Pavlov’s Orienting Response in Frontotemporal Dementia
Abstract
Behavioral variant frontotemporal dementia (bvFTD) is a neurodegenerative disorder with initial disturbances in socioemotional behavior in the absence of a sensitive diagnostic test. This study evaluated Pavlov’s “orienting response” (OR) or “what is it?” reflex as a measure of their ability to refocus attention on socioemotional stimuli and as a potentially distinguishing measure for bvFTD. Ten patients with bvFTD were compared with 18 normal controls (NC) on ORs (defined as initial heart rate [HR] deceleration) to different pictures based on social and emotional (valence) differences from the International Affective Picture Stimuli. HR was measured while participants viewed pleasant-nonsocial (e.g., food), unpleasant-nonsocial (e.g., garbage), pleasant-social (e.g., babies), and unpleasant-social (e.g., violence) pictures. Participants watched each picture for 6 seconds, and the study examined HR changes during the first 2-second OR interval. The results showed significant differences in valence (pleasant-unpleasant) and valence-group interactions, but no effects of nonsocial-social. Whereas the NCs showed the expected HR deceleration (OR) to unpleasant stimuli, the bvFTD patients showed increased HRs without an initial refocusing. Decreased HR slowing to stimuli among the bvFTD patients correlated with increased scores on an emotional blunting scale. These findings suggest that decreased socioemotional behavior in bvFTD may be associated with decreased appreciation of emotional aspects of stimuli as evidenced by decreased ORs to emotional stimuli, regardless of social content. These findings also suggest further investigation of the OR in bvFTD as an early diagnostic measure for this disorder.
Behavioral variant frontotemporal dementia (bvFTD) is a neurodegenerative disorder that results in behavioral disinhibition, apathy, lack of empathy, compulsive behaviors, changes in eating behavior, and impaired executive functions.1 Patients with bvFTD manifest emotional blunting, decreased empathic responses to others, and socioemotional behavioral changes.2 The source of their socioemotional changes is neuropathology in critical frontotemporal areas. These areas control attentional and autonomic systems, and one relatively unexplored association of socioemotional changes in bvFTD is a decrease in the ability to redirect and focus attention on social or emotional stimuli.
The examination of heart rate (HR) responses to arousing pictures among bvFTD patients may clarify whether they have early disruption of the ability to orient attention to social and emotional stimuli.3 Originally named by Ivan Pavlov as an initial HR orienting response (OR), or “what is it?” reflex, the OR involves the refocusing of attention on novel or significant stimuli.4 This initial HR deceleration or “attentional bradycardia” facilitates perceptual processing of these stimuli prior to subsequent preparation of the organism for action.5–8 The OR is primarily mediated by the parasympathetic (vagus) nervous system and is steepest during the first 1–2 seconds of picture presentation.6,9 ORs are expected when viewing all novel pictures, although the elicitation and magnitude of the OR varies with the potential significance or impact of the stimulus, particularly if emotionally significant but also if socially significant.6
The present study evaluated ORs to social and emotional stimuli among bvFTD patients compared with normal controls (NCs). For stimuli, this study used the International Affective Pictures System (IAPS),10 a widely used set of emotional picture stimuli with normative data for valence (ranging from pleasant to unpleasant) and arousal (ranging from calm to excited).10 Among bvFTD patients, this is the first study, to our knowledge, to directly compare social versus nonsocial stimuli while accounting for the affective content (pleasant versus unpleasant) of the stimuli, thus allowing discrimination of the OR effects of sociality separate from the effects of affective valence. Given the neuropathology of bvFTD, we hypothesized that bvFTD patients would exhibit decreased OR responses to the more impactful social and unpleasant stimuli, compared with the nonsocial and pleasant ones.
