Neurodegenerative Dementias After Traumatic Brain Injury
Abstract
Traumatic brain injury (TBI) is often considered to be a risk factor for the later development of neurodegenerative conditions, but some findings do not support a link. Differences in research methods, clinical samples, and limitations encountered when assessing and documenting TBI details likely contribute to the mixed reports in the literature. Despite some variability in findings, a review of the literature does provide support for the notion that TBI appears to be associated with earlier onset of some neurodegenerative disorders, although clearly not everyone with a TBI appears to be at an increased risk. Whereas a mechanistic link remains unknown, TBI has been found to initiate an accumulation of pathological processes related to several neurodegenerative disorders. The authors propose a hypothetical model that relates TBI to the development of pathological burden overlapping with some neurodegenerative conditions, in which onset of cognitive/behavioral impairments is hastened in some individuals, but pathological processes stabilize afterward, resulting in a similar course of decline to individuals with dementia who do not have a history of TBI.
Most individuals survive traumatic brain injury (TBI), and while cognitive, psychiatric, and physiological difficulties are common during the acute phase, there are growing concerns about TBI as a risk factor for the later development of neurodegenerative conditions, even following good initial recovery. It has been posited that TBI either initiates a neurodegenerative process, accelerates underlying neurodegenerative disorders, or disrupts neuronal functioning, and as the survivor ages, leads to development of neurodegenerative disorders long after the occurrence of the TBI.1 However, how or why these phenomena occur remains unclear. The most frequent type of neurodegenerative dementia is Alzheimer’s disease (AD), making up more than half of all cases, followed by dementia with Lewy bodies, and in turn by frontotemporal dementia.2 We present here a critical overview of the literature that explores the relationship between a history of TBI and subsequent development of dementia, and a discussion regarding potential mechanisms.
Research Limitations of the Current Literature
Research on whether TBI is linked to later cognitive decline has proven to be rather challenging. First, neurodegenerative dementias have an insidious onset and may evolve over decades.3 Because of this, findings from nearly all longitudinal studies examining whether older individuals proceed to develop dementia following TBI are limited, as these studies cover relatively short durations (e.g., nearly all within 10 years) of follow-up in comparison to the overall neurodegenerative process. That is, an underlying risk or neurodegenerative process may have already been present at the time of injury, making it difficult to determine the role of TBI in the development of dementia. Studies typically have relied on retrospective examination of TBI information gathered from older individuals presenting for dementia evaluations. One of the biggest concerns is that many of these studies have only gathered a limited history of TBI details, mostly whether there is a lifetime history of TBI resulting in loss of consciousness (LOC). As a result, the severity and the age of the injury are often not or poorly documented. Second, there is no universal standard for classifying TBI, making generalization of the findings and overall interpretation of the literature even more difficult. For example, some have defined TBI based on conventional criteria (mild, moderate, and severe based on duration of LOC and/or posttraumatic amnesia), presence of a history of TBI with LOC, or presence of any TBI, while others have identified TBI according to a history of skull fracture and/or intracranial injury. Each of these TBI definitions may lead to distinctly different correlations with dementia. Third, although several epidemiological studies exist, all have relied on examining a population’s medical records, which raises concerns about accuracy of the diagnoses, since most are diagnosed and their histories of TBI documented outside of specialized dementia clinics.
Alzheimer’s Disease
AD is a neurodegenerative disorder typically comprising brain atrophy first observed in the temporal lobes, which progresses to the frontal and parietal regions before extending to the occipital lobes.4 Amyloid-β plaques and hyperphosphorylated tau neurofibrillary tangles are the pathological hallmarks of AD. Classic clinical presentation of AD involves initial changes in learning and memory and aspects of language followed by global cognitive deterioration as the disease spreads.5 Because of the prevalence of AD, it is the most frequently studied type of dementia in relation to a possible link with TBI.
