Pharmacotherapy Effectiveness in Treating Depression After Traumatic Brain Injury: A Meta-Analysis
Abstract
Objective:
Depression is a highly prevalent neuropsychiatric sequela among individuals who have experienced traumatic brain injury (TBI). Despite its high prevalence, there continues to be conflicting evidence surrounding the efficacy of medication for treating depression post-TBI and whether different treatments have distinct effects. The aim of this study was to systematically review and synthesize the available evidence for the effectiveness of pharmacotherapy for depression following a TBI.
Methods:
A meta-analysis was completed using several online databases (PubMed, National Institute of Health and Care Excellence, and Healthcare Databases Advanced Search) to search for clinical trials involving various pharmacological treatments for depression in patients with TBIs. Twelve studies met the inclusion criteria and were assessed using their sample size, treatment duration, treatment used, TBI severity, method of assessment, and medication response. Standardized mean difference effect sizes (Cohen’s d) were calculated for each study using pre- and postintervention scores and pooled using a random effects model to produce a summary effect size.
Results:
Fourteen effect sizes were calculated, and a mild to moderate pooled effect size (Cohen’s d=–0.49, 95% CI: –0.96, –0.02, p=0.02) was found. Ten studies demonstrated effect sizes that were statistically significant, and four were nonsignificant. The weighted pooled effect size was higher for single-group design studies (Cohen’s d=–1.35, 95% CI: –2.14, –0.56, N=5) compared with independent-group designs (Cohen’s d=0.001, CI: –0.59, 0.58; N=9).
Conclusions:
This meta-analysis tentatively supports the view that pharmacological treatment may be effective in reducing depressive symptoms in those with depression following TBI. However, evidence from randomized controlled trials alone demonstrated no beneficial effect. The limitations are also discussed.
Traumatic brain injury (TBI) is increasingly one of the largest causes of fatalities globally and produces significant pervasive consequences (1). The sequelae of TBI often extend beyond the immediate physical injury to neuropsychiatric and cognitive complaints that have long-term effects on functioning and quality of life. The most commonly reported neuropsychiatric complaint is depression, which can occur in up to 77% of people with TBI (2).
Depression post-TBI has been associated with extensive adverse outcomes such as impaired psychosocial functioning, impaired cognitive performance, and increased risk of other psychiatric disorders, such as anxiety disorders (3). It is also more common for those with post-TBI depression to experience greater postconcussive symptoms (headaches, sleep disturbances, cognitive and memory impairment) than those who do not develop depression following a TBI (4). The heightened need for research into depression post-TBI is highlighted by Bombardier (5), who found 53.1% of 559 TBI patients developed major depressive disorder (MDD) within 1 year. Therefore, early treatment of depression is crucial to meet the needs of the high proportion of post-TBI patients affected by it.
The evidence for the effectiveness of pharmacotherapy for depression in the TBI population is conflicted and equivocal, with no overall trend extrapolated. Many studies within the current literature lack methodological rigor, with limited sample sizes and nonrandomized control groups. Currently, the first-line treatment of depression post-TBI is selective serotonin reuptake inhibitors (SSRIs), which may be attributed to the evidence base surrounding their use in MDD. SSRIs display a safer and more tolerated side effect profile than tricyclic antidepressants (TCAs). However, the efficacy of different SSRIs and TCAs varies. TCAs present an amplified risk of toxicity in overdose and side effects such as sedation and urinary retention; therefore, they are largely a second-line intervention. Findings by Jorge and Arciniegas (6) and Fann et al. (7) have suggested that SSRIs are the most effective pharmacotherapy in treating depression post-TBI. However, because of limited evidence and inconsistencies in the quality of research, they could not establish clinical guidelines on their use. This again emphasizes the need for more statistically powerful, placebo-included randomized controlled trials to validate this association, which can further inform the delivery of high-quality evidence recommendations.
Kant et al. (8) highlighted other beneficial features to antidepressant (SSRI) treatment, such as reducing comorbid irritability and aggression and improving cognitive functioning (e.g., memory). These topographies of treatment increase its usefulness and practicality in those with TBI, as it can alleviate additional neuropsychiatric sequelae that frequently coexist with TBIs.
