Cognitive Performance and Apathy Predict Unemployment in Huntington’s Disease Mutation Carriers
Abstract
Unemployment is common for those with Huntington’s disease (HD), a genetic neurodegenerative disorder, and affects patients’ quality of life. HD is characterized by motor disturbances, cognitive dysfunction, and psychiatric symptoms. The purpose of this article was to determine which clinical signs of HD are predictive of unemployment. Data for employed (N=114) and unemployed (N=106) HD mutation carriers were used to investigate group differences. Univariate logistic regression analyses, adjusted for age and gender, were performed to determine individual predictors of unemployment. Subsequently, a multivariate logistic regression analysis was performed, entering all significant results from the univariate analyses into one fully adjusted model to determine the strongest predictors. HD mutation carriers with lower cognitive performances and higher apathy scores were more likely to be unemployed than were HD mutation carriers with higher cognitive scores and no signs of apathy. Motor functioning was an independent predictor of unemployment but was not associated with unemployment in the fully adjusted model. Cognitive impairments, especially in the executive domain, and apathy were independent determinants of unemployment in HD mutation carriers. Motor disturbances, the clinical hallmark of HD, did not appear to be the most important predictor for work cessation. These results should be taken into consideration in clinical practice when evaluating HD patients’ ability to work.
Huntington’s disease (HD) is a hereditary neurodegenerative disorder characterized by a progressive decline in motor, cognitive, and psychiatric functioning. The mean age at onset is between 30 and 50 years, with a mean disease duration of 17–20 years.1 The motor signs of HD are characterized by choreatic movements, dystonia, bradykinesia, and hypokinesia, but the presence and severity can vary individually.2 The clinical diagnosis of HD is currently based on the presence of unequivocal motor signs. Cognitive decline primarily involves deficits in the executive domain, eventually resulting in dementia.3,4 Executive functions include planning, organization, cognitive flexibility, and regulating behavior. Depression, irritability, apathy, and obsessive-compulsive disorders are the most common psychiatric and behavioral symptoms of HD.5,6 The cognitive and behavioral deficits can already be present years before the onset of motor signs.7,8
At a relatively young age, individuals with HD become unable to work in their accustomed profession, and this affects their quality of life.9 Around 65% of patients with HD report some degree of decline in occupational performance.10 Gene mutation carriers without a clinical diagnosis also report changes in their work function, but they often attribute these changes to their working environment and personality traits and not to signs of HD.11 These results are not limited to the impact of total working cessation, but a reduction in work hours and increased sick leave were also reported as negative changes.12 Cognitive and behavioral deficits—such as mental slowing, inattention, cognitive inflexibility, and apathy—have been associated with a decline in everyday functioning in patients with HD.10,13–17 Only one study, to our knowledge, has reported that cognitive and motor impairments are correlated with unemployment, but these results should be interpreted with caution because of the small sample size.18 The impact of HD symptoms on the ability to work is still unknown. Therefore, the aim of our study was to determine which HD-related signs are specifically associated with unemployment in a large cohort of Dutch HD mutation carriers.
Methods
Participants
Baseline data of 220 HD mutation carriers (cytosine-adenine-guanine [CAG] repeat length ≥36) who participated in the Enroll-HD study at the Leiden University Medical Center (LUMC) were included. The Enroll-HD study is an on-going international, longitudinal, observational study to improve the understanding of HD, identify markers of disease progression, assist in recruitment of clinical trials, and improve the health of HD patients.19 Data are collected on demographics, motor, cognitive, and neuropsychiatric symptoms. Participants are asked to attend annual visits. Additional details about the Enroll-HD study design are described elsewhere.19,20
In this study, only participants with complete data on all assessments were included. Both participants with and without a clinical motor diagnosis (i.e., premanifest gene carriers and manifest HD) were included. The Medical Ethical Committee of the LUMC approved this study, and written informed consent was obtained from all participants.
Current working status was determined on the basis of clinical information provided by the participants. Participants who worked either fulltime (i.e., 36–40 hours per week) or part-time (fewer than 36 hours per week) in a paid job were categorized as employed (N=114). Participants who reportedly retired because of their ill health, caused by signs of HD, were categorized as unemployed (N=106). The previous occupations of the unemployed participants were divided into physically demanding and nonphysically demanding jobs on the basis of the authors’ judgment. Construction workers, nurses, and farmers are examples of physically demanding jobs. Examples of nonphysically demanding jobs are receptionists, administrative workers, and computer programmers. An overview of all job classifications is provided in Table S1 in the data supplement that accompanies the online version of this article.