TBI History and Risk for Alzheimer’s Disease
Many researchers have reported a history of TBI to be associated with an increased risk for developing AD,6 though not all have found an association, including those with well-designed investigations. In one study, Mehta et al.7 examined whether a self-reported remote TBI increased the incidence of AD in individuals from a large sample (N=6,645) in the Netherlands who were age 55 years or older. These authors reported that a history of TBI with LOC, duration of LOC, time since injury, and multiple TBIs were unrelated to the development of AD. Moreover, Dams-O’Connor et al.8 examined individuals aged 65 years and older from Seattle, Washington, every 2 years for an average of 7 years, assessing for the development of AD (N=4,225). Subjects who self-reported a history of TBI with LOC were found to have a similar risk for developing AD as those without a TBI history, regardless of whether the TBI occurred in early, mid-, or mid-to-late life. Thus, the mixed reports in the literature have led some to question if a history of TBI is actually a risk factor for developing AD.
TBI is a heterogeneous condition with several features that could have important implications for subsequent risk of neurodegeneration (e.g., TBI severity, age at injury, multiple injuries, accuracy of diagnosis). Because studies differ on criteria for TBI classification, follow-up duration, and sampling procedures, one of the biggest challenges in reviewing this literature is assessing for the optimal study design. When one critically evaluates the literature, there are several studies that appear to minimize confounds. This can be seen in a study by Plassman et al.,9 who provided some of the earliest and most convincing evidence for TBI as a risk factor for developing AD. In that investigation, the authors evaluated World War II veterans who had a documented history of TBI of any severity (N=548) during military service about 40 years earlier for the risk of developing dementia and compared them to a cohort of veterans who had sustained a non-TBI medical condition (N=1,228). Patients with a remote history of moderate and severe TBI were found to have a 2- and 4-fold higher likelihood of developing AD later in life, while those with a history of mild TBI did not. In addition, Barnes et al.10 compared older veterans with a diagnosis of AD and a history of TBI of any severity (though a majority of TBIs were described as moderate to severe) within the 10 years preceding the AD diagnosis to those with no TBI history. Veterans with a history of TBI were found to have a 1.6-fold increased risk for developing AD during a 9-year follow-up. Thus, much of the evidence for TBI as a risk factor for the later development of AD stems from more serious injuries, while a potential association with milder injuries has received less attention, with more questions remaining.
Evidence-Based Considerations With Meta-Analytic Studies
Meta-analyses of the hypothesized TBI-AD link have produced mixed results. For example, a recent meta-analysis of 28 studies concluded that a history of any head injury was not a significant risk factor for a diagnosis of AD,11 but a previous meta-analysis of 15 case-control studies revealed that a history of TBI with any LOC was a risk.12 Since TBI is a heterogeneous condition, the larger meta-analysis (with and without LOC) could have produced a false negative because any potential association with moderate to severe TBI was likely buried within a large and nonspecific TBI group. Alternatively, TBI’s effect on increasing the absolute risk for developing dementia was found to be only slightly elevated, with 16% of patients with mostly moderate to severe TBI being reported to later develop dementia, compared with 10% of those without a TBI history.10 Therefore, it would be a mistake to assume that everyone with a TBI is at an increased risk for developing AD. However, it does appear that some individuals who sustain a TBI are at increased risk of later developing AD, for reasons that may relate to injury severity as well as other factors which require further investigation.
TBI History and Age at Onset of Alzheimer’s Disease
In addition to increasing the risk of developing AD in some individuals, TBI also appears to confer a risk of earlier age of onset of dementia. LoBue et al.13 recently conducted a retrospective study of the National Alzheimer’s Coordinating Center dataset to determine if a history of TBI with LOC that occurred more than 1 year prior to evaluation was related to the age at onset of AD, independent of gender and apolipoprotein ɛ4 status. First, they found that those with a history of TBI (N=571) with LOC had an age of symptom onset of AD and diagnosis that was 2.5 years earlier than those without a TBI history (N=7,054). Second, this association was seen in both females and males and was unchanged even after accounting for effects related to apolipoprotein ɛ4 status. These findings suggest that a history of TBI reduces cognitive reserve, which could later interact with neurodegenerative processes to lower the threshold for onset of symptoms, or might accelerate the onset of AD in some individuals by contributing directly to an underlying neurodegenerative process.
