Neural Correlates of Error Monitoring in Adult Attention Deficit Hyperactivity Disorder After Failed Inhibition in an Emotional Go/No-Go Task
Abstract
The authors’ aim was to investigate the modulation of event-related potentials (ERPs) by the affective content of stimuli in adult attention deficit hyperactivity disorder (ADHD) patients during error monitoring. By obtaining ERPs from 26 adult ADHD patients and 14 healthy controls in an emotional go/no-go task, the authors investigated two error-related ERP components, the error-related negativity (ERN) and error positivity (Pe). In ADHD patients, the ERN amplitude decreased for negative stimuli after failed response inhibition (“no-go response”) and Pe amplitude decreased for neutral stimuli compared with the controls. These findings suggest that ADHD patients differ from controls both in the early and in the later stages of error processing.
Interest in the neural correlates of error monitoring has increased in the past two decades. Two response-locked event‐related potential (ERP) components, error-related negativity (ERN [or Ne])1–3 and error positivity (Pe),4 have been identified to be closely related to error monitoring. The ERN is a response-locked ERP component commonly observable 20–100 ms after committing different types of errors, such as commission and omission errors or choice error, irrespective of whether the committed error is consciously perceived.5 There is an agreement that ERN reflects the activity of a generic response-monitoring system,6 as well as the unconscious error detection or response conflict, whenever there is a mismatch between the intended and produced responses.1,5 It has a frontocentral maximum and is thought to be generated by the dorsal part of the anterior cingulate cortex (ACC).7 The Pe follows the ERN at approximately 200–500 ms after the error has occurred. It has a centroparietal topography and is thought to be generated by the rostral region of the ACC. Several hypotheses on its functional significance can be found in the literature, including a role in conscious error recognition, adjustment of response strategies after committing an error, and the emotional evaluation of the error.5,8,9
Various experimental paradigms, including the choice reaction time task, go/no-go task, or stop task, are used in practice in the research of motor response inhibition impairment in attention deficit hyperactivity disorder (ADHD). Higher commission error rates,10,11 higher intrasubject variability in reaction time,12–14 and deficient post-error slowing15 are the most consistent findings across these paradigms both in childhood ADHD and in adult ADHD. These findings facilitated further research in potential deficits in error monitoring and neural correlates of error processing in ADHD.
Empirical data available on the error-related ERP components in adult ADHD are limited. Based on a meta-analysis, Geburek et al. concluded that the Pe amplitude was significantly decreased in adult ADHD patients, while the differences did not reach statistical significance in the ERN amplitude compared with controls.16 A potential explanation of the differences seen in childhood and adulthood could be that adult ADHD patients possess more resources of conscious task processing and self-focusing than children, and impairment in early error processing (ERN) may be partially compensated in the subsequent conscious error processing step.
The ability to adequately inhibit inappropriate responses in the context of emotional inputs is essential for social functioning. The emotional content of stimuli can interfere with response inhibition and places extra demands on neural resources. Previous studies investigating the influence of long-lasting affective states or traits in healthy individuals found that individuals scoring high on negative affect scales display enhanced ERN amplitudes and decreased Pe amplitudes after commission error.17,18 Notably, higher impulsivity scores are associated both with lower ERN amplitudes and with lower Pe amplitudes.19,20 Short-lasting emotional factors have also been shown to modulate ERN amplitude, but the results of previous studies are more inconsistent in this regard. Enhanced ERN amplitude to negative affect induction was reported in two studies21,22 and in a flanker task for trials with superimposed pleasant pictures compared with unpleasant and neutral ones.23 In contrast, decreased ERN amplitudes were reported after the presentation of pleasant compared with neutral movie clips prior to a choice reaction time task.24 In two other studies, the mood and fear induction procedure did not modulate the amplitude of ERN.25,26
In the present study, our aim was to investigate the affective modulation of error monitoring in adult ADHD patients compared with healthy controls. There is growing evidence indicating that besides the cognitive impairments, patients with ADHD frequently manifest deficits in emotion regulation.27 Although emotional dysregulation is correlated with all the core domains of ADHD, it shares a strong relationship with symptoms of hyperactivity and/or impulsivity.28–30 Even though researchers have turned to emotional stimulus processing with increasing interest, published data on event-related potentials in emotional processing are scarce, both in adult and in child ADHD literature, and are restricted to stimulus-related ERPs. In our study, we applied a go/no-go task to investigate motor response inhibition in terms of behavioral performance and error-related potentials. Using emotional pictures as stimuli, our goal was to investigate how the affective valence of the stimuli modulates response inhibition and error-related ERPs.