Alternative pharmacotherapy options, such as monoamine oxidase inhibitors (MAOIs) and serotonin noradrenaline reuptake inhibitors (SNRIs), are less established but may offer new avenues of treatment. These are not often used because of the strict guidelines the patient must adhere to while taking them, including strict diet restriction. This is problematic in the TBI population, as the common cognitive consequences often affect medication compliance. SNRIs have a dual action on serotonin and noradrenaline reuptake inhibition and could have potential. However, this class of antidepressants is currently considered a second- or third-line treatment in MDD, and there is limited evidence for their use specifically for depression post-TBI. This highlights the critical need for more comprehensive research into the potential effectiveness of SNRIs and MAOIs for treating depression post-TBI.
Evidence for the use of psychostimulants in TBI patients with depression is lacking, with more focus on the treatment of cognitive deficits following a TBI. Gaultiere and Evans (9) found that methylphenidate showed some symptomatic relief, but some participants developed a tolerance to its administration over time.
Anticonvulsants such as lamotrigine have also shown specific benefit in treating the depressive symptoms in bipolar disorder, which may be generalized to future research into its potential in MDD (10). There is a larger evidence base for the use of anticonvulsants such as valproate, carbamazepine, and phenytoin in treating other neuropsychiatric sequelae of TBI, including aggression, agitation, and behavioral issues. However, there are also contradictory results from Smith et al. (11), who showed that they produced negative effects on cognition and motor performance.
A limited number of studies have examined pharmacotherapy effectiveness in treating depression in those with TBI in comparison to studies examining MDD in the general population. A recent meta-analysis investigating pharmacological interventions for MDD in primary care found 66 eligible randomized controlled trials (12). This meta-analysis included a total of 15,161 participants, which is larger than the total sample size investigated in the TBI population. This emphasizes the imbalance of research in contrast to MDD and the insufficient number of studies focusing on effects in the TBI sample.
The methodological rigor of research should also be considered when evaluating the evidence base for the effectiveness of pharmacotherapy in the treatment of depression in the TBI population. One way to assess the quality of studies is by grading them using systems such as the Cochrane Grading Recommendations Assessment, Development and Evaluation criteria. This can evaluate studies for methodological flaws such as small sample sizes and lack of randomized control. It is useful in research because it can assist in producing evidence-based guidelines. A systematic review by Fann et al.(7) found only one study with the highest grade of research (assessed using American Academy of Neurology criteria). This highlights not only the need for more research but also the need for studies that are conducted at a higher level of quality.
Overall, the current studies examining the treatment for depression post-TBI all implemented different outcome measures, sample sizes, periods of treatment, TBI severity, and time between TBI and the onset of depression. This makes comparison across studies especially difficult. There is a need to amalgamate the current research to provide clear guidelines that inform the treatment of depression following TBI. The aim of the current meta-analysis is to converge existing studies and provide a synthesized effect size to measure the effectiveness of pharmacotherapy for depression following a TBI.
Methods
Search Strategy
Online searches of published studies were conducted through several databases with the criteria of being peer-reviewed, written in English, published after 1980, and including only human participant studies. The searches were performed through PubMed, Cochrane Database, Google Scholar, and the National Institute of Health and Care Excellence Healthcare Databases Advanced Search to search through the following simultaneously: Allied and Complementary Medicine Database, British Nursing Index, Cumulative Index to Nursing and Allied Health Literature, Embase, Health Business Elite, Health Management Information Consortium, Medline, and PsychINFO. The following terms were used: “antidepressants,” “depression treatment,” “depression,” “traumatic brain injury,” and “TBI.” One author (AS) independently reviewed all abstracts and full texts and extracted the relevant data. This process was overseen by author RC to ensure objectivity.
Inclusion Criteria
To ensure that relevant articles were selected, the following inclusion criteria were chosen to refine the studies obtained. This included studies in which the depressive symptoms were measured as a secondary outcome so that a larger number of studies could be incorporated in the already depleted area of research. Unpublished and ongoing studies were not searched for and therefore not included. Research was not contained to a specific setting such as secondary care. The inclusion criteria for the current meta-analysis were as follows:
Including adults above 18 years of age.