TBI History and Cognitive Decline in Alzheimer’s Disease
Since TBI may contribute to an acceleration of neurodegenerative processes that underlie AD, it is possible that TBI influences the course of decline in AD. In one study that followed individuals diagnosed with AD for up to 11 years, Gilbert et al.14 reported that a history of TBI involving head injury resulting in LOC, medical attention, or posttraumatic amnesia within 10 years of AD onset was linked to more rapid decline on a measure of dementia severity after AD onset, but more remote TBIs were not. As such, it would appear that although a remote history of TBI may be a risk factor for earlier onset of AD, a remote history of TBI does not affect the course of cognitive decline in AD.
Mild Cognitive Impairment
Mild cognitive impairment (MCI) is often a transitional stage between normal aging and AD, constituting a period during which a patient complains of cognitive difficulties and there is subtle neuropsychological decline, but criteria for dementia are not met.15 MCI thus represents an earlier threshold prior to clinical dementia onset, and it has recently been reported that TBI may also be associated with the development of MCI. In the first study to address this, LoBue et al.16 compared subjects with MCI (N=3,187) to a cohort of participants with normal cognition (N=3,244) from a large, multicenter national database. They found that a self-reported history of TBI with LOC without chronic deficit, occurring more than 1 year prior, was related to a 1.3 fold increased risk for an MCI diagnosis. This relationship remained even after accounting for sociodemographic (i.e., age and education), genetic (apolipoprotein ɛ4), and vascular factors, although the link between TBI and MCI was heavily influenced by a history of depression, and to a lesser extent, sex. The reasons for this are unclear, but a possible explanation may relate to the association between TBI and depression,17 as a higher prevalence of a history of depression was observed in those with a TBI history. Furthermore, individuals with a history of TBI and LOC were diagnosed with MCI approximately 2.5 years earlier than those without a TBI history, indicating that a history of TBI may be linked to an earlier onset of MCI potentially from reducing cognitive reserve and/or contributing to the pathological processes related to neurodegeneration. To our knowledge, only one other study has examined the association between TBI and earlier onset of MCI, and similar results were found.18
Dementia with Lewy Bodies
Dementia with Lewy bodies (DLB) is a neurodegenerative disorder associated with diffuse accumulation of Lewy bodies and alpha-synuclein proteins within the brain.19 Classic clinical presentation of DLB involves impaired and fluctuating cognition, development of extrapyramidal symptoms, and visual hallucinations. Despite DLB being the second most common dementia, very little is known about TBI as a risk factor. In the one small (N=147) study that has been published, Boot et al.20 reported that a history of TBI was not associated with an increased risk for a diagnosis of DLB when compared with controls or to patients with AD. However, Crane et al.21 examined individuals aged 50 years and older from three cohorts (N=1,652) and found that a history of TBI with LOC≤1 hour was found to be associated with the presence of Lewy bodies in frontal and temporal areas of the brain at autopsy, while a TBI history with LOC >1 hour was not. Interestingly, this association was reversed when the authors only examined subjects with injuries occurring several decades earlier (before age 25), but there were only four cases with more severe injuries and presence of any Lewy body pathology. Thus, it is unclear if a history of TBI is related to later development of DLB, and further investigation is clearly needed.
Frontotemporal Dementia
Frontotemporal dementia (FTD) comprises three main related dementia types, including behavioral variant FTD, primary progressive aphasia, and progressive nonfluent aphasia, depending on which areas of the brain are most affected.22,23 Individuals may present with changes in personality and emotional expression, becoming impulsive, socially inappropriate, and apathetic (behavioral variant), or changes in speech articulation (primary progressive aphasia) or loss of semantic knowledge (progressive nonfluent aphasia). FTD, as its name implies, characteristically involves degeneration of the frontal lobes and the anterior temporal regions, with nearly all cases being pathologically characterized by either accumulation of TAR DNA-binding protein inclusions or tau.24 Due to the irregular inner surface of the skull and location of the brain within the intracranial vault, TBI has a well-known propensity for affecting frontal and temporal brain regions and their underlying connections, prompting several researchers to investigate TBI’s potential influence on developing this form of dementia.