Methods
Participants
We enrolled 26 patients meeting DSM-IV criteria for adult ADHD (men, N=20; women, N=6; mean age=26.7 years [SD=5.7]; inattentive type: N=12; hyperactive/impulsive type: N=7; combined type: N=7) and 14 healthy controls (men, N=11; women, N=3; mean age=31.5 years [SD=11.4]), matched by age (SD=5 years), gender, and level of education. Participants provided written, informed consent in accordance with procedures approved by the Institutional Review Board of Semmelweis University, Budapest, Hungary. Patients were recruited from the adult ADHD outpatient clinic of the Department of Psychiatry and Psychotherapy of Semmelweis University. Controls were recruited from the office and medical staff at the University and their acquaintances. Participants in both the ADHD and the healthy control groups completed the 66-item version of the Conners’ Adult ADHD Rating Scales (CAARS).31 The 90-item Symptom Checklist32 (SCL-90R) was used to select controls with no current psychiatric comorbidity. Among the ADHD patients, two had depression in their medical history, while dysthymia occurred in one patient, somatization disorder occurred in one patient, and panic disorder also occurred in one patient. Patients taking stimulant treatment (N=10) were off medication at least 24 hours before testing. Lack of history of psychiatric disease was required for inclusion in the control group. The main exclusion criteria for participants in the control group were any present or past neurologic disorder and history of head injury with loss of consciousness.
Stimuli and Procedures
Subjects performed the task in a dimly lit, sound-attenuated room. The computer screen for stimuli was placed at a viewing distance of approximately 100 cm. We applied an emotional go/no-go response inhibition task, which was presented by the Presentation 13.0 software (Neurobehavioral Systems, Inc., Albany, Calif.). We used pictures from the International Affective Picture System (IAPS [http://www4.ncsu.edu/~dgruehn/page7/page8/page8.html]) as stimuli; they comprised images with positive, negative, and neutral affective contents. The neutral, positive, and negative images were not different in physical characteristics (luminance, contrast and spatial frequency, confirmed by the Delplanque procedure33), but they differed in terms of arousal (since negative pictures in the IAPS are associated with higher arousal than the neutral or positive ones).34,35 Each participant was instructed to respond with a “go” button when a picture appeared on the screen and to withhold responding when the picture was repeated in the consecutive trial. Furthermore, participants were instructed to respond as quickly and accurately as possible. Each block consisted of 240 stimuli comprising 85% (160) of go stimuli and 15% (80) of no-go stimuli. All participants completed three experimental blocks. All stimuli were presented for 800 ms and were followed by an interstimulus interval of 600 ms.
EEG Recording and Preprocessing
The BioSemi recording system (sample rate=1024 Hz, band-pass filter=0.5−70 Hz) with average reference was used to acquire EEG. A standard BioSemi 128-electrode head cap system (https://www.biosemi.com/), with electrodes labeled in four blocks of 32 electrodes, was applied.
Data were analyzed off-line using Electro-magnetic Source Signal Imaging (EMSE Suite v.5.0, Source Signal Imaging, Inc., San Diego) and the Statistical Analysis System (SAS9.4) software. EEG data were filtered between 0.5 and 70 Hz using zero-phase shift-forward and reverse IR Butterworth-filter. Additionally, the 48–52 Hz Parks-McClellan stop-band notch filter was applied in order to remove any potential electrical interference from the 50-Hz line. Artifacts due to blinks and eye movements were removed manually and with the electrooculography artifact removal procedure. Epoch selection for the analyses was conducted manually, as well as applying automatic artifact rejection criteria. Response-locked data were segmented into epochs of 200 ms from before response to 400 ms after response. The time period immediately following the motor response was the main focus of our interest, and it was investigated in inferential statistical analyses. The difference between the two groups in terms of trial count (no-go trials with incorrect responses) was statistically significant (p<0.05). Subjects who committed at least six errors were included in the analysis.
ERP Analysis and Behavioral Measures
The ERN was defined as the average amplitude in microvolts occurring in the window from 20 ms to 70 ms post-response. The Pe was defined as the average amplitude in microvolts peak within 100–300 ms of the response.4 The ERN was measured in FCz and Cz, and the Pe at Cz and Pz electrodes on the basis of previous research.2 Performance was assessed with measures of the mean reaction time and commission error rate. Mean reaction time was calculated as the average of mean reaction times for correct go trials.