Assessments
The total motor score of the Unified Huntington’s Disease Rating Scale (UHDRS-TMS) was used to assess the degree of motor disturbances.21 Here, higher scores indicated more motor impairments (range=0–124). The UHDRS total functional capacity (UHDRS-TFC) was used as a measure of general functioning.21 Higher scores indicated more functional disability. The cognitive scores included the total number of correct responses on the written Symbol Digit Modalities Test (SDMT),22 which was used to measure psychomotor speed and visual attention; the correct responses on the Stroop test (color, word, and interference),23 measuring speed of processing and executive functions; and the completion time in seconds of the Trail-Making Test, Part B (TMT-B),24 which was used to assess cognitive flexibility and executive functions. Lower total scores on the SDMT and Stroop test indicated worse performances. Lower completion times on the TMT-B indicated better performances. Behavioral and psychiatric problems were evaluated with the short version of the Problems Behaviors Assessment (PBA-s).25,26 The PBA-s is a semistructured interview assessing the following 11 symptoms: depression, suicidal ideation, anxiety, irritability, aggressive behavior, apathy, perseverative thinking, obsessive behavior, paranoid thinking, hallucinations, and disorientation. Severity and frequency scores are rated for each item on a 5-point scale (range=0–4), with a score of zero meaning that the symptom is absent and a score of four indicating that the symptom is causing severe problems in daily life. Scores per item are derived by multiplying the severity and frequency of each item. In this study the following previously defined constructs were used: depression, irritability, apathy, psychotic behavior, and executive dysfunction.19 Higher scores reflected more psychiatric and behavioral problems.
Statistical Analyses
Differences between unemployed and employed HD mutation carriers were analyzed with independent-sample t tests, chi-square test, and Mann-Whitney U test, when applicable. Univariate logistic regression analyses were performed for each predictor variable, with working status (employed vs. unemployed) as the dependent variable. Scores on the predictor variables were dichotomized at the median in high and low scores. For the UHDRS-TMS, scores higher than the median indicated more motor impairments. For the SDMT and Stroop test, lower scores indicated worse performances; for the TMT-B, higher scores indicated worse performances. For all neuropsychiatric domains, higher scores indicated more symptoms.
All univariate logistic analyses were adjusted for age and gender. Subsequently, the significant predictors resulting from the univariate logistic regression analyses were included in a multivariate logistic regression analysis, resulting in a fully adjusted model. The multivariate logistic regression analysis included age, gender, years of education, and HD group (premanifest or manifest) as covariates. All predictors were entered in one block (method=enter). This means that each predictor is entered into the model after correction for all other predictors that were included in the model. Significant results are, therefore, corrected for all predictors included. The significance threshold was set at p<.05 for all analyses. Statistical analyses were conducted by using the Statistical Package for Social Sciences (SPSS, version 23.0 for Windows).
Results
Demographic and clinical characteristics of all HD mutation carriers, employed, and unemployed participants are reported separately in Table 1. Sixty-seven participants (60 employed and seven unemployed) were in the premanifest stage, and 153 participants (54 employed and 99 unemployed) were in the manifest stage of the disease, on the basis of their UHDRS-TMS (≤5 is premanifest). Of the 153 manifest HD participants, 59 (38.6%) were categorized in disease stage 1, 64 (41.8%) in disease stage 2, 23 (15.0%) in disease stage 3, and seven (4.6%) in disease stage 4, on the basis of the UHDRS-TFC.27 None of the participants were classified in the latest stage, disease stage 5. Of the unemployed, 52 (49.1%) used to work in a physically demanding job, whereas 54 (50.9%) worked in less physically demanding environments (Table 1). The mean duration of unemployment was 6.9 years. The employed HD mutation carriers were significantly younger (mean=42.5 years) than the unemployed HD mutation carriers (mean=51.1 years). There were no significant differences between the two groups in gender and CAG repeat length. The unemployed HD mutation carriers showed significantly worse performances on the SDMT, Stroop test, and TMT-B. They also had significantly higher scores on the UHDRS-TMS and on irritability, apathy, and executive functioning scores of the PBA-s (Table 1).