TBI History and Risk for Frontotemporal Dementia
Deutsch, Mendez, and Tang25 examined a large sample of subjects (N=1,016) with behavioral variant FTD, primary progressive aphasia, and progressive nonaffluent aphasia from the National Alzheimer’s Coordinating Center dataset. Subjects who self-reported a history of TBI with ≥5 minutes of LOC occurring more than 1 year prior to evaluation were found to have a 1.7-fold higher risk for a diagnosis of FTD than those without a history of TBI. Based upon the hypothesis that some individuals with a TBI history may show an increased likelihood for later cognitive decline, the authors examined whether it was possible that individuals with a history of TBI might also demonstrate more pronounced cognitive impairment. However, no neuropsychological differences were seen between subjects with and without a history of TBI diagnosed with primary progressive aphasia or progressive nonaffluent aphasia, and those with a TBI history diagnosed with behavioral variant FTD actually performed significantly better on a working memory task and showed lower dementia severity ratings. Although the authors concluded this finding might not be clinically relevant, since all other neuropsychological performances were similar, when this finding is combined with those in the other subtypes it appears that a remote history of TBI is not associated with greater levels of cognitive impairment in FTD.
Two other research groups found similar, though not identical, results. Rosso et al.26 examined several lifetime medical, cardiovascular, and cerebrovascular conditions, including a history of TBI, as risk factors for being diagnosed with behavioral variant FTD. The authors compared patients with a diagnosis of behavioral variant FTD to demographically matched controls, of whom only a small proportion had a history of TBI of any severity (19 vs. 10 cases, respectively). Despite a limited sample size, a history of TBI was found to be associated with a significantly higher risk for a diagnosis of behavioral variant FTD, whereas other medical factors were not. In a follow-up investigation, Kalkonde et al.27 compared veterans with behavioral variant FTD to those with any other dementia type. While TBI cases were again limited, patients with a history of TBI with loss of consciousness showed a significantly higher likelihood for being diagnosed with behavioral variant FTD compared with all other types of dementia combined.
TBI History and Onset of Frontotemporal Dementia
As with AD, in addition to a higher absolute risk of FTD, TBI may accelerate the onset of symptoms. LoBue et al.28 examined age of symptom onset and age of diagnosis in behavioral variant FTD subjects from the National Alzheimer’s Coordinating Center dataset (N=678). They found that individuals with a history of TBI with LOC occurring more than 1 year prior had an approximately 3 years earlier age of symptom onset and diagnosis of FTD than did those without a TBI history. The reasons for this association are poorly understood but, as in other conditions reported earlier, may relate to TBI lowering the threshold for later impairment by reducing cognitive reserve and/or accelerating the development of pathological processes.
Chronic Traumatic Encephalopathy
Chronic traumatic encephalopathy (CTE) is a neuropathological condition characterized by hyperphosphorylated tau in the form of neurofibrillary tangles, astrocytic tangles, and neurites deep within the sulci of the brain.29 It has been described as a neurodegenerative dementia, previously referred to as “punch drunk syndrome,” dementia pugilistica, and traumatic encephalopathy, but it has always been controversially related to repetitive mild TBI. The notion of CTE dates back to a 1928 case report by Martland, who described a professional boxer who developed symptoms similar to Parkinson’s disease.30 Since then, only 158 cases of CTE have been described, and a thorough review of CTE can be found in Gardner, Iverson, and McCrory.31 While there are no accepted clinical criteria for a diagnosis of CTE, due to the overlap of symptoms with other conditions, the clinical symptoms thought to be associated with CTE have varied over time. Speech, movement, and memory changes were historically reported in autopsy-identified cases, but alterations in personality and emotional expression along with cognitive decline have mostly been described in cases within the past 10 years. Given the relatively few cases identified, the incidence of CTE may be quite low, and the absence of large population-based studies contributes to our poor understanding of the syndrome.
