable analyses | Hierarchical logistic regression model with time postinjury as covariate | SCID-I at 49 d | Nagelkerke R2=0.18 | Age (OR: 0.99, SE: 0.05, p>0.05); preinjury depression (OR: 0.28, SE: 0.67, p>0.05); substance abuse (OR: 0.25, SE: 0.67, p<0.05); time postinjury (OR: 1.00, SE: 0.001, p>0.05); gender (OR: 0.50, SE: 0.52, p>0.05); employment (OR: 0.49, SE: 0.71, p>0.05); education (OR: 0.52; SE: 0.48, p>0.05), family history of depression (OR: 0.28, SE=0.67, p>0.05); medical history (OR: 1.49, SE: 0.55, p>0.05); focal CT abnormalities (OR: 0.77, SE: 0.55, p>0.05); mechanism of injury (OR: 1.66, SE: 1.62, p>0.05) |
| Whelan-Goodinson et al.52 | ED | 100 | 46 | 13 | Significant in univariable analyses | Logistic regression model | SCID-I at 0.5 to 5 y | χ2(6)=29.10, p<0.001, Nagelkerke R2=0.35; correct classification absent depression: 80.4%; correct classification presence depression: 67.4%; overall correct classification: 74.2% | Gender (B=0.48; p=0.10); pain (B=–0.97, p=0.06); postinjury unemployment (B=0.48; p=0.39); preinjury depression (B=1.87; p=0.01); years of education (B=1.87; p=0.01); time postinjury (B=0.32, p=0.06) |
| PTSD | |||||||||
| Alway et al.37 | ED | 203 | 27 | 5 | Not reported | Multivariable random-effects logistic regression model adjusting for time postinjury | SCID-I at different follow-up points, 3 m to 5 y | Not reported | Age (OR: 0.99; 95% CI: 0.95 to 1.03); female gender (OR: 0.31; 95% CI: 0.05 to 2.08); years of education (OR: 1.06; 95% CI: 0.80 to 1.42); preinjury psychiatric disorder (OR: 0.84; 95% CI: 0.23 to 3.15); PTA (days; OR: 0.98; 95% CI: 0.95 to 1.02) |
| Ashman et al.54 | Unknown | 188 | 30; 18; 21c | 3 | Not reported | Linear random- effects longitudinal model | SCID-I at 3 m to 4 y | Not reported | Age (OR: 0.98; p=0.22), time postinjury (OR: 1.07, p=0.74), and time of enrollment in the study (OR: 0.59, p=0.003) |
| Caspi et al.50 | 2.9 y postinjury | 120 | 22 | 4 | Not reported | Logistic regression model adjusted for co-occurring depressive (BDI) and anxiety (BAI) symptoms | SCID-I at 3 y | Goodness of fit: 83.42, p<0.001; Nagelkerke R2=0.42, p<0.001 | Memory for the traumatic event (OR: 2.8; 95% CI: 1.8 to 8.9); male gender (OR: 0.5, p>0.05); history of psychiatric illness (OR: 0.5, p>0.05), age (OR: 1.2, p>0.05) |
| Gil et al.35 | 1 m postinjury | 120 | 17 | 16 | Significant in univariable analyses | Logistic regression model with variables that had shown significant association in univariable analyses | SCID-I at 6 m | Nagelkerke R2=0.38, p<0.001 | Memory of traumatic event (OR: 2.2, 95% CI: 1.0 to 10.1); |
| acute posttraumatic symptoms (CAPS; OR: 5.3; 95% CI: 1.1 to 9.3); acute posttraumatic symptoms (PSS; OR: 5.2; 95% CI: 1.0 to 9.4); depressive symptoms (1 wk; OR: 5.1; 95% CI: 1.0 to 9.2); | |||||||||
| anxiety symptoms (1 wk; OR: 4.9; 95% CI: 1.0 to 9.1), history of psychiatric disorders (OR: 3.7; 95% CI: 1.1 to 8.9) | |||||||||
TABLE 4. Multivariable Models of Major Depressive Disorder (MDD) and Posttraumatic Stress Disorder (PTSD) After Traumatic Brain Injury (TBI)a
Four studies used a multivariable model to predict PTSD. Models included on average 7.7 cases (range: 1.1–19) per predictor. Again, none of the studies described how they handled missing values in predictors. Nagelkerke R2 was reported for two models35,50 and ranged from 0.38 to 0.42. Both models included memory of the traumatic event and history of psychiatric disorders. None of the multivariable models for MDD and PTSD used internal or external validation to improve the generalizability.