Statistical Analysis
The primary statistical analysis for group difference between ADHD and control subjects was based on the random regression hierarchical linear model. Amplitude (voltage) values within the time window of interest (20–70 ms, and 100–300 post-response for ERN and Pe, respectively) for error-related activity were used as dependent variables in the hierarchical linear model. We applied group, time (sampling point), and their interaction as independent variables; age, gender, and level of education served as covariates. The group main effect served as the principal interest in the analyses. A separate analysis was performed for each of the two time windows (ERN and Pe) in each brain region of interest (i.e., FCz, Cz, and Pz). For scalp areas that yielded a significant group difference in the primary analysis after correction for multiple testing, we conducted additional analyses to test whether psychopathological variables served as covariates in explaining the significant alterations in error-related activity. Covariates that we tested included the total score on the CAARS hyperactivity, impulsivity, inattention, and problems with self-concept domains. Group comparisons for behavioral data (error rates and reaction times), which had a skewed distribution, were investigated by generalized linear model analysis, since this method allows for the investigation of non-normally distributed variables. The Hochberg procedure was applied for correction for multiple testing. The statistical significance of the group difference was tested using Wald’s chi-square statistic. Continuous demographical variables (e.g., age) were tested with analysis of variance using F statistics; categorical variables (e.g., gender) were tested using chi-square analysis.
Results
Demographic and Basic Descriptive Characteristics
Basic demographic and clinical characteristics of the study population are presented in Table 1. As shown in the table, the study groups were similar on basic demographic variables, including age and gender. Approximately three-quarters of the sample consisted of males. As expected, the ADHD group had higher severity of general psychopathology as measured by the SCL-90R scale, and this group displayed higher severity on all specific symptom dimensions, including the CAARS factors of inattention, hyperactivity, impulsivity, and problems with self-concept.
| Characteristic | Control Group (N=14) | ADHD Group (N=26) | χ2 (df=1) | p |
|---|---|---|---|---|
| Categorical variable (N%) | ||||
| Demographic | ||||
| Male, N (%) | 11 (78.6%) | 20 (76.9%) | 0.01 | 0.91 |
| Continuous variables: mean (SD) | Fa | p | ||
| Age (years) | 31.5 (11.4) | 26.7 (5.7) | 3.26 | 0.08 |
| CAARSb | ||||
| Hyperactivity | 10.3 (6.5) | 19.7 (6.5) | 12.05 | 0.0015 |
| Impulsivity | 8.5 (4.7) | 16.6 (6.6) | 11.42 | 0.0019 |
| Inattention | 10.0 (7.4) | 20.8 (8.4) | 11.59 | 0.0018 |
| Problems with self-concept | 4.9 (5.4) | 8.1 (5.4) | 2.41 | 0.1306 |
| Wald χ2 (df=1) | p | |||
| Reaction time, (SD) | ||||
| Negative | 413.2 (53.7) | 410.9 (70.0) | 0.23 | 0.6324 |
| Neutral | 405.8 (53.2) | 399.5 (66.0) | 0.07 | 0.7932 |
| Positive | 409.3 (48.3) | 404.7 (68.6) | 0.11 | 0.7472 |
| Commission error (%), (SD) | ||||
| Negative | 23.6 (17.9) | 40.07 (17.9) | 5.00 | 0.0316 |
| Neutral | 21.7 (15.8) | 41.1 (16.2) | 9.46 | 0.0040 |
| Positive | 30.4 (21.1) | 40.7 (17.1) | 1.52 | 0.2251 |
TABLE 1. Basic Demographic and Clinical Characteristics of the Study Sample
Task Performance
The ADHD group made significantly more commission errors compared with the healthy control group for the neutral and negative stimuli, while the numeric difference of a similar magnitude did not obtain statistical significance for positive valence (Wald χ2=9.46, df=1, p=0.0040 for neutral stimuli; Wald χ2=5.00, df=1, p=0.0316 for negative stimuli; Wald χ2=1.52, df=1, p=0.2251 for positive stimuli). There were no group differences with regard to the reaction times.
Error-Related ERP Activity
To illustrate the group differences in error-related ERP waveforms, response-locked average ERPs to neutral and emotionally valenced stimuli are displayed in the frontal, central, and parietal areas (Figure 1). The shaded areas show the time windows in which we examined ERN and Pe. Error-related activity had similar waveforms in both groups. Significant group differences with negative stimuli were detectable for the ERN at the FCz, Cz, and Pz electrodes (for FCz: F=14.15, df=1, 37, p=0.0013; for Cz: F=288.74, df=1, 37, p<0.0001; for Pz: F=75.65, df=1, 37, p<0.0001). For neutral stimuli, we found a significant ERN amplitude difference only at the Cz electrode (F=12.17, df=1, 37, p=0.0028). We found no differences in ERN for the positive stimuli. The analysis of the Pe revealed a significant group difference for neutral stimuli at electrodes FCz, Cz, and Pz (for FCz: F=54.81, df=1, 37, p<0.0001; for Cz: F=109.29, df=1, 37, p<0.0001; for Pz: F=86.26, df=1, 37, p<0.0001). Group difference for Pe was not observable for the positive and negative stimuli. The interaction between group and time did not reach statistical significance in either of the above analyses. Numeric results for the error-related ERPs are summarized in Table 2.