| Characteristic | Whole Group (N=220) | Employed (N=114) | Unemployed (N=106) | p |
|---|---|---|---|---|
| Demographic | ||||
| Gender (male/female) (% male) | 96/124 (43.6%) | 54/60 (47.4%) | 42/64 (39.6%) | 0.247 |
| Age (years) | 46.6±11.1 (19–75) | 42.5±10.5 (19–66) | 51.1±10.0 (27–75) | <0.001 |
| Years of education | 14.8±3.4 (8–25) | 15.5±3.4 (8–25) | 14.1±3.3 (8–23) | 0.002 |
| Duration of unemploymentb | NA | NA | 6.9±6.6 (5; 0–29) | NA |
| Years to retirement after clinical diagnosis | NA | NA | –1.5±6.1 (0; –24–11) | NA |
| Job demands: physical/nonphysical (% physical) | NA | NA | 52/54 (49.1%) | NA |
| Clinical | ||||
| CAG repeat length | 43.1±2.9 (36–60) | 42.8±3.1 (36–60) | 43.5±2.7 (36–55) | 0.107 |
| UHDRS-TMS | 21.1±21.2 (0–104) | 9.54±11.3 (0–57) | 33.6±22.4 (0–104) | <0.001 |
| UHDRS-TFC | 11.0 (1–13) | 13.0 (7–13) | 9.0 (1–13) | <0.001 |
| Cognitive scores | ||||
| SDMTc | 39.7±16.1 (0–74) | 48.9±11.8 (17–74) | 29.8±14.2 (0–63) | <0.001 |
| Stroop colorc | 58.5±18.4 (16–100) | 67.8±15.0 (33–100) | 48.6±16.5 (16–83) | <0.001 |
| Stroop wordc | 78.2±22.9 (26–135) | 90.0±17.1 (46–135) | 65.6±21.5 (26–117) | <0.001 |
| Stroop interferencec | 34.5±12.6 (3–67) | 41.1±10.0 (14–67) | 27.5±11.2 (3–56) | <0.001 |
| TMT-Bd | 94.9±67.6 (23–240) | 62.8±41.9 (23–240) | 129.5±73.0 (28–240) | <0.001 |
| Neuropsychiatrice | ||||
| Depression score | 4.9±5.8 (0–28) | 4.7±5.3 (0–22) | 5.2±6.4 (0–28) | 0.531 |
| Irritability score | 2.6±3.5 (0–18) | 2.1±2.8 (0–12) | 3.2±4.0 (0–18) | 0.019 |
| Apathy score | 1.4±2.9 (0–16) | 0.75±1.9 (0–9) | 2.2±3.6 (0–16) | <0.001 |
| Psychotic score | 0.1±1.2 (0–18) | 0.0±0.1 (0–1) | 0.2±1.8 (0–16) | 0.326 |
| Executive functioning score | 1.5±3.3 (0–18) | 0.6±2.1 (0–16) | 2.5±4.1 (0–18) | <0.001 |
TABLE 1. Demographic, Clinical, and Neuropsychiatric Characteristics of the Huntington's Disease Participantsa
The univariate logistic regression analyses showed that after adjusting for age and gender, the UHDRS-TMS, SDMT, Stroop test, TMT-B, and irritability, apathy, and executive functioning scores were predictive of unemployment (Table 2). Scores on the SDMT were the strongest predictor (odds ratio [OR]=8.09, 95% confidence interval [CI]=4.18–15.64, p<0.001), meaning that HD mutation carriers with a total score of less than 42 on the SDMT are 8.09 times more likely of being unemployed than are HD mutation carriers with higher scores (Table 2). Depression and psychotic scores on the PBA-s were not significantly related to unemployment. The multivariate logistic regression analysis, including all significant predictors in one fully adjusted model, revealed that scores on the TMT-B, apathy, and executive functioning domains were significantly related to unemployment (Table 2, Figure 1). Here, apathy was the most significant predictor, with HD mutation carriers who showed signs of apathy having 3.26 times more chance of being unemployed than would HD mutation carriers without apathy (OR=3.26, CI=1.30–8.14, p=0.011). All other independent variables were no longer associated with unemployment in this adjusted model.