Methods
Participants
After institutional review board approval, bvFTD patients and their caregivers were recruited from the UCLA Behavioral Neurology Program and Clinic. All participants and caregivers gave informed consent for participation. The bvFTD patients were community-based individuals who underwent a clinical evaluation and had mild to moderate impairment as defined by their Mini-Mental State Examination (MMSE) scores and Clinical Dementia Rating Scale Sum of Boxes (CDR-SB) scores. The bvFTD participants (N=10) in this study presented with progressive behavioral changes, such as declines in social interpersonal conduct, impairment in regulation of personal conduct, emotional blunting, and loss of insight into their disease, and they met criteria for “clinically probable bvFTD” based on the International Consensus Criteria for bvFTD.1 The clinical diagnosis was confirmed by the presence of predominant frontal and anterior temporal involvement on magnetic resonance imaging, fluorodeoxy-glucose positron emission tomography, or both. Exclusion criteria were the presence of lesions on neuroimaging and medications that could affect HR, including beta blockers, psychostimulants, asthma medications, and decongestants. Five bvFTD patients were on sertraline, and one was taking citalopram. Although these selective serotonin reuptake inhibitors may have a small effect on baseline HR, there was no effect from review of their recorded HRs on and off of these medications or on the presence of sympathetic acceleration in HR.
The normal controls (NCs) were recruited from the community through fliers. Eighteen NCs participated and were comparable in age, gender, and education to the bvFTD patients. None of the NCs had a history of neurologic or psychiatric disease or were using medications that could interfere with the psychophysiological testing.
Socioemotional Measures
The caregivers of the bvFTD patients completed the following two questionnaires to assess social dysfunction and emotional blunting:
Socioemotional Dysfunction Scale (SDS): The measure is a 25-item informant-based rating scale that is primarily derived from the Social Competency Questionnaire (SCQ),11 which measures socially effective behavior, including extraversion, warmth, social influence, insight, openness, appropriateness, and maladjustment. Informants (caregivers) rate items regarding the participant’s current social behavior, compared with their typical premorbid behavior, on a 5-point Likert scale (1-to-5=very inaccurate to very accurate). For example, “Makes inappropriate comments to others.” The 25 items are summed yielding a total raw score, with higher scores suggestive of greater social dysfunction.
Scale for Emotional Blunting (SEB): The measure is a 16-item informant scale of emotional behavior completed on the bvFTD patients by their caregivers. The SEB was initially developed to characterize negative symptoms in schizophrenia,12,13 but has proven to be an effective instrument in assessing the presence of emotional blunting in bvFTD.2,14 Each behavioral symptom is scored on a 3-point scale on which 0=”condition absent,” 1=”slightly present or doubtful,” and 2=”clearly present.” Items are summed into three domains: absence of pleasure-seeking behavior (behavior), affective blunting (affect), and cognitive blunting (thought), for example, “reclusive, avoids social contact.”
Psychophysiological Assessment
Stimuli presentation.
The participants were instructed to view the pictures displayed on a 32-inch 60-Hz LCD HDTV monitor, presented at level with their field of vision and located 2 feet from them. Forty digital color images were chosen from the standardized IAPS. The pictures were classified into four categories based on their established valence (pleasant versus unpleasant) and the presence of socially relevant stimuli (social versus nonsocial). The IAPS pictures selected as “social” depicted humans, and those as “nonsocial” did not.15 The mean valence (rated 1–9, most unpleasant to most pleasant) and arousal (rated 1–9, least arousing to most arousing) ratings were derived from normative data on the images.10 Social and nonsocial pictures were matched on valence (4.83±1.53 versus 4.99±1.48, respectively, n.s.). By design, the pleasant stimuli had a valence >6 (7.64±0.72), and the unpleasant stimuli had a valence <3 (2.19±0.52) (analysis of variance [ANOVA] F[3, 396]=775.59, p<0.001; post hoc pleasant-unpleasant significance). There remained differences in arousal ratings (social=5.78±1.01; nonsocial=4.90±0.91; pleasant=4.64±0.82; unpleasant=6.04±0.97; F[3, 396]=775.59, p<0.001; post hoc analysis showed arousal ratings to be significantly higher in social compared with nonsocial and in unpleasant compared with pleasant pictures).