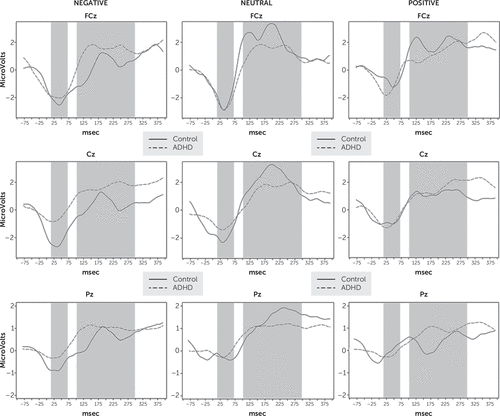
FIGURE 1. Grand Mean Error-Related Event-Related Potential (ERP) Averages Across Electrodes in the Frontal, Central, and Parietal Areas Capturing Differential ERP Responses to Affective Stimuli (Neutral, Negative, and Positive) During Error-Related Negativity (ERN) and Error Positivity (Pe) in Healthy Controls (N=14) and Patients With Adult Attention Deficit Hyperactivity Disorder (N=26)a
a Grand mean of response-locked average ERPs for patients (dashed line) and healthy controls (solid line) are shown. Time windows of interests, displayed as shaded area, were 20–70 ms for ERN and 100–300 ms for Pe after motor response.
| Error-Related Activity Amplitude µV (SE)b | Group Difference | ||||
|---|---|---|---|---|---|
| Error-Related Activity | Channel | Control Group | ADHD Group | F (df=1, 37) | pc |
| Error-Related Negativity (ERN)d | FCz | ||||
| Negative | –2.8 (0.2) | –2.2 (0.1) | 14.15 | 0.0013* | |
| Neutral | –1.5 (1.4) | –1.7 (0.9) | 0.01 | 0.9116 | |
| Positive | –1.3 (1.1) | –1.6 (0.8) | 0.06 | 0.8098 | |
| Cz | |||||
| Negative | –3.0 (0.1) | –0.5 (0.1) | 288.74 | <0.0001* | |
| Neutral | –0.8 (0.1) | –0.4 (0.1) | 12.17 | 0.0028* | |
| Positive | –1.1 (0.7) | –1.15 (0.5) | 0.00 | 0.9464 | |
| Pz | |||||
| Negative | –1.1 (0.1) | –0.4 (0.0) | 75.65 | <0.0001* | |
| Neutral | –0.2 (0.3) | –0.1 (0.2) | 0.08 | 0.7806 | |
| Positive | 0.1 (0.4) | –0.2 (0.3) | 0.44 | 0.5086 | |
| Error positivity (Pe)e | FCz | ||||
| Negative | –0.1 (0.9) | 1.0 (0.7) | 1.29 | 0.2568 | |
| Neutral | 2.9 (0.1) | 2.0 (0.1) | 54.81 | <0.0001* | |
| Positive | 1.4 (0.9) | 1.0 (0.7) | 0.17 | 0.6835 | |
| Cz | |||||
| Negative | 1.2 (0.7) | 1.8 (0.7) | 0.54 | 0.4609 | |
| Neutral | 3.1 (0.1) | 2.2 (0.1) | 109.29 | <0.0001* | |
| Positive | 1.2 (0.7) | 1.6 (0.5) | 0.25 | 0.6163 | |
| Pz | |||||
| Negative | 0.8 (0.5) | 1.1 (0.3) | 0.28 | 0.5964 | |
| Neutral | 1.7 (0.0) | 1.3 (0.0) | 86.26 | <0.0001* | |
| Positive | 0.4 (0.5) | 0.9 (0.3) | 0.80 | 0.3721 | |
TABLE 2. Group Differences in Error-Related Activitya
Covariates of Altered Error-Related Activity in the ADHD Group
For the ERP group differences that reached the level of statistical significance, we performed a follow-up analysis based on CAARS symptom dimensions, within the ADHD group. After adjustments for multiple comparisons, we found associations between ERN amplitude and impulsivity factor at the FCz electrode and between CAARS hyperactivity factor at the Cz electrode. Investigation of the direction of the relationship indicated larger ERN amplitude among those ADHD subjects who had higher severity on impulsivity compared with patients who had lower severity on it. Lower hyperactivity was associated with larger ERN amplitude compared with higher hyperactivity. We found no association with CAARS dimensions in terms of Pe amplitude. The relationships between error-related activity and CAARS symptom dimensions are presented in Table 3.