| Employed (N=114) | Unemployed (N=106) | Univariate Logistic Regression | Multivariate Logistic Regression | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Measure | N | % | N | % | Odds Ratio | 95% CI | p | Odds Ratio | 95% CI | p |
| UHDRS-TMS >15 | 32 | 28 | 78 | 74 | 5.20 | 2.66–10.18 | <0.001 | 0.85 | 0.31–2.36 | 0.756 |
| SDMT score <42 | 28 | 25 | 83 | 78 | 8.09 | 4.18–15.64 | <0.001 | 2.22 | 0.80–6.13 | 0.125 |
| Stroop color score <60 | 32 | 28 | 81 | 76 | 6.24 | 3.30–11.81 | <0.001 | 2.37 | 0.86–6.50 | 0.095 |
| Stroop word score <80 | 31 | 27 | 79 | 75 | 5.50 | 2.88–10.53 | <0.001 | 1.27 | 0.45–3.57 | 0.656 |
| Stroop interference score <36 | 31 | 27 | 79 | 75 | 6.04 | 3.22–11.36 | <0.001 | 0.65 | 0.22–1.87 | 0.421 |
| TMT-B score >66 | 28 | 25 | 82 | 77 | 8.07 | 4.20–15.50 | <0.001 | 2.88 | 1.09–7.61 | 0.033 |
| Depression score >2 | 59 | 52 | 52 | 49 | 0.88 | 0.49–1.57 | 0.668 | |||
| Irritability score >1 | 43 | 38 | 59 | 56 | 2.40 | 1.32–4.37 | 0.004 | 1.46 | 0.66–3.23 | 0.353 |
| Apathy score >0 | 24 | 21 | 45 | 43 | 3.52 | 1.80–6.88 | <0.001 | 3.26 | 1.30–8.14 | 0.011 |
| Psychotic score >0 | 2 | 2 | 2 | 2 | 0.75 | 0.10–5.87 | 0.786 | |||
| Executive functioning score >0 | 22 | 19 | 42 | 40 | 3.12 | 1.60–6.11 | 0.001 | 2.61 | 1.10–6.19 | 0.029 |
TABLE 2. Clinical Correlates of Unemployment in Huntington’s Disease (HD) Mutation Carriersa
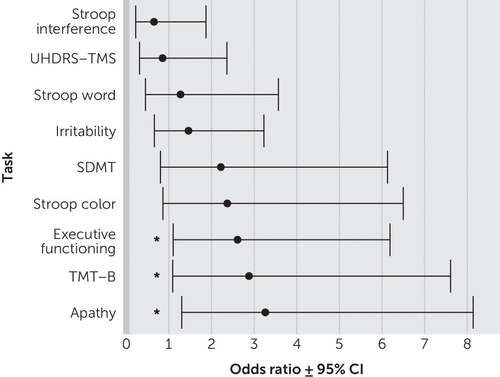
FIGURE 1. Predictors of Unemployment in Huntington’s Disease (HD) Mutation Carriersa
a Odds ratios and 95% confidence intervals (CIs) are based on the multivariate logistic regression analysis. Abbreviations: SDMT=Symbol Digit Modalities Test; TMT-B=Trail-Making Test, Part B; UHDRS-TMS=Unified Huntington’s Disease Rating Scale-Total Motor Score.
* p<0.05.
Discussion
This study identified significant predictors of unemployment in HD mutation carriers. Our results showed that worse performances on cognitive tasks reflecting speed of processing and cognitive flexibility are independent determinants of unemployment. Additionally, apathy and executive functioning, evaluated with the PBA-s, are also predictors of unemployment. Motor functioning was not a significant independent predictor of unemployment. This suggests that although motor functioning is considered the clinical hallmark of HD, impairments in cognitive and psychiatric domains are more important to assess when discussing occupational changes. The association between motor functioning and unemployment diminished in the multivariate analysis because of the stronger influence of cognition. However, the cognitive task that was significantly related to unemployment (i.e., TMT-B) also includes a motor component, and this should be considered when interpreting the results.