Overlapping Symptoms
The current clinical description of individuals with CTE involves impulsivity, irritability/aggressiveness, depression, suicidality, a variety of cognitive problems, and headaches.29 A majority of these features are part of the constellation of symptoms described clinically with persistent posttraumatic symptoms.32 Additionally, many of the symptoms believed to be related to CTE overlap with those seen in other psychiatric conditions, most notably depression and well-known types of dementia. For example, in classic behavioral variant FTD, individuals present with impulsivity, irritability, reduced empathy, and symptoms of depression, and may show a variety of cognitive difficulties.5 Because the described symptoms of CTE are not specific to the syndrome, there are currently no established clinical criteria for recognizing CTE in vivo. Whereas a history of repetitive TBI has been reported in many cases of CTE, this is also the case with FTD and other conditions, as reported above. Thus, it is likely that some cases of clinically suspected CTE have been misclassified in the absence of neuropathological data.
Controversy Over Chronic Traumatic Encephalopathy
The National Institutes of Health convened a consensus conference in 2013 to determine whether CTE could be reliably distinguished from other neuropathological processes. In a pilot investigation, seven neuropathologists were selected to blindly evaluate brain tissue from 25 cases, including 10 presumed to have CTE (70% also showed presence of some AD-related pathology), 13 considered to have a tau-related dementia (e.g., AD, progressive supranuclear palsy, and corticobasal degeneration), and two with normal age-related changes.33 Although it is unclear how the 25 cases were selected for review, there was good agreement in classification and high agreement (>90%) for recognizing CTE, leading the consensus panel to develop the initial neuropathological criteria for this entity. Although the neuropathological findings of CTE have been well described, the implications of CTE-related pathology and its link with TBI are actually unclear. In a sample of 61 athletes with a history of repetitive mild TBI, for example, 25% had clinical symptoms and “pure” CTE pathology, but nearly 25% had clinical symptoms without CTE pathology, and approximately 25% had CTE pathology without clinical symptoms.34 It is also important to note that for the 61 CTE cases identified within the past 10 years who participated in athletic sports, almost 75% had neuropathological processes present that were related to other well-known neurodegenerative disorders, similar to that seen in the NIH consensus conference findings. Furthermore, a routine neuropathology service examined 111 cases under the age of 60 presenting for autopsy for CTE; while 39 cases were recognized as having CTE pathology, nearly 50% had a history of substance abuse along with a history of TBI, and approximately 25% had a history of substance abuse alone.35 For more detailed discussions of clinical and neuropathological issues in CTE, see Gardner, Iverson, and McCrory;31 Noy, Krawitz, and Del Bigio;35 and Iverson, Gardner, McCrory, Zafonte, and Castellani.36 As a result, CTE has been highly debated, with some hypothesizing that repetitive mild TBI can activate a neurodegenerative process leading to CTE, some emphasizing that cognitive/behavioral changes are not clearly linked to CTE pathology, and others proposing instead that TBI accelerates the expression of well-known neurodegenerative conditions.