Discussion
This systematic review provides an overview of univariable predictors of and multivariable models for MDD and PTSD following TBI. We included 26 studies and found that the development of MDD was associated with female gender and a preinjury depression. Postinjury MDD might also be associated with postinjury unemployment status, early postinjury psychiatric symptoms, a higher GCS, and a lower brain volume. The development of PTSD was associated with a shorter PTA and a memory of the traumatic event. It may also be associated with early symptoms (e.g., depression, anxiety, ASD). Only a few studies used a multivariable model to predict MDD or PTSD, of which the majority were of limited quality.
This systematic review included studies over the last 23 years from all over the globe and therefore provides a complete overview of current knowledge of predictors and multivariable models for MDD and PTSD following TBI. Some notes should, however, be made regarding the completeness and applicability of the evidence. First, the majority of predictors were examined in only one study and therefore were not included in our meta-analyses. For many predictors, we consequently cannot draw firm conclusions. A possible solution might have been to include studies with self-reported outcome measurements, because these studies are more common and usually include more patients. However, self-reported measurements are less reliable for TBI patients.16–18 For example, a 2006 study found that the diagnosis of PTSD varied from 59% to 3% when using self-reported measurements and structured diagnostic interviews, respectively.20 For MDD, a similar range is reported.22 In self-reported measurements, the overlap between TBI and the psychiatric disorder is usually not captured. For example, focus on the memory gap following coma without great distress could be inappropriately labeled as intrusive in a self-reported measurement.20 Also the symptoms of sleep problems, irritability, and concentration problems, which might be indicative of postconcussive syndrome, might be scored as hyperarousal symptoms in self-reported instruments. Reliability of self-reported measurements might further be hampered by memory deficits, low self-awareness, and attention problems.17–22 This is illustrated in a 2001 case report.63 The inclusion of self-reported measurement might therefore have resulted in the reporting of invalid predictors, compromising the quality of this systematic review.
A second note that could be made regarding the completeness and applicability of evidence is that only a minority of studies used a multivariable model. The majority of results are consequently based on univariable associations. As a consequence, we cannot exclude the possibility that some of the associations that we found were influenced by other factors. Also, factors that are nonsignificant in this review might comprise important predictors after correction for confounders. Third, the majority of studies included patients with mild, moderate, and severe TBI and did not stratify or correct for TBI severity. Lastly, the majority of studies were underpowered, which might have resulted in nonsignificant findings in the narrative synthesis. This problem was partly captured by performing meta-analyses. This was, however, only possible for 18 and six predictors of MDD and PTSD, respectively.
The risk of bias for most studies developing multivariable models was high. Models included on average six to eight cases for every predictor, while it is recommended to include at least 10.64,65 Including too many predictors enhances the risk of finding too extreme estimates (“statistical overfitting”), limiting generalizability of findings.66 Additionally, the majority of studies did not report how they handled missing data and how they selected candidate predictors. Also, none of the studies used internal or external validation. As a consequence, none of the multivariable models could be applied to clinical practice yet.
We found a significant association between female gender and the likelihood of developing MDD in our meta-analysis. This is in line with systematic reviews about gender and depression in the general population; females have approximately twice as high a risk of developing major depression as do males.67,68 However, this significant association was not found in three studies that were not included in the meta-analysis.43,55,56 These studies were, however, underpowered because they included only 48, 10, and 21 cases, respectively.
MDD was also associated with the presence of a preinjury depression, which might be due to the high recurrence rates in MDD. A large prospective study reported that up to 85% of the patients with prior MDD developed a new MDD episode during a 15-year follow-up period.69 Recurrence of MDD can be triggered by a stressful life event, such as a TBI, although causation is usually multifactorial.70,71
Furthermore, MDD was more prevalent among those reporting postinjury unemployment and early postinjury psychiatric symptoms. This has also been shown in systematic reviews in the general population.72,73 Unemployment can result in reduced social interactions and status, which may subsequently result in depression.74
Higher-admission GCS, referring predominately to moderate TBI patients in comparison with severe TBI patients, might also be associated with higher odds of MDD. However, we did not find an association between 24 hours GCS and MDD and also failed to find an association between GCS as a categorical variable and MDD in the narrative synthesis. As a consequence, the association between GCS and MDD remains uncertain.
Lastly, MDD after TBI might also be associated with lower brain volume. This was in line with a 2012 meta-analysis about gray matter abnormalities in MDD.75 Because this was only assessed in two studies that used relatively low sample sizes, these finding should be interpreted with caution.