Not being able to work affects a patient’s quality of life, so it is important to consider potential working cessation carefully and try to postpone unemployment if possible. Adjusting or restructuring the work responsibilities to cognitively less demanding tasks might result in longer job participation. This will not be applicable to all professions, and not all employers may be willing to change the working environment. Patients should be advised about other possible activities that they can undertake, such as volunteer work, to prevent social isolation.
Cognitive functioning is increasingly being considered when forming the clinical HD diagnosis.9 Our results strengthen the idea that regular evaluation of cognitive and behavioral functioning is necessary, because these factors influence daily life activities, in particular work abilities. Apathy is very common and progressive in HD mutation carriers, even in early stages of the disease.5,6,28–30 It has also been related to a decline in general functioning.17 Our study showed that apathy is specifically related to unemployment, which is often incorporated in measures of general functioning. Cognitive functioning and psychiatric behavior—in particular, apathy—are also significantly related.29,31 Cognitive apathy has been defined as poor planning and organizational skills and lower cognitive flexibility.28 This might explain our results showing that both cognitive functioning and apathy are predictive of unemployment. However, the TMT and apathy scores remained significant predictors in the fully adjusted model. Thus, when we take this correlation between apathy and cognition into consideration, these constructs are still independent determinants of unemployment.
Interestingly, depression was not a predictor of unemployment in both analyses, although depression has been strongly linked to quality of life, and up to 50% of the HD patients report depressive symptoms at some point during the disease.5,17,28 In our study, half of the HD mutation carriers reported depressive symptoms, but this was comparable for both the employed and unemployed participants (52% and 49%, respectively). One explanation might be that depressive symptoms are more manageable with psychotropic medication than are other psychiatric symptoms and therefore interfere less with daily life activities. The progression of depressive symptoms is also nonlinear, and the severity decreases over time.6,28 The lack of a relationship between psychotic scores and unemployment might be explained by the low presence of psychotic behavior in our cohort. In general, psychotic behavior has a relatively low prevalence in HD (3%−11%) and is often reported in later disease stages.5,9
When we categorized the professions of the unemployed in our cohort into physical and nonphysical demanding jobs, unemployed individuals mostly worked in nonphysical jobs (50.9% vs. 49.1%). This suggests that physical activity alone might not be a reason to quit working, which is in line with our results that cognitive impairment is more predictive of work cessation than is motor function. However, this distinction remains arbitrary, and the influence of different job demands should be further explored. In addition, only unemployed participants, and no participants who reduced their working capacity, were included in this study. Furthermore, patients with more cognitive and psychiatric symptoms might not participate in research, resulting in a potential bias. Our study still revealed significant determinants of unemployment, suggesting that cognitive and psychiatric signs influence the ability to work. Most participants with manifest HD in the studied cohort were classified as in disease stages 1 and 2 (80.4%), so evaluating cognitive and psychiatric symptoms that might affect work is already important at an early stage of the disease. Longitudinal studies are needed to determine how disease progression is related to changes in work ability. To reduce potential cultural influences, we included only participants from one study site. The cohort contained more than 200 participants, which is considered large enough to investigate group differences. Still, more specific information regarding job types and classifications is necessary to thoroughly explore differences between professions and cultures. Asking participants to categorize their own job as either physical or nonphysical has previously been studied and could also be an interesting approach for future studies in HD.32
To our knowledge, this study is a first effort to determine strong predictors of unemployment in HD mutation carriers and to provide results for all types of professions. In conclusion, our study shows that cognitive impairments, especially in the executive domain, and apathy are independent determinants of unemployment in HD mutation carriers. Motor disturbances, the clinical hallmark of HD, do not seem to be the most important predictor of work cessation. This should be taken into consideration in the clinical practice when evaluating the ability to work.