Clinical trials measuring the effect of all pharmacological treatments (i.e., all drug types) for depression following a TBI.
Have a diagnosis of depression before the study initiation.
Sample to include participants with a TBI of any severity (mild, moderate, severe).
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines were used for the process of conducting the meta-analysis (13). Initially, 98 studies were identified. Duplicates and studies that did not meet the inclusion criteria were omitted, leaving 24 studies. If the information in the abstract was not sufficient, the full paper was reviewed. Of these, six were discounted because they did not meet the inclusion criteria; one study was excluded because it was a continuation of a previous study not focusing on depression, and a further six studies were excluded because of the use of preventative treatment. However, two additional articles were discovered through independent Internet searches and through other journal articles. Of these studies, one was excluded due to insufficient descriptive data provided (after the author was contacted), leaving 12 articles that fully met the inclusion criteria. A diagram summarizing the selection of studies is provided in Figure 1.
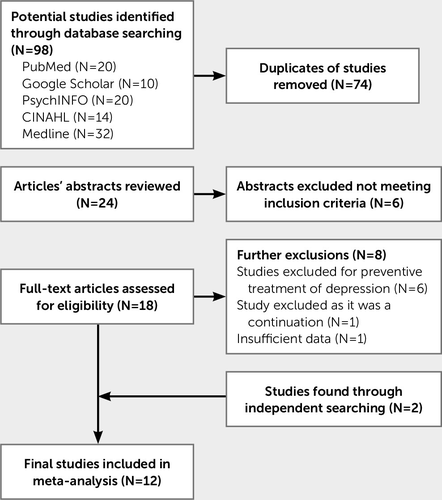
FIGURE 1. Flowchart of the study selection and exclusion process
Statistical Analyses
The statistical analysis was calculated using standardized mean differences for both randomized controlled trials (two independent groups) and single intervention groups, using different calculations for both.
The standardized mean difference was calculated (Cohen’s d) for the pre- and postintervention and control scores by using the extracted means and standard deviations from the literature (14). This resulted in an effect size for each study measuring treatment effectiveness for depression following a TBI. Correlations between the pre- and postintervention scores were based upon the literature review of the correlation efficient of the outcome measure (test-retest reliability r value). Where the r value was not derived from the available literature, the recommended value of 0.5 from the Cochrane Handbook was employed (15). To assess whether this would bias results, a sensitivity analysis was conducted using r values of 0.3 and 0.8 to assess impact on effect size. There were no large differences in results, so 0.5 was used in such instances. For example, the test-retest reliability of the Hamilton Depression Rating Scale (HAM-D) and DSM-III-R were set at 0.5, as there was no appropriate estimate in the TBI population. The Patient Health Questionnaire-9 was set at 0.76, which was extracted from a study in the TBI population (16).
The standard deviation of pooled change scores was not reported by any of the studies. Therefore, this was calculated using the standard deviation of changed scores for the treatment group as well as the placebo and control groups. The effect sizes were then calculated, as were the standard error, variance, and the weight of each effect size. Alternative calculations for the effect size in single-group design were also completed. According to Cohen, (17) a small effect size is interpreted as Cohen’s d=0.25, a medium effect as Cohen’s d=0.5, and a large effect as Cohen’s d=0.75. For the purpose of this meta-analysis, a negative effect size (–)indicates a reduction in depression scores.
Pooled Analysis
All effect sizes were weighted and pooled to obtain an average effect size for both the two-group and one-group designs combined. Subsequently, a 95% CI was calculated for the average weighted effect size produced. A random effects model was then applied to the analysis, and heterogeneity was assessed using a chi-squared-based Q-statistic test and I2 value (15).
When multiple assessments were used, the most reliable and validated method was included. For instance, in cases where both the HAM-D and Beck Depression Inventory were used, the HAM-D was included to keep the data extracted as consistent as possible.
In order to assess publication bias, a funnel plot was created and was assessed visually, which is displayed in Figure 2. An asymmetrical funnel plot indicates the presence of publication bias.