PTSD was more likely among patients with a shorter PTA and those with a memory of the traumatic event. A shorter PTA (less amnesia) and memory of the event basically mean the same thing, and it is suggested that amnesia for the traumatic event minimizes the establishment of cognitive representations and so reduces the likelihood of intrusive symptoms.50 However, one out of three studies found a significant association between the occurrence of LOC and PTSD, and the two studies assessing the association between PTSD and GCS did not find a significant effect, which might be contradictory to our findings on PTA and memory of the event; i.e., LOC and a low GCS are usually accompanied by at least some PTA. The difference in findings could be attributable to the lack of power in individual studies in the narrative synthesis. Future research is important in confirming the possible association between memory of the traumatic event and PTSD. PTSD was further significantly associated with ASD and early PTSD symptoms. Although studies could not be pooled because of different outcomes reported, four individual studies found a significant association between ASD or PTSD symptoms after 1 month and PTSD after 6 or 12 months. This was in line with a systematic review about predictors of sequelae in mild TBI patients76 and a review about predictors of PTSD using self-reported outcome measurements.16
Strengths of this systematic review include the comprehensive search strategy, the restriction to structured diagnostic interviews, and the performance of meta-analyses, which improved the statistical power. Additionally, we combined results from the meta-analyses, narrative syntheses, and multivariable models to obtain conclusions about the significance of predictors. We thereby integrated all available sources of evidence. A limitation of the use of meta-analyses is that there was between-studies variation in time span, TBI severity, and outcome measurement, resulting in estimates that are difficult to interpret. Also, the use of the I2 statistic to interpret heterogeneity in the meta-analyses could be considered a limitation. Although the I2 statistic is the best heterogeneity measurement available, it might be biased and not very precise in small meta-analyses.77,78 Therefore, overlap in CIs should also be considered when interpreting heterogeneity between studies. A third limitation concerns our screening process, which was conducted by one study author. We, however, performed an audit and found a 100% concordance between study authors, indicating that screening by two independent reviewers would probably not have resulted in the inclusion of any additional studies.
The results of this systematic review imply that there is still limited knowledge regarding which patients develop MDD and PTSD after TBI. We therefore cannot recommend yet which patients should receive additional follow-up or preventive treatment and advise physicians to be aware regarding all patients who sustained TBI. Physicians could be extra aware regarding female patients with a preinjury history of depression and postinjury unemployment or psychiatric symptoms. Also, a reduction in brain volume might indicate a risk of developing MDD postinjury. Furthermore, patients with a shorter PTA, with a clear memory of the traumatic event, and with early posttraumatic symptoms might be at higher risk of developing PTSD post-TBI.
More research is needed to confirm the relevance of these predictors of MDD and PTSD after TBI and to develop a multivariable model that could be implemented in hospitals and rehabilitation centers. Future prognostic studies should include a more homogenous group of TBI patients (e.g., only those with mild TBI). It is also recommended that future studies include a large sample size and a limited set of candidate predictors. Selection of candidate predictors could be based on current review, theory, or clinical knowledge about etiology of psychiatric disorders. Additionally, the confirmation of specific predictions among different patient samples is critically important to increase our knowledge about predictors of psychiatric sequelae post-TBI.
Conclusions
Our systematic review showed that MDD after TBI was associated with female gender, preinjury depressive disorder, postinjury unemployment, early postinjury psychiatric symptoms, and a lower brain volume, whereas PTSD was related to PTA, a memory of the traumatic event, and early posttraumatic symptoms. Currently, available multivariable models of MDD and PTSD after TBI suffer from methodological shortcomings. The findings of the current review, together with clinical knowledge about etiology of psychiatric disorders, could form the basis for future development of a prognostic model from a large sample of TBI patients using solid methodology.