1 : Huntington’s disease: a clinical review. Orphanet J Rare Dis 2010; 5:40Crossref, Medline, Google Scholar
2 : Chorea associated with Huntington’s disease: to treat or not to treat? Mov Disord 2014; 29:1414–1418Crossref, Medline, Google Scholar
3 : Cognitive impairment in Huntington disease: diagnosis and treatment. Curr Neurol Neurosci Rep 2011; 11:474–483Crossref, Medline, Google Scholar
4 : A review of cognition in Huntington’s disease. Front Biosci (Schol Ed) 2013; 5:1–18Crossref, Medline, Google Scholar
5 : Psychopathology in verified Huntington’s disease gene carriers. J Neuropsychiatry Clin Neurosci 2007; 19:441–448Link, Google Scholar
6 : Neuropsychiatric symptoms in a European Huntington’s disease cohort (REGISTRY). J Neurol Neurosurg Psychiatry 2014; 85:1411–1418Crossref, Medline, Google Scholar
7 : Detection of Huntington’s disease decades before diagnosis: the Predict-HD study. J Neurol Neurosurg Psychiatry 2008; 79:874–880Crossref, Medline, Google Scholar
8 : Onset of Huntington’s disease: can it be purely cognitive? Mov Disord 2014; 29:1342–1350Crossref, Medline, Google Scholar
9 : Huntington disease. Nat Rev Dis Primers 2015; 1:15005Crossref, Medline, Google Scholar
10 :
11 : Couples’ attributions for work function changes in prodromal Huntington disease. J Genet Couns 2010; 19:343–352Crossref, Medline, Google Scholar
12 : Work and recreational changes among people with neurological illness and their caregivers. Disabil Rehabil 2008; 30:600–610Crossref, Medline, Google Scholar
13 : Rate of functional decline in Huntington’s disease. Neurology 2000; 54:452–458Crossref, Medline, Google Scholar
14 ; Cognitive and psychiatric aspects of Huntington disease contribute to functional capacity. J Nerv Ment Dis 2004; 192:72–74Crossref, Medline, Google Scholar
15 : Cognitive deficits predict poorer functional capacity in Huntington’s disease: but what is being measured? Neuropsychology 2015; 29:268–273Crossref, Medline, Google Scholar
16 : Cognitive and functional decline in Huntington’s disease: dementia criteria revisited. Mov Disord 2010; 25:1163–1169Crossref, Medline, Google Scholar
17 : Behavioural abnormalities contribute to functional decline in Huntington’s disease. J Neurol Neurosurg Psychiatry 2003; 74:120–122Crossref, Medline, Google Scholar
18 : Determinants of functional disability in Huntington’s disease: role of cognitive and motor dysfunction. Mov Disord 2014; 29:1351–1358Crossref, Medline, Google Scholar
19 : Data analytics from Enroll-HD, a global clinical research platform for Huntington’s disease. Mov Disord Clin Pract 2017; 4:212–224Crossref, Google Scholar
20
21
22 : Symbol Digits Modalities Test. Los Angeles, CA, Western Psychological Services, 1991Google Scholar
23 : Studies of interference in serial verbal reactions. J Exp Psychol 1935; 18:643–662Crossref, Google Scholar
24 : Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills 1958; 8:271–276Crossref, Google Scholar
25 : Behavioural problems in Huntington’s disease using the Problem Behaviours Assessment. Gen Hosp Psychiatry 2008; 30:155–161Crossref, Medline, Google Scholar
26 : Reliability and factor structure of the Short Problem Behaviors Assessment for Huntington’s disease (PBA-s) in the TRACK-HD and REGISTRY studies. J Neuropsychiatry Clin Neurosci 2015; 27:59–64Link, Google Scholar
27 : Huntington disease: clinical care and evaluation. Neurology 1979; 29:1–3Crossref, Medline, Google Scholar
28 : Longitudinal evaluation of neuropsychiatric symptoms in Huntington’s disease. J Neuropsychiatry Clin Neurosci 2012; 24:53–60Link, Google Scholar
29 : “Frontal” behaviors before the diagnosis of Huntington’s disease and their relationship to markers of disease progression: evidence of early lack of awareness. J Neuropsychiatry Clin Neurosci 2010; 22:196–207Link, Google Scholar
30 : Neuropsychiatric symptoms are very common in premanifest and early stage Huntington’s disease. Parkinsonism Relat Disord 2016; 25:58–64Crossref, Medline, Google Scholar
31 : Behavior in Huntington’s disease: dissociating cognition-based and mood-based changes. J Neuropsychiatry Clin Neurosci 2002; 14:37–43Link, Google Scholar
32 : Retention in physically demanding jobs of individuals with low back pain: study protocol for a randomised controlled trial. Trials 2015; 16:166Crossref, Medline, Google Scholar