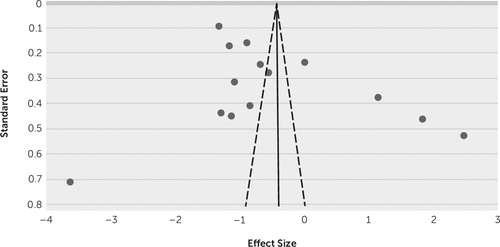
FIGURE 2. Funnel plot to assess publication biasa
a The summary effect size is indicated by the straight line, and dashed diagonal lines represent the 95% confidence intervals.
Results
Study Characteristics
Out of the 12 studies analyzed, four compared pharmacological effectiveness to placebo groups, one compared treatment in the TBI sample to a functional depressive group, and two studies included control groups. Five studies compared the pre- and posttreatment effects with no control group. All placebo and control groups contained depressed TBI patients who were given either a placebo or no treatment. Dinan and Moyabed (18) was the only study to compare treatment effectiveness in non-TBI patients, instead using functionally depressed patients.
There was considerable variation in the measurements of treatment effectiveness, but it was most commonly measured using HAM-D scores in nine studies. Depression was diagnosed with the DSM in all but one study, which used the Patient Health Questionnaire-9.
Two independent groups.
Fann et al. (19) and Zhang and Wang (20) used multiple outcome measures including HAM-D, Maeir subscale, and Beck Depression Inventory. For consistency the HAM-D pre- and postscores were assessed.
In the Saran (21) study participants received 4 weeks of amitriptyline and then a washout period of 1 week, followed by initiation of treatment with phenelzine. To gain separate effect sizes for each drug type (amitriptyline [TCA] and phenelzine [MAOI]), each condition was assessed separately. As this was a crossover trial, there were concerns regarding any possibility of carryover treatment effects (15).
Dinan and Moyabed (18) were contacted for further data concerning the comparison group of TBI patients to functionally depressed patients. However, as the authors no longer had access to the data, the pre- and postintervention scores were used from a previous meta-analysis (22).
Single-treatment groups.
In the study by Fann et al. (23), all participants initially received 1 week of placebo before switching to sertraline (after no significant drop of more than 50% in HAM-D scores). However, the baseline scores were given before the placebo and no additional data were included. Therefore calculations used the baseline score and the postintervention scores given at study’s end.
In the trial conducted by Wroblewski et al. (24), 10 patients were randomized to start with either placebo or desipramine and then evaluated using two different measures. However, various data were missing for both outcomes. Only eight participants had complete data that could be evaluated using DSM-III criteria and seven for the affect/mood scale. Therefore, the measure with the most data (DSM-III) was used. The means and standard deviations were calculated from the patient-level data provided.
In the Perino et al. (25) study, the participants received both carbamazepine and citalopram and were split into two groups according to time elapsed since TBI. The global pre- and postscores for both groups were combined.
Rapoport et al. (26) continued the study for 10 weeks, from the initial 6 weeks, for 26 of the 54 participants. However, the most complete data (6-week stage) were used in the analysis.
Kanetani et al.’s (27) postintervention scores were not provided in the study but were taken from a previous meta-analysis (22).
Meta-Analysis
The results of pre- to posttreatment differences are presented in Table 1, showing the effect size for each study (Cohen’s d), standard error, 95% CI, and weights (inverse of the variance). These analyses are presented in Figure 3 in the form of a forest plot, which demonstrates the effect size and 95% CI as well as the overall synthesized effect size for all studies.