1 : Position statement: definition of traumatic brain injury. Arch Phys Med Rehabil 2010; 91:1637–1640Crossref, Medline, Google Scholar
2 : Epidemiology of traumatic brain injury in Europe. Acta Neurochir (Wien) 2015; 157:1683–1696Crossref, Medline, Google Scholar
3 : Neuropsychiatric complications of traumatic brain injury: a critical review of the literature (a report by the ANPA Committee on Research). J Neuropsychiatry Clin Neurosci 2007; 19:106–127Link, Google Scholar
4 : Depression following adult, non-penetrating traumatic brain injury: a meta-analysis examining methodological variables and sample characteristics. Neurosci Biobehav Rev 2014; 47:1–15Crossref, Medline, Google Scholar
5 : Clinical and neuropsychological correlates of major depression following post-traumatic brain injury, a prospective study. Asian J Psychiatr 2014; 12:118–124Crossref, Medline, Google Scholar
6 : Traumatic brain injury and its neurobehavioral sequelae. Neurol Clin 2011; 29:35–47, [vii.]Crossref, Medline, Google Scholar
7 : Prevalence and risk factors of anxiety and depressive disorders following traumatic brain injury: a systematic review. J Neurotrauma 2016Crossref, Google Scholar
8 : The manifestation of anxiety disorders after traumatic brain injury: a review. J Neurotrauma 2015; 32:411–421Crossref, Medline, Google Scholar
9 : Impact of depression and post-traumatic stress disorder on functional outcome and health-related quality of life of patients with mild traumatic brain injury. J Neurotrauma 2015; 32:853–862Crossref, Medline, Google Scholar
10 : The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J Clin Psychiatry 2015; 76:155–162Crossref, Medline, Google Scholar
11 : Health care costs associated with posttraumatic stress disorder symptoms in women. Arch Gen Psychiatry 2003; 60:369–374Crossref, Medline, Google Scholar
12 : Traumatic brain injury in the Netherlands: incidence, costs and disability-adjusted life years. PLoS One 2014; 9:e110905Crossref, Medline, Google Scholar
13 : Effect of psychological interventions on depressive symptoms in long-term rehabilitation after an acquired brain injury: a systematic review and meta-analysis. Arch Phys Med Rehabil 2013; 94:1386–1397Crossref, Medline, Google Scholar
14 : Immediate and early behavioral interventions for the prevention of acute and posttraumatic stress disorder. Curr Opin Psychiatry 2011; 24:526–532Crossref, Medline, Google Scholar
15 : Prevention of posttraumatic stress disorder in intensive care unit patients. Dimens Crit Care Nurs 2012; 31:69–72Crossref, Medline, Google Scholar
16 : Psychosocial and psychological factors associated with post-traumatic stress disorder following traumatic brain injury in adult civilian populations: a systematic review. Brain Inj 2014; 28:1–14Crossref, Medline, Google Scholar
17 : The clinical significance of major depression following mild traumatic brain injury. Psychosomatics 2003; 44:31–37Crossref, Medline, Google Scholar
18 : Mild traumatic brain injury and anxiety sequelae: a review of the literature. Brain Inj 2006; 20:117–132Crossref, Medline, Google Scholar
19 : Evaluation of the Neurobehavioral Functioning Inventory as a depression screening tool after traumatic brain injury. J Head Trauma Rehabil 2005; 20:512–526Crossref, Medline, Google Scholar
20 : Errors in self-report of post-traumatic stress disorder after severe traumatic brain injury. Brain Inj 2006; 20:93–99Crossref, Medline, Google Scholar
21 : Psychiatric comorbidity following traumatic brain injury. Brain Inj 2007; 21:1321–1333Crossref, Medline, Google Scholar
22 : Updates and current perspectives of psychiatric assessments after traumatic brain injury: a systematic review. Front Psychiatry 2016; 7:95Crossref, Medline, Google Scholar
23 : Blast-related traumatic brain injury: what is known? J Neuropsychiatry Clin Neurosci 2006; 18:141–145Link, Google Scholar
24 : The pathobiology of blast injuries and blast-induced neurotrauma as identified using a new experimental model of injury in mice. Neurobiol Dis 2011; 41:538–551Crossref, Medline, Google Scholar
25 : Military traumatic brain injury: a review. Alzheimers Dement 2014; 10(Suppl):S97–S104Crossref, Medline, Google Scholar
26 : A predictive screening index for posttraumatic stress disorder and depression following traumatic injury. J Consult Clin Psychol 2008; 76:923–932Crossref, Medline, Google Scholar
27 : Clinical Prediction Models. New York, Springer Sciences and Business Media, 2009Crossref, Google Scholar