| FCz | Cz | Pz | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| µv (SE) | Difference | µv (SE) | Difference | µv (SE) | Difference | ||||||||
| Event-Related Potential, Emotional condition | Covariatea | Lowb | Highb | F | p | Lowb | Highb | F | p | Lowb | Highb | F | p |
| ERNc, Negative | |||||||||||||
| Hyperactivity | –2.5 (0.5) | –1.4 (1.2) | 0.78 | 0.3777 | –1.5 (0.6) | 2.9 (2.3) | 4.69 | 0.0306* | –0.8 (0.2) | –0.1 (0.6) | 1.61 | 0.2050 | |
| Inattention | –2.5 (0.5) | –1.4 (1.5) | 0.61 | 0.4333 | –1.0 (0.7) | –1.6 (1.4) | 0.16 | 0.6933 | –0.8 (0.2) | –0.3 (0.6) | 0.63 | 0.4263 | |
| Impulsivity | –2.5 (0.5) | –5.0 (1.3) | 4.07 | 0.0438* | –1.1 (0.7) | –3.5 (1.2) | 3.78 | 0.0523 | –0.7 (0.2) | –1.8 (0.6) | 3.79 | 0.0518 | |
| Problems with self-concept | 0.0 (1.3) | 4.0 (3.6) | 3.08 | 0.0793 | 0.4 (1.3) | 3.2 (3.3) | 1.72 | 0.1898 | –0.4 (0.5) | 0.2 (1.5) | 0.35 | 0.5550 | |
| Ped, Neutral | |||||||||||||
| Hyperactivity | 1.8 (0.7) | 3.7 (1.6) | 1.28 | 0.2579 | 2.8 (0.6) | 5.3 (1.8) | 2.52 | 0.1127 | 1.4 (0.2) | 1.6 (0.6) | 0.11 | 0.7398 | |
| Inattention | 2.6 (0.7) | 0.4 (1.8) | 1.38 | 0.2399 | 2.9 (0.6) | 4.0 (1.4) | 0.65 | 0.4189 | 1.5 (0.3) | 1.2 (0.6) | 0.29 | 0.5888 | |
| Impulsivity | 2.1 (0.7) | 0.1 (1.8) | 1.40 | 0.2368 | 3.1 (0.7) | 3.0 (1.3) | 0.01 | 0.9246 | 1.4 (0.2) | 1.1 (0.6) | 0.27 | 0.6016 | |
| Problems with self-concept | 3.2 (1.5) | 5.3 (4.3) | 0.53 | 0.4687 | 2.3 (1.1) | 0.6 (2.9) | 0.72 | 0.3962 | 1.3 (0.5) | 1.2 (1.5) | 0.02 | 0.8974 | |
TABLE 3. Covariates for Error-Related Negativity (ERN) and Error Positivity (Pe) With Conners’ Adult ADHD Rating Scales (CAARS) Domains in the ADHD Group
Discussion
The present study investigated error monitoring after failed inhibition in adult ADHD patients in comparison to healthy controls. To gain a better understanding of how an affective cue modifies the processing of an inhibition error, we applied a go/no-go task with emotional stimuli. We evaluated performance and ERP correlates of error monitoring (ERN and Pe).
Behavioral results showed no difference between the ADHD and control groups in terms of mean reaction time. Comparable data on commission errors in the ADHD literature are limited, as in most studies the applied stimuli had no emotional content. Consistent with the data from the literature available for neutral stimuli,10,11 in our study the patient group committed significantly more errors for neutral IAPS pictures than the controls. This may be attributable to inhibition impairment; however, since the task requires efforts with regard to working memory and focused attention, it may also be related, at least in part to difficulties with these functions.
In the case of emotionally valenced stimuli, we found a significantly increased commission error rate in the ADHD group compared with controls for negative stimuli and no significant difference for positive stimuli. The emotional content of the stimuli has been shown to serve as a distractor that captures attention in a bottom-up fashion, thereby disrupting the focus on goal-relevant information.36 In a study that used induction of short-term affect before the target stimuli,22 there was a significant main effect of valence: participants responded slower after unpleasant pictures. Considering that there was no significant valence effect on error rates in that study, this finding suggests that processing pictures with negative valence requires more effort; it requires more time to avert an increase in error rate. Due to the attention inhibition deficit, ADHD patients have poor ability to remain focused in the presence of distracting emotional information. The fact that in our study, the ADHD group exhibited similar reaction times to controls but significantly worse performance in response to negatively valenced pictures suggests an enhanced susceptibility and insufficient inhibition with respect to negatively valenced stimuli. Based on our results, this effect seems to be less pronounced when exposed to positive stimuli, and although ADHD patients tended to commit more errors even under such circumstances, there was no significant difference in performance between the two groups.