| Study and summary | Na | Effect sizeb | SE | 95% CI | Weight | Treatment |
|---|---|---|---|---|---|---|
| Fann et al. (19) | 62 | 0.019 | 0.239 | –0.45, 0.488 | 1.345 | Sertraline |
| Ansari et al. (33) | 80 | –1.145 | 0.171 | –1.480, –0.809 | 1.407 | Sertraline |
| Ashman et al. (34) | 41 | –0.546 | 0.278 | –1.090, –0.001 | 1.318 | Sertraline |
| Fann et al. (23) | 15 | –3.624 | 0.710 | –5.016, –2.232 | 0.843 | Sertraline |
| Lee et al. (35) | 30 | –0.831 | 0.409 | –1.633, –0.030 | 1.178 | Sertraline |
| Lee et al. (35) | 30 | –1.283 | 0.437 | –2.140, –0.426 | 1.146 | Methylphenidate |
| Zhang and Wang (20) | 33 | –1.069 | 0.315 | –1.686, –0.451 | 1.281 | Methylphenidate |
| Rapoport et al. (26) | 54 | –0.883 | 0.160 | –1.197, –0.569 | 1.414 | Citalopram |
| Perino et al. (25) | 20 | –0.673 | 0.248 | –1.158, –0.187 | 1.346 | Citalopramc |
| Wroblewski et al. (24) | 8 | –1.119 | 0.451 | –2.003, –0.235 | 1.130 | Desipramine |
| Dinan and Moyabed (18) | 26 | 1.151 | 0.463 | 0.415, 1.886 | 1.216 | Amitriptyline |
| Saran (21) | 20 | 1.843 | 0.526 | 0.935, 2.751 | 1.116 | Amitriptyline |
| Saran (21) | 20 | 2.477 | 0.093 | 1.446, 3.509 | 1.043 | Phenelzine |
| Kanetani et al. (27) | 10 | –1.316 | 0.093 | –1.498, –1.133 | 1.449 | Milnacipran |
| Summary | –0.491 | 0.241 | –0.963, –0.019 |
TABLE 1. Pre- to posttreatment differences in the studies analyzed
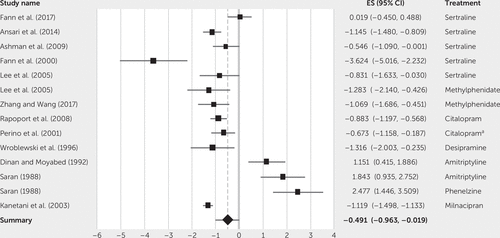
FIGURE 3. Forest plot of the effect size (ES) and 95% confidence intervals (CIs) in the included studiesa
a Carbamazepine was administered with citalopram in the study.
Most of the studies had a negative effect size. The weighted effect size for all studies was –0.49 (Cohen’s d; SE=0.24, 95% CI: –0.96, –0.02). The calculated Z score was –2.04 (p=0.02), which determined that the mean effect size was larger than zero. The studies were highly heterogenous (I2=91.94%, τ2=0.68). Studies without a control or placebo group were lower in heterogeneity (I2=81.45%, τ2=0.19).
When additional analyses were performed, the mean effect size for studies that included a comparison group was nonsignificant (Cohen’s d=0.001, 95% CI:–0.59, 0.58) when compared with single-group design studies (Cohen’s d=–1.35, 95% CI: –2.14, –0.56). This should be interpreted in the context that these studies included no control or placebo group.
The 95% CIs of the overall effect size do not cross the zero threshold. Therefore the results of the current meta-analysis are considered statistically significant. However, it should be highlighted that the margin is very close. The null hypothesis can be rejected and the alternative hypothesis that there is an effect can be accepted. Although there is a large variation in the studies included and very small sample sizes, 10 studies have shown statistically significant results with effect sizes larger than the pooled estimate. Four studies are statistically nonsignificant. The nonsignificant studies included very small sample sizes, were nonrandomized, and had no control groups, apart from the randomized controlled trial conducted by Fann et al. (23).
The forest plot (Figure 3) demonstrates the pharmacological treatment that had the greatest effect size was the Fann et al. (23) study, which examined sertraline. Although this study was nonrandomized, it had very large CIs and the lowest weight, which indicates that it lacks precision.
The largest number of studies was performed on sertraline (N=5), all of which produced large effect sizes apart from the most recent study by Fann et al. (19). In the Fann et al. study (19), both the treatment group and the placebo group had significant changes in depressive symptoms. The variation in results could be from the underpowered studies (ranging from 20 to 80 participants), the varying dosage (from 25 mg/day up to 200 mg/day), and the period of treatment (ranging from 4 weeks to 12 weeks). Fann et al. (19) had the longest period of treatment, highest dosage, and second largest sample size of 62 TBI patients. However, their sample contained a larger number of severe TBI patients with high levels of psychiatric comorbidity. When the pooled effect size was calculated for sertraline, it produced a large effect size: Cohen’s d=–1.02 (95% CI: –1.76, –0.28, p=0.004).