Superlab Pro (Cedrus Corporation, San Pedro, Calif.) software was used to display pictures in a randomized order. Two blocks of passive picture viewing were conducted with an intersession interval of 10 minutes. In each block, the participants passively viewed five pictures for a total of 6 seconds each in each category (20 pictures). The pictures were present in random order and counterbalanced. Interstimulus interval between two pictures was 22 seconds during which a blank screen was displayed on the monitor. The participants were monitored using a video camera during the sessions and observed for activity and gaze. The recordings were made while their gaze was maintained on the stimuli. The participants were debriefed at the end of the second session and assessed for any adverse reactions.
Psychophysiological recording and data reduction.
All participants underwent psychophysiological recordings. The recording devices were attached to the participants while they were seated in a chair. HR was measured by placing disposable electrodes on the dorsal aspect of both wrists in lead II configuration (EL 503, Biopac, Inc., Goleta, Calif.). The procedure was done at approximately the same time of day (10:30 a.m.) for all participants, who had refrained from caffeine 2 hours prior to testing. HR was continuously recorded using the Biopac base module (150MP system), the HR module (EKG 100C, Biopac Inc., Goleta, Calif.), and Biopac AcqKnowledge 4.1 software. HR acquisition parameters were set at no high-pass and low-pass filter, and the sampling rate was 1,000 Hz. This study obtained continuous recording of HR while calculating interbeat intervals (detection of R wave) for beats/minute, for each second, and the data were processed using MATLAB 2006a. Artifact detection and removal was conducted using MATLAB 2006a and visual inspection.
The baseline HR was derived from the last prestimulus second prior to stimulus presentation, and HR change scores were computed for each category relative to the second before viewing the picture. Similar to previous studies,16 the mean HR changes were based on the phasic HR change during each second interval of viewing. Visual inspection of the HR changes confirmed that maximum declines occurred within the first two seconds consistent with an OR.5–7
Statistical Analysis
T-test and chi-square test were the assessments conducted to examine group differences in continuous variables (e.g., age, education, and MMSE scores) and categorical variables (e.g., ethnicity and gender), respectively. The ORs were then compared across the groups in the four picture categories. Although small numbers, the distribution of the ORs within groups were sufficiently normal such that ANOVA was used to evaluate group differences in HR change associated with valence (pleasant versus unpleasant) and sociability (social versus nonsocial). Given the nonlinear responses on HR analysis (i.e., increasing deceleration or acceleration) and a number of relative outliers for both bvFTD and NCs (not excluded from analysis), standard errors were plotted along with mean HR change values. Additional multiple linear regression and correlations examined the relationship of OR and independent variables (group, MMSE, CDR-SB, SDS, and SEB scores).
Results
No significant differences were observed in age at examination, gender, race, and education in years between the bvFTD and the NC groups. As expected, the bvFTD patients had lower MMSE scores compared with NCs (see Table 1).
| Characteristic | bvFTD Group (N=10) | NC Group (N=18) | p |
|---|---|---|---|
| Age (years); mean (SD) | 60.8 (9.4) | 55.0 (8.4) | n.s. |
| Male, N (%) | 4 (40) | 7 (39) | n.s. |
| Caucasian, N (%) | 10 (100) | 15 (83) | n.s. |
| Education (years); mean (SD) | 15.9 (2.9) | 15.9 (1.7) | n.s. |
| Mini-Mental State Examination score; mean (SD)a | 25.9 (3.2) | 29.5 (0.8) | <0.0001 |
| Clinical Dementia Rating Sum of Boxes score; mean (SD) | 5.62 (2.16) | ||
| Socioemotional Dysfunction Scale score; mean (SD) | 146 (34) | ||
| Scale for Emotional Blunting (total) score; mean (SD) | 23.5 (5.9) |
TABLE 1. Clinical Characteristics of Patients With Behavioral Variant Frontotemporal Dementia (bvFTD) Versus Normal Controls (NCs)
Psychophysiology Data
Overall, for the four picture categories, there were significant HR changes for valence with a valence-group interaction (see Table 2). For the four categories, the bvFTD patients had HR changes of 0.616 (SE=1.04), compared with the NCs changes of –0.107 (SE=0.533) (i.e., across categories, the bvFTD group failed to show an initial OR deceleration, whereas the NC group showed significant HR deceleration consistent with expected ORs). ANOVA post hoc (LSD, Tukey’s) analysis showed significant group differences on viewing the unpleasant pictures (see Figure 1A), with an early HR acceleration without significant OR (2.828; SE=1.427) among the bvFTD patients compared with an expected OR (−0.696; SE=0.944) among the NCs. Post hoc analysis did not reveal significant differences for pleasant pictures (bvFTD: −1.597; SE=1.455 versus NC: 0.482; SE=0.496), social stimuli (bvFTD: 0.735; SE=1.472 versus NC: −0.034; SE=0.629), and nonsocial stimuli (bvFTD: 0.447; SE=1.493 versus NC: −0.18; SE=0.866) (see Figure 1A and 1B).