Possible Mechanisms of Delayed Neurodegeneration After TBI
A mechanistic link underlying the apparent association between TBI and the later development of neurodegenerative conditions has not been well established. Neuroimaging studies have found that beyond the acute phase of injury (between 5 and 30 months), there is atrophy of frontal and temporal connections in small samples of moderate to severe TBI patients.37,38 Between 1 and 4 years following injury, moderate to severe TBI patients have shown greater diffuse white matter atrophy compared with age-matched controls.39 It is thought that damage to white matter tracts may initiate an accumulation of pathological processes related to neurodegenerative dementias,40 with autopsy studies reporting a presence of tau neurofibrillary tangles in nearly 1 of 3 individuals surviving a moderate to severe TBI for longer than a year, compared with only 1 of 10 age-matched controls.41 Animal research also provides evidence for this notion, as TBI models have shown mice to develop phosphorylated tau hours (up to 48) after a moderate to severe TBI and/or repetitive mild injuries, which showed progression over months and resulted in neuronal cell death.42
We have integrated available evidence into a model to represent the role TBI may play in developing neurodegenerative dementias (see Figure 1). We speculate that TBI contributes to the development of pathological burden overlapping several neurodegenerative processes. The pathological burden may interact with aging-related neuronal cell death (e.g., apoptosis), and when the overall brain changes surpass a threshold, cognitive/behavioral impairments become clinically manifest (represented in Figure 1 with the Clinical Threshold line). Because individuals with a remote history of TBI may develop greater pathological burden and white matter damage, our model predicts that some will reach the threshold for onset of symptoms earlier than those without a TBI history. Specifically, as shown, there may be some individuals with a remote TBI history who develop MCI at an earlier age before progressing to AD, while others may have a direct acceleration of onset of symptoms related to AD and behavioral variant FTD. However, the pathological burden may plateau shortly after onset of a dementia process, resulting in a similar course of decline between those with and those without a history of TBI. In contrast, individuals who proceed to DLB might show a similar course regardless of whether they have a remote history of TBI or not. This model best supports descriptions in the literature indicating that although a history of TBI can accelerate onset of AD and FTD in some individuals, a remote history of TBI does not appear to confer greater levels of impairment or more rapid decline in most individuals. Other plausible mechanisms besides triggering or accelerating neurodegenerative cascades include a TBI-induced static injury that reduces cognitive reserve.43
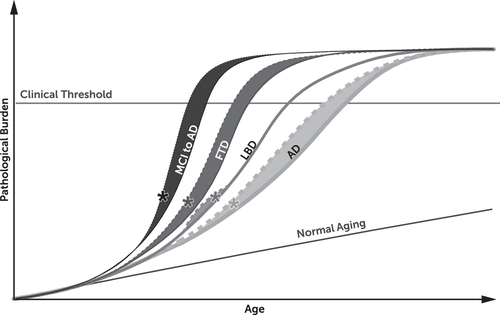
FIGURE 1. A Potential Role for Traumatic Brain Injury (TBI) in Developing Neurodegenerative Dementiasa
a Asterisk indicates sustaining a history of TBI. AD=Alzheimer’s disease; FTD=frontotemporal dementia; LBD=Lewy body dementia; MCI to AD=developing mild cognitive impairment and progressing to Alzheimer's disease.
Future Directions
TBI appears to have associations with the subsequent development of some neurodegenerative conditions. However, drawing further conclusions has been limited by 1) the insidious nature of neurodegenerative disorders and 2) research methods and limitations of available studies and findings. Moving forward, more comprehensive assessment of a TBI history (e.g., TBI severity, age at injury, multiple injuries) in individuals presenting for dementia evaluation is needed. Without due consideration of these factors, researchers will not be able to determine which ones potentiate TBI’s effects on the subsequent development of dementia. Next, studying neuropathologically confirmed cases of different types of dementia may help resolve mixed findings in the literature. Since several lines of evidence point to TBI accelerating the onset of some neurodegenerative conditions, it will also be important for future studies to thoroughly examine whether TBI influences the course of cognitive decline. It is likely that longitudinal neuroimaging (e.g., tau imaging) after TBI will be needed to reveal 1) whether TBI initiates a progressive development of neurodegenerative pathology and/or if such changes eventually stabilize and 2) if persistent neurodegenerative changes occur in milder injuries. Research on TBI and its relationship with the later development of neurodegenerative conditions is in its infancy, and it is likely that future studies incorporating one or more of the above suggestions will yield insight into this important issue.