Both studies that looked at amitriptyline produced nonsignificant effect sizes with larger CIs that were more positive than the pooled estimate. Citalopram and methylphenidate were studied in only two trials each, but both showed large effect sizes and therefore significantly reduced depressive symptoms.
HAM-D was the most commonly used outcome measure, but there was variation on the version used (either the 17-item or 21-item scale). Eleven effect sizes were calculated using the reported pre- and postintervention HAM-D scores. The mean reduction of these scores in the treatment groups was 10.17, and standard deviation was 4.46. Six of the studies reported a statistically significant decrease in HAM-D scores.
Publication Bias
The funnel plot seen in Figure 2 demonstrates large asymmetry and therefore publication bias. When applying the Orwin fail-safe N formula, the number of studies needed to decrease the effect size to less than Cohen’s d=0.2 is 48. All but four studies are clustered in the top area, which means these studies have more precise estimated effects. However, 95% of studies do not lie between the 95% CIs and therefore show between-trial heterogeneity.
Discussion
These results indicate that pharmacological treatment may be mildly to moderately effective in treating depression post-TBI. Specifically, treatments such as citalopram, sertraline, and methylphenidate may be effective in reducing depressive symptoms in patients with TBI. The overall small to moderate effect size (Cohen’s d=–0.49) suggests that there is a significant reduction in symptoms from pre- to postintervention scores. However, this overall result should be interpreted with caution, because there is a lack of evidence in the form of large randomized controlled trials but rather includes a small sample of studies with poor methodological quality.
The findings from the current meta-analysis are supported by a previous meta-analysis (28) that found similar results. They evaluated antidepressant treatment effectiveness in nine studies treating depression, finding significant effects in favor of antidepressants. However, the authors could not draw definite conclusions on treatment efficacy due to the limited number of studies available in the literature.
This stance is reflected in another meta-analysis conducted by Barker-Collo et al. (22), who analyzed both pharmacological and nonpharmacological treatments and found that treatment did decrease depressive symptoms. They came to a similar conclusion that there is insufficient evidence to conclusively recommend treatment options to TBI patients. The current meta-analysis does offer an expansion on the included literature but still contains limitations; interpretation of the results should acknowledge this.
While the current meta-analysis was under review, the authors were made aware of a newly published meta-analysis by Kreitzer et al.(29). Although the overall conclusions slightly differ, the results of both meta-analyses are very similar. This is encouraging from a scientific replicability standpoint. For example, Kreitzer et al. (29) acknowledged significant reductions in depression scores for individuals after pharmacotherapy (mean change in HADS scores of –11.2). However, similar to the current meta-analysis, Kreitzer et al. (29) found no significant reduction in depression scores when considering the evidence from randomized controlled trials alone, for which Kreitzer et al. (29) derived their conclusion. In contrast, the current meta-analysis derived its conclusion from the entire evidence base, including both randomized controlled trials and single-group design studies, which indicated a small to medium effect for pharmacological treatments for depression following TBI. In addition to an overall omnibus effect size for all studies, the current meta-analysis also provided separate effect sizes for both single-group design studies and randomized controlled trials for the reader’s consideration.
The most consistent modest effect size was for sertraline, followed by methylphenidate and citalopram. A pooled estimate calculated for the sertraline studies (Cohen’s d=–1.02) produced a large effect size, even with the included outlier. Therefore, these findings suggest that sertraline, with the largest number of studies included and effect size, is potentially the most efficacious pharmacotherapy in treating depression after a TBI. Furthermore, the results from methylphenidate and milnacipran could have further implications in guiding potential pharmacological options, especially with the additional benefits on cognitive impairments and tolerability.
The studies that had nonsignificant effect sizes consisted of control groups such as in Fann et al. (19). In their paper, Fann et al. (19) discussed several possibilities for such nonsignificant effect sizes. One hypothesis is that the control group had more contact with staff, which may have produced the reduction in depressive symptoms. Fann et al. (19) highlighted the high prevalence of social isolation in the TBI population and hypothesized that frequent contact with health care staff might have reduced social isolation and decreased depression scores. This could be important in the context of rehabilitation; researching what nonpharmacological mechanism caused reductions in reported depression could potentially lead to subsequent application in nonpharmacological therapies.