| Source | Type III Sum of Squares | df | Mean Square | F | Sig. |
|---|---|---|---|---|---|
| Corrected model | 1082.353a | 8 | 135.294 | 3.904 | 0.000 |
| Intercept | 6.247 | 1 | 6.247 | 0.180 | 0.671 |
| Valence | 160.480 | 1 | 160.480 | 4.631 | 0.032 |
| Sociability | 3.348 | 1 | 3.348 | 0.097 | 0.756 |
| Group | 42.234 | 1 | 42.234 | 1.219 | 0.270 |
| Valence-by-sociability | 9.636 | 1 | 9.636 | 0.278 | 0.598 |
| Valence-by-group | 648.276 | 1 | 648.276 | 18.706 | 0.000 |
| Sociability-by-group | 0.048 | 1 | 0.048 | 0.001 | 0.970 |
| Valence-by-sociability-by-group | 0.217 | 1 | 0.217 | 0.006 | 0.937 |
| Error | 47340.825 | 1366 | 34.657 | ||
| Total | 48451.845 | 1375 | |||
| Corrected total | 48423.179 | 1374 |
TABLE 2. Analysis of Variance for Orienting Responses
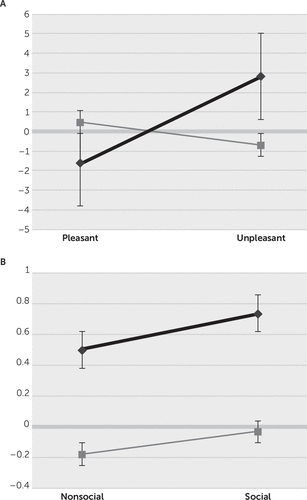
FIGURE 1. Orienting Responses (ORs) With More Negative Values Indicating Greater ORs for Behavioral Variant Frontotemporal Dementia (bvFTD) Patients Compared With Normal Controls (NCs)a
a Plots of ORs on the y-axis are shown, with more negative values indicating greater ORs for bvFTD patients (black line) compared with NCs (gray line). Graph A) indicates ORs to pleasant versus unpleasant picture stimuli. Graph B) indicates ORs to nonsocial versus social picture stimuli. Only the group differences in ORs to unpleasant pictures reached statistical significance. Error bars are standard errors.
Comparison With Behavioral Measures
A multiple linear regression of OR was further conducted with the MMSE and CDR-SB as dementia variables, the SDS and SEB as socioemotional variables, and group. The model was not significant (adjusted R2=−0.003; F[5, 1530]=0.033, n.s.). Subsequent bivariate correlations were conducted across the entire 6-second recording interval for these measures. There were no significant correlations with the MMSE, the CDR-SB, and the SDS; however, within the bvFTD group, the OR significantly positively correlated with the total SEB scores (r=0.09; p=0.017). In other words, increased HR reflected a lack of OR (initial HR deceleration), and this corresponded to increasing scores on the measure for emotional blunting.