In summary, there is evidence to support a link between a history of TBI and the later development of neurodegenerative conditions, but individual risk cannot be determined. TBI appears to be associated with earlier onset of some neurodegenerative disorders but may not influence the course of decline. Additionally, the mechanistic link between TBI and neurodegenerative conditions remains unknown. Future studies need to include more detailed information about TBI history and injury characteristics as well as modern neuropsychological, neuroimaging, and biomarker assays.
1 : Dementia risk after traumatic brain injury vs nonbrain trauma: the role of age and severity. JAMA Neurol 2014; 71:1490–1497Crossref, Medline, Google Scholar
2 : Relative frequencies of Alzheimer disease, Lewy body, vascular and frontotemporal dementia, and hippocampal sclerosis in the State of Florida Brain Bank. Alzheimer Dis Assoc Disord 2002; 16:203–212Crossref, Medline, Google Scholar
3 : Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol Aging 1997; 18:351–357Crossref, Medline, Google Scholar
4 : Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 1991; 82:239–259Crossref, Medline, Google Scholar
5 : Neuropsychological deficits in frontotemporal dementia and Alzheimer’s disease: a meta-analytic review. J Neurol Neurosurg Psychiatry 2007; 78:917–928Crossref, Medline, Google Scholar
6 : 2015 Alzheimer’s disease facts and figures. Alzheimers Dement 2015; 11:332–384Crossref, Medline, Google Scholar
7 : Head trauma and risk of dementia and Alzheimer’s disease: The Rotterdam Study. Neurology 1999; 53:1959–1962Crossref, Medline, Google Scholar
8 : Risk for late-life re-injury, dementia and death among individuals with traumatic brain injury: a population-based study. J Neurol Neurosurg Psychiatry 2013; 84:177–182Crossref, Medline, Google Scholar
9 : Documented head injury in early adulthood and risk of Alzheimer’s disease and other dementias. Neurology 2000; 55:1158–1166Crossref, Medline, Google Scholar
10 : Traumatic brain injury and risk of dementia in older veterans. Neurology 2014; 83:312–319Crossref, Medline, Google Scholar
11 : Meta-analysis of modifiable risk factors for Alzheimer’s disease. J Neurol Neurosurg Psychiatry 2015; 86:1299–1306Medline, Google Scholar
12 : Head injury as a risk factor for Alzheimer’s disease: the evidence 10 years on; a partial replication. J Neurol Neurosurg Psychiatry 2003; 74:857–862Crossref, Medline, Google Scholar
13 : Traumatic brain injury history is associated with earlier age of onset of Alzheimer disease. Clin Neuropsychol 2017; 31:85–98Crossref, Medline, Google Scholar
14 : The association of traumatic brain injury with rate of progression of cognitive and functional impairment in a population-based cohort of Alzheimer’s disease: the Cache County Dementia Progression Study. Int Psychogeriatr 2014; 26:1593–1601Crossref, Medline, Google Scholar
15 : Mild cognitive impairment: transition from aging to Alzheimer’s disease; in Alzheimer’s Disease: Advances in Etiology, Pathogenesis and Therapeutics. Edited by Iqbal K, Sisodia SS, Winblad B. Chichester, UK, John Wiley & Sons, Ltd, 2002Google Scholar
16 : Self-reported traumatic brain injury and mild cognitive impairment: increased risk and earlier age of diagnosis. J Alzheimers Dis 2016; 51:727–736Crossref, Medline, Google Scholar
17 : Psychiatric comorbidity following traumatic brain injury. Brain Inj 2007; 21:1321–1333Crossref, Medline, Google Scholar
18 : Traumatic brain injury and age at onset of cognitive impairment in older adults. J Neurol 2016; 263:1280–1285Crossref, Medline, Google Scholar
19 : Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 2005; 65:1863–1872Crossref, Medline, Google Scholar