28 : Systematic review of prognostic models in traumatic brain injury. BMC Med Inform Decis Mak 2006; 6:38Crossref, Medline, Google Scholar
29 : Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6:e1000097Crossref, Medline, Google Scholar
30 : Critical appraisal and data extraction for systematic reviews of prediction modelling studies: the CHARMS checklist. PLoS Med 2014; 11:e1001744Crossref, Medline, Google Scholar
31 (eds): Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. The Cochrane Collaboration, 2011. Available at http://handbook.cochrane.org/. Accessed April 22, 2014Google Scholar
32 : Assessing bias in studies of prognostic factors. Ann Intern Med 2013; 158:280–286Crossref, Medline, Google Scholar
33 (eds): Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. http://handbook.cochrane.org/Google Scholar
34 Review Manager (RevMan): [Computer program] Version 5.3. Copenhagen, the Nordic Cochrane Centre, the Cochrane Collaboration, 2014Google Scholar
35 : Does memory of a traumatic event increase the risk for posttraumatic stress disorder in patients with traumatic brain injury? A prospective study. Am J Psychiatry 2005; 162:963–969Crossref, Medline, Google Scholar
36 : Depression in patients with acute traumatic brain injury. Am J Psychiatry 1992; 149:918–923Crossref, Medline, Google Scholar
37 : Factors associated with posttraumatic stress disorder following moderate to severe traumatic brain injury: a prospective study. Depress Anxiety 2016; 33:19–26Crossref, Medline, Google Scholar
38 : Relationship between acute stress disorder and posttraumatic stress disorder following mild traumatic brain injury. Am J Psychiatry 1998; 155:625–629Crossref, Medline, Google Scholar
39 : Coping style and post-traumatic stress disorder following severe traumatic brain injury. Brain Inj 2000; 14:175–180Crossref, Medline, Google Scholar
40 : Neuropsychiatric consequences of traumatic brain injury: a comparison between two age groups. Brain Inj 2007; 21:301–307Crossref, Medline, Google Scholar
41 : Psychiatric disorders and health-related quality of life after severe traumatic brain injury: a prospective study. J Neurotrauma 2012; 29:1029–1037Crossref, Medline, Google Scholar
42 : Predictive and associated factors of psychiatric disorders after traumatic brain injury: a prospective study. J Neurotrauma 2011; 28:1155–1163Crossref, Medline, Google Scholar
43 : Axis I psychopathology in individuals with traumatic brain injury. J Head Trauma Rehabil 1998; 13:24–39Crossref, Medline, Google Scholar
44 : Predicting depression following mild traumatic brain injury. Arch Gen Psychiatry 2005; 62:523–528Crossref, Medline, Google Scholar
45 : Age and major depression after mild traumatic brain injury. Am J Geriatr Psychiatry 2003; 11:365–369Crossref, Medline, Google Scholar
46 : Psychiatric disorders after traumatic brain injury. Brain Inj 1996; 10:319–327Crossref, Medline, Google Scholar
47 : Head injury and loss of consciousness raise the likelihood of developing and maintaining PTSD symptoms. J Trauma Stress 2013; 26:727–734Crossref, Medline, Google Scholar
48 : Prevalence and predictors of post-traumatic stress disorder in adults one year following traumatic brain injury: a population-based study. Brain Impair 2013; 14:425–435Crossref, Google Scholar
49 : White matter changes in posttraumatic stress disorder following mild traumatic brain injury: a prospective longitudinal diffusion tensor imaging study. Chin Med J (Engl) 2016; 129:1091–1099Crossref, Medline, Google Scholar
50 : Memory of the traumatic event is associated with increased risk for PTSD: A retrospective study of patients with traumatic brain injury. J Loss Trauma 2005; 10:319–335Crossref, Google Scholar
51 : Axis I and II psychiatric disorders after traumatic brain injury: a 30-year follow-up study. Am J Psychiatry 2002; 159:1315–1321Crossref, Medline, Google Scholar
52 : Predictors of psychiatric disorders following traumatic brain injury. J Head Trauma Rehabil 2010; 25:320–329Crossref, Medline, Google Scholar
53 : Post-traumatic stress disorder symptoms following a head injury: does amnesia for the event influence the development of symptoms? Brain Inj 2001; 15:775–785Crossref, Medline, Google Scholar
54 : Psychiatric challenges in the first 6 years after traumatic brain injury: cross-sequential analyses of Axis I disorders. Arch Phys Med Rehabil 2004; 85(Suppl 2):S36–S42Crossref, Medline, Google Scholar
55 : Neuroanatomical correlates of depression in post traumatic brain injury: preliminary results of a pilot study. J Neuropsychiatry Clin Neurosci 2010; 22:231–235Link, Google Scholar