In comparison, a recent study by Cipriani et al. (30) investigating the efficacy of 21 different antidepressants in treating MDD showed modest effect sizes for all antidepressants in reducing depressive symptomology. Although this exhibits a significant difference in response compared with depression post-TBI, the study drew from a substantial evidence base, which is severely lacking in regard to the TBI population. Consequently, it is likely that similar results of treatment efficacy may be found if there is further comprehensive research into depression post-TBI.
In contrast, Saran (21) found that both amitriptyline and phenelzine had greater effects in reducing HAM-D scores in those without a minor closed head injury. Similarly, Dinan and Moyabed (18) reported an 85% response to amitriptyline in those with just MDD, compared with 31% responding to treatment in those with depression post-TBI. Cipriani et al. (29) found that amitriptyline produced the greatest effect size in non-TBI MDD, which is interesting compared to the response to amitriptyline in TBI patients. These findings indicate that depression in TBI may respond differently to treatment compared with the non-TBI population. However, the studies in the TBI population had very low methodological quality, lacked large sample size, and included a crossover trial, so assumptions cannot be conclusive.
A meta-analysis by Price et al. (31) assessing treatment of depression in neurological disorders (stroke, Parkinson’s disease, multiple sclerosis, epilepsy, and TBI) found statistically significant effects of antidepressants compared with placebo. The meta-analysis also found a small difference in antidepressant responsiveness in this neurological sample when compared with the non-neurologically depressed population. However, as there was only one TBI study included, these conclusions cannot be applied to the whole TBI population. This was acknowledged by the authors, who stated that lack of trials only permitted evidence on Parkinson’s disease and stroke.
The current meta-analysis evaluated only pharmacological treatments. It is important to note that pharmacological treatments alone are not the only options in treating depression in TBI patients. Future research could investigate medication effectiveness in the setting of other interventions, such as cognitive-behavioral therapy, magnetic field stimulation, and psychoeducation. This is because treating neuropsychiatric sequelae such as depression in TBI patients requires a multifactorial approach to assist TBI patients in their recovery and increase their quality of life.
Although this study considered the proposed effectiveness of the antidepressants, it is also imperative to look at the population to whom the treatment will be administered. Their existing comorbidities and current medication use will inevitably affect which treatment they receive. This is further complicated by the possibility that the characteristics of depression post-TBI and related comorbidities could affect treatment effectiveness. For example, most depressed patients post-TBI also experience executive dysfunction, although the causation for this is not clear-cut (it is due either to the TBI damage or specific symptoms from the depression). Goryln et al. (32) found that deficits in executive function could influence treatment adherence and predict patients’ response to SSRIs. This also presents the idea of neuropsychological testing to enable identification of those who are at risk of not responding to treatment.
A potential limitation to this meta-analysis is the incorporation of single-group designs. This makes it challenging to delineate the treatment effectiveness from the natural course of depression in the context of TBI rehabilitation and recovery. This is also highlighted by Kreitzer et al. (29). Incidentally, single-group design studies produced a large pooled effect size (Cohen’s d=–1.35). However, this is limited in the context of the Cochrane recommendations results from nonrandomized studies should be interpreted with caution. This is because it results in a higher risk of selection bias and can produce effect size estimates that indicate the more extreme ends of the effects of treatment than randomized trials do.
Another limitation is the inability to control for the variation in the time since injury or the type and severity of TBI, which otherwise might produce more meaningful results for TBI patients. This undoubtedly contributed to the high heterogeneity, including the different outcome measures and length of treatment. For example, the time from injury varied from 30 days to 18.6 years, and the period of treatment varied from 4 to 30 weeks. The main concern of the shorter administration periods is not allowing for the optimal dosage and adjustment needed for therapeutic levels, such as in the Saran (21) study. For instance, the SSRI sertraline generally shows a latency period of 2 weeks before it has an effect. Perhaps more significant results could be found if periods of treatment were longer.