On a neurophysiological level, the two groups differed significantly in ERN amplitude for negatively valenced stimuli, with significant amplitude reduction in the patient group. For positive stimuli, there were no significant differences, and for neutral stimuli we found a significant ERN amplitude difference at the Cz electrode.
As in prior ERP studies, no stimuli with emotional valence were applied; comparison can only be made with those of our results that were obtained under the neutral condition. In four published studies,37–40 the authors did not demonstrate a significant decrease in ERN amplitude, while in three studies41–43 a significant reduction in ERN amplitude was found in the ADHD group compared with controls. The reduction of the ERN in the ADHD participants was consistent across these studies, and the meta-analysis of their data aggregated these statistical findings into a significant result.16
The question of why negative stimuli elicit significantly reduced ERN in the ADHD group arises. One explanation of these findings could be that negative stimuli serve as more potent distractors: by capturing attention they distract from the goal-relevant information. Earlier publications reported of a reduction in ERN amplitude following an error in healthy control subjects during conditions of dual attention constraints.44–46 In our study, due to the enhanced susceptibility to distractors characterizing ADHD, negative emotional content is expected to distract the attention from the task. Consequently, the error appears as less salient, which in turn could result in a lower ERN amplitude.
It is important to note that in our study, impulsivity was positively associated with higher ERN amplitude in the fronto-central area for the negative condition. Thus, it is possible that the impulsivity/emotional lability and reactivity47,48 characterizing ADHD mitigates the impact of the aforementioned effect of distraction from goal-relevant information by emotional stimuli during the process of error detection. Further symptom dimensions of CAARS (inattention, hyperactivity, problems with self-concept) showed the same direction as the ADHD group effect; however, statistical significance was reached only in the case of hyperactivity.
We found that patients had significantly lower Pe amplitude than controls in the neutral condition, whereas they did not differ from controls for emotionally valenced stimuli. While the decrease in the Pe amplitude is a consistent finding in child ADHD studies that applied neutral stimuli,49,50 in adults this observation is much less consistent.16 Wiersema et al. reported a significant reduction of Pe in 23 adult ADHD patients in a visual go/no-go task and a negative correlation with the ADHD symptom severity as measured by the Adult Self-Report scale and the Wender Utah Rating Scale.39 However, the ADHD subjects and matched controls showed no significant differences in terms of behavioral results, such as reaction time, accuracy, and post-error slowing. Herrmann et al. investigated a younger (mean age=25.2 years) and an older subgroup (mean age=40.9 years) of ADHD patients and control subjects.42 Commission error rate and post-error slowing were significantly higher only in the younger ADHD subgroup compared with the age-matched controls, while reduced Pe amplitude was found for the whole ADHD sample. With increasing age, improved performance was found among the ADHD patients for both aforementioned behavioral measures, while no effect of age was observed for controls. For the Pe amplitude, no group interaction was present with age. These findings emphasize the importance of developmental factors in ADHD and suggest that as opposed to the impairments in behavioral measures, the amplitude reduction of Pe is a stable impairment that persists over time.51
In our study, patients did not differ from controls in terms of Pe amplitude for emotionally valenced stimuli. Available data indicate that the magnitude of the Pe amplitude is proportional to the extent of awareness. When accuracy is emphasized in the instructions given for solving a task, the Pe amplitude increases.52 One explanation for this could be that even though the emotional valence of the stimulus served as distracting information, it reduced the monotony of the task. Furthermore, by positively affecting the motivational components in ADHD patients, it resulted in increased awareness of the errors committed and improved attempts at following instructions. Higher monotony of neutral pictures results in a more pronounced deficit in error monitoring as indicated by the significantly lower Pe observed in the ADHD group.
A limitation of our study is the small sample size in the control group as a result of a lower rate of qualification for the analyses, which was due to difficulty in enrolling control subjects with a sufficient number of error trials for the investigation. Furthermore, IAPS images were not matched on arousal ratings, and negative pictures in general are associated with higher arousal than the neutral or the majority of the positive pictures. However, balancing the pictures across emotion categories for arousal is difficult and may lead to selection bias (e.g., use of pictures with specific semantic categories). The ADHD sample included a subgroup of patients who were medicated with psychostimulants. This limits generalizability with regard to unmedicated samples despite the fact that patients discontinued their medication 24 hours prior to the ERP study. Notwithstanding these limitations, our study is the first, to our knowledge, to investigate the neurobiological basis of how affective cues modify cognitive control, response inhibition, and the error monitoring in adult ADHD patients.