56 : Cognitive impairment associated with major depression following mild and moderate traumatic brain injury. J Neuropsychiatry Clin Neurosci 2005; 17:61–65Link, Google Scholar
57 : Major depression following traumatic brain injury. Arch Gen Psychiatry 2004; 61:42–50Crossref, Medline, Google Scholar
58 : Two-year prospective evaluation of the relationship between acute stress disorder and posttraumatic stress disorder following mild traumatic brain injury. Am J Psychiatry 2000; 157:626–628Crossref, Medline, Google Scholar
59 : Relationship between depression and psychosocial functioning after traumatic brain injury. Arch Phys Med Rehabil 2004; 85(Suppl 2):S43–S53Crossref, Medline, Google Scholar
60 : The nature, frequency and course of psychiatric disorders in the first year after traumatic brain injury: a prospective study. Psychol Med 2011; 41:2099–2109Crossref, Medline, Google Scholar
61 : Hippocampal volume and mood disorders after traumatic brain injury. Biol Psychiatry 2007; 62:332–338Crossref, Medline, Google Scholar
62 : The evolution of post-traumatic stress disorder following moderate-to-severe traumatic brain injury. J Neurotrauma 2016; 33:825–831Crossref, Medline, Google Scholar
63 : Errors in diagnosing post-traumatic stress disorder after traumatic brain injury. Brain Inj 2001; 15:39–46Crossref, Medline, Google Scholar
64 : Reporting and methods in clinical prediction research: a systematic review. PLoS Med 2012; 9:1–12Crossref, Medline, Google Scholar
65 : Stepwise selection in small data sets: a simulation study of bias in logistic regression analysis. J Clin Epidemiol 1999; 52:935–942Crossref, Medline, Google Scholar
66 : Some prognostic models for traumatic brain injury were not valid. J Clin Epidemiol 2006; 59:132–143Crossref, Medline, Google Scholar
67 : Gender differences in depression. Critical review. Br J Psychiatry 2000; 177:486–492Crossref, Medline, Google Scholar
68 : Depression and gender. An international review. Am Psychol 1997; 52:25–31Crossref, Medline, Google Scholar
69 : Recurrence after recovery from major depressive disorder during 15 years of observational follow-up. Am J Psychiatry 1999; 156:1000–1006Medline, Google Scholar
70 : Multiple risk factors predict recurrence of major depressive disorder in women. J Affect Disord 2015; 180:52–61Crossref, Medline, Google Scholar
71 : Recurrence of major depressive disorder and its predictors in the general population: results from the Netherlands Mental Health Survey and Incidence Study (NEMESIS). Psychol Med 2013; 43:39–48Crossref, Medline, Google Scholar
72 : A meta-analysis of risk factors for depression in adults and children after natural disasters. BMC Public Health 2014; 14:623Crossref, Medline, Google Scholar
73 : Health effects of employment: a systematic review of prospective studies. Occup Environ Med 2014; 71:730–736Crossref, Medline, Google Scholar
74 : The individual experience of unemployment. Annu Rev Psychol 2012; 63:369–396Crossref, Medline, Google Scholar
75 : Gray matter abnormalities in major depressive disorder: a meta-analysis of voxel based morphometry studies. J Affect Disord 2012; 138:9–18Crossref, Medline, Google Scholar
76 : Systematic review of multivariable prognostic models for mild traumatic brain injury. J Neurotrauma 2015; 32:517–526Crossref, Medline, Google Scholar
77 : The dilemma of heterogeneity tests in meta-analysis: a challenge from a simulation study. PLoS One 2015; 10:e0127538Medline, Google Scholar
78 : The heterogeneity statistic I2 can be biased in small meta-analyses. BMC Med Res Methodol 2015; 15:35Crossref, Medline, Google Scholar
79 : Depression following traumatic brain injury: A 1 year longitudinal study. J Affect Disord 1993; 27:233–243Crossref, Medline, Google Scholar
80 : Are there symptoms that are specific for depressed mood in patients with traumatic brain injury? J Nerv Ment Dis 1993; 181:91–99Crossref, Medline, Google Scholar
81 : The temporal relationship between depression, anxiety and functional status after traumatic brain injury: A cross-lagged analyses. J Int Neuropsychol Soc 2011; 17:781–787Crossref, Medline, Google Scholar
82 : Alexithymia after traumatic brain injury: Its relation to magnetic resonance imaging findings and psychiatric disorders. Psychosom Med 2005; 67:807–812Crossref, Medline, Google Scholar
83 : Depression in patients with acute traumatic brain injury. Am J Psychiatry 1992; 149:918–923Crossref, Medline, Google Scholar