20 : Risk factors for dementia with Lewy bodies: a case-control study. Neurology 2013; 81:833–840Crossref, Medline, Google Scholar
21 : Association of traumatic brain injury with late-life neurodegenerative conditions and neuropathologic findings. JAMA Neurol 2016; 73:1062–1069Crossref, Medline, Google Scholar
22 : Clinical and pathological diagnosis of frontotemporal dementia: report of the Work Group on Frontotemporal Dementia and Pick’s Disease. Arch Neurol 2001; 58:1803–1809Crossref, Medline, Google Scholar
23 : Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 1998; 51:1546–1554Crossref, Medline, Google Scholar
24 : Frontotemporal dementia: a bridge between dementia and neuromuscular disease. Ann N Y Acad Sci 2015; 1338:71–93Crossref, Medline, Google Scholar
25 : Interactions between traumatic brain injury and frontotemporal degeneration. Dement Geriatr Cogn Disord 2015; 39:143–153Crossref, Medline, Google Scholar
26 : Medical and environmental risk factors for sporadic frontotemporal dementia: a retrospective case-control study. J Neurol Neurosurg Psychiatry 2003; 74:1574–1576Crossref, Medline, Google Scholar
27 : Medical and environmental risk factors associated with frontotemporal dementia: a case-control study in a veteran population. Alzheimers Dement 2012; 8:204–210Crossref, Medline, Google Scholar
28 : Traumatic brain injury history is associated with earlier age of onset of frontotemporal dementia. J Neurol Neurosurg Psychiatry 2016; 87:817–820Crossref, Medline, Google Scholar
29 : The neuropathology of chronic traumatic encephalopathy. Brain Pathol 2015; 25:350–364Crossref, Medline, Google Scholar
30 : Punch drunk. J Am Med Assoc 1928; 91:1103–1107Crossref, Google Scholar
31 : Chronic traumatic encephalopathy in sport: a systematic review. Br J Sports Med 2014; 48:84–90Crossref, Medline, Google Scholar
32 : A review of post-concussion syndrome and psychological factors associated with concussion. Brain Inj 2015; 29:228–237Crossref, Medline, Google Scholar
33 : The first NINDS/NIBIB consensus meeting to define neuropathological criteria for the diagnosis of chronic traumatic encephalopathy. Acta Neuropathol 2016; 131:75–86Crossref, Medline, Google Scholar
34 : The spectrum of disease in chronic traumatic encephalopathy. Brain 2013; 136:43–64Crossref, Medline, Google Scholar
35 : Chronic traumatic encephalopathy-like abnormalities in a routine neuropathology service. J Neuropathol Exp Neurol 2016; 75:1145–1154Crossref, Medline, Google Scholar
36 : A critical review of chronic traumatic encephalopathy. Neurosci Biobehav Rev 2015; 56:276–293Crossref, Medline, Google Scholar
37 : Use of diffusion tensor imaging to examine subacute white matter injury progression in moderate to severe traumatic brain injury. Arch Phys Med Rehabil 2008; 89(Suppl):S45–S50Crossref, Medline, Google Scholar
38 : Magnetic resonance imaging evidence of progression of subacute brain atrophy in moderate to severe traumatic brain injury. Arch Phys Med Rehabil 2008; 89(Suppl):S35–S44Crossref, Medline, Google Scholar
39 : Longitudinal volumetric changes following traumatic brain injury: a tensor-based morphometry study. J Int Neuropsychol Soc 2012; 18:1006–1018Crossref, Medline, Google Scholar
40 : Axonal pathology in traumatic brain injury. Exp Neurol 2013; 246:35–43Crossref, Medline, Google Scholar
41 : Widespread τ and amyloid-β pathology many years after a single traumatic brain injury in humans. Brain Pathol 2012; 22:142–149Crossref, Medline, Google Scholar
42 : Antibody against early driver of neurodegeneration cis p-tau blocks brain injury and tauopathy. Nature 2015; 523:431–436Crossref, Medline, Google Scholar
43 : Chronic traumatic encephalopathy; in Behavioral Neurology of Dementia, 2nd ed. Edited by Miller B, Boeve B. Cambridge, England, Cambridge University Press, 2014Google Scholar