Discussion
This study investigated attentional orienting responses to socioemotional stimuli among patients with bvFTD compared with NCs. We examined the Pavlovian OR of initial HR deceleration as a measure of perceived importance and registration of social and emotional stimuli. The analysis reveals that NCs, but not bvFTD patients, showed the expected OR to unpleasant pictures, the most arousing of the four stimulus groups. A lack of HR deceleration among the bvFTD patients correlates with the degree of emotional blunting. In these patients, the finding of decreased “attentional bradycardia” may reflect diminished appreciation of the emotional significance of unpleasant stimuli, or, alternatively, the dampened emotions in bvFTD contribute to diminished autonomic attentional reorientation to emotional stimuli.8,17,18
The early location of neuropathology in bvFTD supports the view that socioemotional dysfunction in this dementia may have roots in the inability to appreciate, attend, and respond to stimuli with emotional content.19 The neuropathology in bvFTD focuses on the major frontal control areas that control attentional changes, such as the anterior cingulate cortex and resting autonomic nervous system (ANS) levels.2,17,18,20 Other mesial or paralimbic frontal lobe structures affected in bvFTD are involved in controlling or regulating sympathetic and parasympathetic responses, including the ventral mesial prefrontal cortex and its connection to the amygdala for vagally mediated HR deceleration.21,22 Moreover, patients with bvFTD have decreased ANS responses to unpleasant emotions, including disgust and fear conditioning to aversive stimuli,23,24 which also seems to correspond to emotional blunting.
The OR may be an excellent reflex for assessing perceived alterations in the socioemotional significance of stimuli. The OR is an ANS-mediated reflex relating attention and focused perception on stimuli with emotion and interoceptive awareness, particularly if negative or threatening.25 In previous studies, healthy adults subjected to emotionally unpleasant stimuli have shown greater initial ORs, when compared with neutral or pleasant stimuli.6,16,26–28 In contrast, the nonthreatening emotional aspects of pleasurable stimuli may not require as much of an initial attentional reorientation; hence, they may have minimal ORs and faster onset of sympathetic reactivity with early increased HRs, as in our NCs.3,6,29–31 Paradoxically, although not statistically significant, the bvFTD patients showed an OR to pleasant stimuli, possibly reflecting their increased drive to orient to appetitive items.1 This HR acceleration, often generally termed a “defense reaction” but also an appetitive reaction, follows the OR when present and actually reflects sympathetic ANS reactivity in preparation for action.26,27,32 In bvFTD, the mitigation of ORs and the tendency to go rapidly to sympathetic reactivity after viewing unpleasant stimuli is consistent with the neuropathology and symptoms of bvFTD and may be a good measure for distinguishing these patients early in their course.33
This study showed that, compared with NCs, patients with bvFTD had impaired ORs to the unpleasant emotional content of the stimuli but not to their social content. Although there have been few prior studies on ORs to social (images of people in various situations) stimuli, differential orienting to social versus nonsocial stimuli is relevant to survival, reproduction, and social life,34 and prior studies have shown varying responses to social stimuli depending on their “sociality”34,35. Differential responses to social stimuli, however, could actually be consequent to emotional arousal and not to social content, as in the bvFTD patients in this study.6,26,36–38 For example, in one study, exposure to social positive film clips resulted in larger skin conduction responses (SCRs), a sympathetic rather than OR measure, compared with nonsocial positive clips, but nonsocial negative clips resulted in larger SCRs than social negative clips,38 and in another study, SCRs increased on presenting negative social images (e.g., mutilation), compared with positive social images.39 The present study did not find a significant effect of social versus nonsocial pictures, perhaps because of the lesser importance of reorienting attention to social stimuli compared with emotional valence.
In bvFTD patients, emotional blunting appears to correlate with their decreased initial orienting but does not establish directionality. Further studies with larger samples can establish cause and effect (i.e., whether critical frontal pathology in bvFTD results in dampened responses to the emotional aspects of stimuli with decreased ORs or whether decreased ORs contribute to the dampening of emotions). The presence of an OR response to pleasant stimuli among these patients suggests that the lack of autonomic response to emotional stimuli could drive the affective blunting. Establishing this directionality could have major implications for prevailing theories of emotion.