In conclusion, behavioral performance and ERP correlates of error monitoring (ERN and Pe) are a prominent field of adult ADHD research. There is growing evidence indicating that besides cognitive impairments, patients with ADHD have deficits in emotional processing and emotion regulation, although the number of studies investigating the association between these areas is rather limited. In our study, the Pe amplitude decreased significantly in the ADHD group when we applied neutral stimuli, and the ERN amplitude showed a reduction for stimuli with negative emotional valence. While these results are in line with previous results in the literature, they underline the need to further investigate how the emotional content of the stimuli interferes with the process of error monitoring in ADHD. Beyond behavioral data, electrophysiological examination of error monitoring is essential for the characterization of the neurobiological basis of the self-monitoring deficit characteristic in ADHD.
1 : Effects of crossmodal divided attention on late ERP components, II: Error processing in choice reaction tasks. Electroencephalogr Clin Neurophysiol 1991; 78:447–455Crossref, Medline, Google Scholar
2 : Effects of errors in choice reaction tasks on the ERP under focused and divided attention, in Psychophysiological Brain Research. Edited by Brunia CHM, Gaillard AWK, Kok A. Tilburg, The Netherlands, Tilburg University Press, 1990, pp 192–195Google Scholar
3 : The error-related negativity: an event-related brain potential accompanying errors. Psychophysiology 1990; 27:S34Medline, Google Scholar
4 : Event-related potential correlates of errors in reaction tasks. Electroencephalogr Clin Neurophysiol Suppl 1995; 44:287–296Medline, Google Scholar
5 : ERP components on reaction errors and their functional significance: a tutorial. Biol Psychol 2000; 51:87–107Crossref, Medline, Google Scholar
6 : The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychol Rev 2002; 109:679–709Crossref, Medline, Google Scholar
7 : The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiol Behav 2002; 77:477–482Crossref, Medline, Google Scholar
8 : Error-related brain potentials are differentially related to awareness of response errors: evidence from an antisaccade task. Psychophysiology 2001; 38:752–760Crossref, Medline, Google Scholar
9 : Dissociable components of error processing: on the functional significance of the Pe vis-a-vis the ERN/Ne. J Psychophysiol 2005; 19:319–329Crossref, Google Scholar
10 : Interactions among variables in the P300 response to a continuous performance task in normal and ADHD adults. J Am Acad Audiol 2004; 15:666–677Crossref, Medline, Google Scholar
11 : Error monitoring in children with ADHD or reading disorder: An event-related potential study. Biol Psychol 2010; 84:176–185Crossref, Medline, Google Scholar
12 : Examining predictors of reaction times in children with ADHD and normal controls. J Int Neuropsychol Soc 2010; 16:138–147Crossref, Medline, Google Scholar
13 : Reaction time distribution analysis of neuropsychological performance in an ADHD sample. Child Neuropsychol 2006; 12:125–140Crossref, Medline, Google Scholar
14 : Mean response times, variability, and skew in the responding of ADHD children: a response time distributional approach. Acta Psychol (Amst) 2000; 104:167–190Crossref, Medline, Google Scholar
15 : Post-error slowing in patients with ADHD: A meta-analysis. J Atten Disord 2014; 20:1004–1016Crossref, Medline, Google Scholar
16 : Electrophysiological indices of error monitoring in juvenile and adult attention deficit hyperactivity disorder (ADHD): A meta-analytic appraisal. Int J Psychophysiol 2013; 87:349–362Crossref, Medline, Google Scholar
17 : Error-related psychophysiology and negative affect. Brain Cogn 2004; 56:189–197Crossref, Medline, Google Scholar
18 : Mood, personality, and self-monitoring: negative affect and emotionality in relation to frontal lobe mechanisms of error monitoring. J Exp Psychol Gen 2000; 129:43–60Crossref, Medline, Google Scholar
19 : Reduced punishment sensitivity in neural systems of behavior monitoring in impulsive individuals. Neurosci Lett 2006; 397:130–134Crossref, Medline, Google Scholar
20 : Error processing and impulsiveness in normals: evidence from event-related potentials. Brain Res Cogn Brain Res 2005; 24:317–325Crossref, Medline, Google Scholar
21 : Feelings of helplessness increase ERN amplitudes in healthy individuals. Neuropsychologia 2013; 51:613–621Crossref, Medline, Google Scholar
22 : Modulation of the error-related negativity by induction of short-term negative affect. Neuropsychologia 2009; 47:83–90Crossref, Medline, Google Scholar
23 : Affective context-induced modulation of the error-related negativity. Neuroreport 2006; 17:329–333Crossref, Medline, Google Scholar
24 : Positive affect modulates flexibility and evaluative control. J Cogn Neurosci 2011; 23:524–539Crossref, Medline, Google Scholar
25 : What are the influences of orthogonally-manipulated valence and arousal on performance monitoring processes? The effects of affective state. Int J Psychophysiol 2013; 87:327–339Crossref, Medline, Google Scholar
26 : The effects of fear on performance monitoring and attentional allocation. Psychophysiology 2005; 42:261–268Crossref, Medline, Google Scholar
27 : Emotional dysregulation in adults with attention-deficit/hyperactivity disorder-validity, predictability, severity, and comorbidity. J Clin Psychol 2017; 73:99–112Crossref, Medline, Google Scholar
28 : Emotional dysregulation as a core feature of adult ADHD: its relationship with clinical variables and treatment response in two methylphenidate trials. J ADHD Relat Disord 2010; 1:53–64Google Scholar
29 : Emotional dysregulation in adult ADHD and response to atomoxetine. Biol Psychiatry 2005; 58:125–131Crossref, Medline, Google Scholar
30 Stieglitz RD, Rösler M: Different psychopathological dimensions in adult ADHD. Presented at the Proceedings of the 3rd World Congress on ADHD, 2011Google Scholar
31 Conners CK, Erhardt D, Sparrow E: Conners’ Adult ADHD Rating Scales (CAARS), Technical Manual. New York, Multi Health Systems, 1999Google Scholar
32 : SCL-90-R: Symptom Checklist-90-R. Administration, Scoring, and Procedures Manual, 3rd ed. Minneapolis, Minnesota, National Computer Systems, 1994Google Scholar
33 : Spatial frequencies or emotional effects? A systematic measure of spatial frequencies for IAPS pictures by a discrete wavelet analysis. J Neurosci Methods 2007; 165:144–150Crossref, Medline, Google Scholar
34 : Age-related differences in valence and arousal ratings of pictures from the International Affective Picture System (IAPS): do ratings become more extreme with age? Behav Res Methods 2008; 40:512–521Crossref, Medline, Google Scholar
35 : Multidimensional normative ratings for the International Affective Picture System. Behav Res Methods 2007; 39:326–334Crossref, Medline, Google Scholar
36 : Automatic attention to emotional stimuli: neural correlates. Hum Brain Mapp 2004; 22:290–299Crossref, Medline, Google Scholar
37 : Electrophysiological indices of abnormal error-processing in adolescents with attention deficit hyperactivity disorder (ADHD). J Child Psychol Psychiatry 2010; 51:66–76Crossref, Medline, Google Scholar
38 : The neural correlates of deficient error awareness in attention-deficit hyperactivity disorder (ADHD). Neuropsychologia 2009; 47:1149–1159Crossref, Medline, Google Scholar
39 : ERP correlates of error monitoring in adult ADHD. J Neural Transm (Vienna) 2009; 116:371–379Crossref, Medline, Google Scholar
40 : Neural activity associated with executive functions in adolescents with attention-deficit/hyperactivity disorder (ADHD). Int J Psychophysiol 2009; 74:19–27Crossref, Medline, Google Scholar
41 : Error monitoring in college students with attention-deficit/hyperactivity disorder. J Psychophysiol 2009; 23:113–125Crossref, Google Scholar
42 : Neural correlates of performance monitoring in adult patients with attention deficit hyperactivity disorder (ADHD). World J Biol Psychiatry 2010; 11:457–464Crossref, Medline, Google Scholar
43 : Performance monitoring is altered in adult ADHD: a familial event-related potential investigation. Neuropsychologia 2009; 47:3134–3142Crossref, Medline, Google Scholar
44 : Mental fatigue, motivation and action monitoring. Biol Psychol 2006; 72:123–132Crossref, Medline, Google Scholar
45 : Mental fatigue and impaired response processes: event-related brain potentials in a Go/NoGo task. Int J Psychophysiol 2009; 72:204–211Crossref, Medline, Google Scholar
46 : The effects of uncertainty in error monitoring on associated ERPs. Brain Cogn 2004; 56:215–233Crossref, Medline, Google Scholar
47 : Abnormal affective responsiveness in attention-deficit/hyperactivity disorder: subtype differences. Biol Psychiatry 2009; 65:578–585Crossref, Medline, Google Scholar
48 : Aspects of social and emotional competence in adult attention-deficit/hyperactivity disorder. Neuropsychology 2003; 17:50–58Crossref, Medline, Google Scholar
49 : Self-regulation in ADHD: the role of error processing. Clin Psychol Rev 2010; 30:951–961Crossref, Medline, Google Scholar
50 : The effects of performance-based rewards on neurophysiological correlates of stimulus, error, and feedback processing in children with ADHD. Psychophysiology 2013; 50:1157–1173Crossref, Medline, Google Scholar
51 : Development of response-monitoring ERPs in 7- to 25-year-olds. Dev Neuropsychol 2004; 25:355–376Crossref, Medline, Google Scholar
52 : Response accuracy rating modulates ERN and Pe amplitudes. Biol Psychol 2014; 96:1–7Crossref, Medline, Google Scholar