Although the present study shows OR differences in bvFTD compared with NCs, there are a number of potential limitations. First, the sample sizes are relatively small. Despite this, our investigation does show several significant alterations in OR among the bvFTD patients compared with NCs. Second, a potential limitation is the lack of participant ratings of the IAPS stimuli for valence and arousal. The brief and sequential presentations did not permit subjective ratings of how the stimuli made the participants feel. Moreover, studies involving bvFTD patients document the unreliability and correspondence to stimuli of their verbal or subject reports and ratings.33,40 Finally, this preliminary study could not exclude more general effects of having dementia, and subsequent work needs to compare bvFTD with other dementia comparison groups.
In conclusion, this preliminary investigation discloses alterations in the initial orienting to socioemotional stimuli in bvFTD patients. These patients have decreased and variable attentional orienting to these stimuli, primarily to the emotional, unpleasant aspects of pictures, prior to initiating a sympathetic response. In addition to clarifying the relationship of ORs to emotion, future studies can explore whether impaired ORs could be an early diagnostic marker of bvFTD.
1 : Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011; 134:2456–2477Crossref, Medline, Google Scholar
2 : The Scale for Emotional Blunting in Patients with Frontotemporal Dementia. Neurocase 2006; 12:242–246Crossref, Medline, Google Scholar
3 : Affective reactions to acoustic stimuli. Psychophysiology 2000; 37:204–215Crossref, Medline, Google Scholar
4 : Heart-rate change as a component of the orienting response. Psychol Bull 1966; 65:305–320Crossref, Medline, Google Scholar
5 : Affective reactions to briefly presented pictures. Psychophysiology 2001; 38:474–478Crossref, Medline, Google Scholar
6 : Natural selective attention: orienting and emotion. Psychophysiology 2009; 46:1–11Crossref, Medline, Google Scholar
7 : Effects of stimulus intensity, risetime, and duration on autonomic and behavioral responding: implications for the differentiation of orienting, startle, and defense responses. Psychophysiology 1999; 36:453–463Crossref, Medline, Google Scholar
8 : Preparing hearts and minds: cardiac slowing and a cortical inhibitory network. Psychophysiology 2009; 46:1170–1178Crossref, Medline, Google Scholar
9 : Sex differences in orienting to pictures with and without humans: evidence from the cardiac evoked response (ECR) and the cortical long latency parietal positivity (LPP). PLoS One 2014; 9:e108224Crossref, Medline, Google Scholar
10 Lang PJ, Bradley MM, Cuthbert BN: International Affective Picture System (IAPS): Affective Ratings of Pictures and Instruction Manual (Technical Report A-8). Gainesville, Fla, University of Florida, 2008Google Scholar
11 : To “act wisely in human relations”: Exploring the dimensions of social competence. Pers Individ Dif 1996; 21:469–481Crossref, Google Scholar
12 : A rating scale for emotional blunting. Am J Psychiatry 1978; 135:226–229Crossref, Medline, Google Scholar
13 : The nature of emotional blunting: a factor-analytic study. Psychiatry Res 1987; 20:57–67Crossref, Medline, Google Scholar
14 : Relationship between positive and negative symptoms and neuropsychological scores in frontotemporal dementia and Alzheimer’s disease. J Int Neuropsychol Soc 2003; 9:698–709Crossref, Medline, Google Scholar
15 : Emotional category data on images from the International Affective Picture System. Behav Res Methods 2005; 37:626–630Crossref, Medline, Google Scholar
16 : Integrity of emotional and motivational states during the prodromal, first-episode, and chronic phases of schizophrenia. J Abnorm Psychol 2010; 119:71–82Crossref, Medline, Google Scholar
17 : Evaluation of emotional blunting in behavioral variant frontotemporal dementia compared to Alzheimer’s disease. Dement Geriatr Cogn Disord 2014; 38:79–88Crossref, Medline, Google Scholar
18 : Skin conductance levels may reflect emotional blunting in behavioral variant frontotemporal dementia. J Neuropsychiatry Clin Neurosci 2014; 26:227–232Link, Google Scholar
19 : Neuroanatomical correlates of behavioural phenotypes in behavioural variant of frontotemporal dementia. Behav Brain Res 2012; 235:124–129Crossref, Medline, Google Scholar
20 : Neuroanatomical correlates of emotional blunting in behavioral variant frontotemporal dementia and early-onset Alzheimer’s disease. J Alzheimers Dis 2014; 41:793–800Crossref, Medline, Google Scholar
21 : Heart rate variability is associated with amygdala functional connectivity with MPFC across younger and older adults. Neuroimage 2016; 139:44–52Crossref, Medline, Google Scholar
22 : Brain mediators of cardiovascular responses to social threat, part I: Reciprocal dorsal and ventral sub-regions of the medial prefrontal cortex and heart-rate reactivity. Neuroimage 2009; 47:821–835Crossref, Medline, Google Scholar
23 : Diminished disgust reactivity in behavioral variant frontotemporal dementia. Neuropsychologia 2012; 50:786–790Crossref, Medline, Google Scholar
24 : Fear conditioning in frontotemporal lobar degeneration and Alzheimer’s disease. Brain 2008; 131:1646–1657Crossref, Medline, Google Scholar
25 : Emotion drives attention: detecting the snake in the grass. J Exp Psychol Gen 2001; 130:466–478Crossref, Medline, Google Scholar
26 : Emotion and motivation, I: defensive and appetitive reactions in picture processing. Emotion 2001; 1:276–298Crossref, Medline, Google Scholar
27 : Uncovering the relationship between defence and orienting in emotion: cardiac reactivity to unpleasant pictures. Int J Psychophysiol 2006; 61:34–46Crossref, Medline, Google Scholar
28 : Emotional scenes and facial expressions elicit different psychophysiological responses. Int J Psychophysiol 2011; 80:173–181Crossref, Medline, Google Scholar
29 : A freezing-like posture to pictures of mutilation. Psychophysiology 2005; 42:255–260Crossref, Medline, Google Scholar
30 : Effects of picture content and intensity on affective physiological response. Psychophysiology 2006; 43:93–103Crossref, Medline, Google Scholar
31 : Arousal and attention: picture size and emotional reactions. Psychophysiology 2007; 44:680–686Crossref, Medline, Google Scholar
32 : Cardiac defense: from attention to action. Int J Psychophysiol 2007; 66:169–182Crossref, Medline, Google Scholar
33 : Understanding emotions in frontotemporal fementia: the explicit and implicit emotional cue mismatch. J Alzheimers Dis 2015; 46:211–225Crossref, Medline, Google Scholar
34 : Beyond arousal and valence: the importance of the biological versus social relevance of emotional stimuli. Cogn Affect Behav Neurosci 2012; 12:115–139Crossref, Medline, Google Scholar
35 : Emotional valence modulates activity in the posterior fusiform gyrus and inferior medial prefrontal cortex in social perception. Neuroimage 2003; 18:675–684Crossref, Medline, Google Scholar
36 : Autonomic responses to social and nonsocial pictures in adolescents with autism spectrum disorder. Autism Res 2014; 7:17–27Crossref, Medline, Google Scholar
37 : Psychophysiological correlates of social judgement in high-functioning adults with autism spectrum disorder. Int J Psychophysiol 2013; 87:88–94Crossref, Medline, Google Scholar
38 : Differential subjective and psychophysiological responses to socially and nonsocially generated emotional stimuli. Emotion 2006; 6:150–155Crossref, Medline, Google Scholar
39 : Autonomic markers of emotional processing: skin sympathetic nerve activity in humans during exposure to emotionally charged images. Front Physiol 2012; 3:394Crossref, Medline, Google Scholar
40 : Loss of emotional insight in behavioral variant frontotemporal dementia or “frontal anosodiaphoria.” Conscious Cogn 2011; 20:1690–1696Crossref, Medline, Google Scholar