A strength of this current meta-analysis is that it has, to the authors’ knowledge, the most up-to-date inclusion and largest compilation of studies. Therefore, this is the largest comprehensive meta-analysis conducted so far on pharmacological treatment options, and not just antidepressants. This can give a wider scope on other pharmacological interventions and their potential effects on depression post-TBI.
To conclude, this meta-analysis suggests that pharmacological treatment may be mildly to moderately effective in treating depression following a TBI. It also demonstrates that sertraline may be the most effective pharmacological treatment option. However, there is still a need for higher quality studies with lower heterogeneity to draw less preliminary and more conclusive inferences concerning TBI treatment. This is important in the context of Fann et al., (7) who found that untreated depression in TBI patients is associated with poorer psychosocial functioning, cognition, and integration into the community. Consequently, if more comprehensive research is established, especially with pharmacotherapy in conjunction with nonpharmacological therapies, it could help future clinical application and rehabilitation of TBI patients.
1 : The impact of traumatic brain injuries: a global perspective. NeuroRehabilitation 2007; 22:341–353Medline, Google Scholar
2 : Major depression in patients with closed head injury. Neuropsychology 1987; 1:7–9. doi:
3 : Association between psychiatric state and outcome following traumatic brain injury. J Rehabil Med 2008; 40:850–857. doi:
4 : Postconcussive symptom report: the relative influence of head injury and depression. J Clin Exp Neuropsychol 2002; 24:981–993. doi:
5 : Rates of major depressive disorder and clinical outcomes following traumatic brain injury. JAMA 2010; 303:1938–1945. doi:
6 : Mood disorders after TBI. Psychiatr Clin North Am 2014; 37:13–29. doi:
7 : Treatment for depression after traumatic brain injury: a systematic review. J Neurotrauma 2009; 26:2383–2402. doi:
8 : Treatment of aggression and irritability after head injury. Brain Inj 1998; 12:661–666. doi:
9 : Stimulant treatment for the neurobehavioural sequelae of traumatic brain injury. Brain Inj 1988; 2:273–290. doi:
10 : Varying uses of anticonvulsant medications. Psychiatry (Edgmont Pa) 2008; 5:31–33Medline, Google Scholar
11 : Neurobehavioral effects of phenytoin and carbamazepine in patients recovering from brain trauma: a comparative study. Arch Neurol 1994; 51:653–660. doi:
12 : Efficacy and acceptability of pharmacological treatments for depressive disorders in primary care: systematic review and network meta-analysis. Ann Fam Med 2015; 13:69–79. doi:
13 : The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009; 339:b2700. doi:
14 : Applied social research methods series, vol. 49: Practical Meta-Analysis. Thousand Oaks, CA, Sage Publications, Inc., 2001Google Scholar
15 Higgins J, Deeks J, Altman D: Special topics in statistics; in Cochrane Handbook for Systematic Reviews of Interventions (Version 5.1.0). Edited by Higgins JPT, Green S. London, England, The Cochrane Collaboration, 2011. www.handbook.cochrane.org. Accessed Dec 4, 2018Google Scholar
16 : Validity of the Patient Health Questionnaire-9 in assessing depression following traumatic brain injury. J Head Trauma Rehabil 2005; 20:501–511. doi:
17 : Statistical Power Analysis for the Behavioral Sciences, 2nd ed. Hillsdale, NJ, Lawrence Earlbaum Associates, 1988Google Scholar
18 : Treatment resistance of depression after head injury: a preliminary study of amitriptyline response. Acta Psychiatr Scand 1992; 85:292–294. doi:
19 : Sertraline for major depression during the year following traumatic brain injury: a randomized controlled trial. J Head Trauma Rehabil 2017; 32:332–342. doi:
20 : Efficacy of methylphenidate for the treatment of mental sequelae after traumatic brain injury. Medicine (Baltimore) 2017; 96:e6960. doi:
21 : Antidepressants not effective in headache associated with minor closed head injury. Int J Psychiatry Med 1988; 18:75–83Crossref, Medline, Google Scholar
22 : Treatment for depression following mild traumatic brain injury in adults: a meta-analysis. Brain Inj 2013; 27:1124–1133. doi:
23 : Sertraline in the treatment of major depression following mild traumatic brain injury. J Neuropsychiatry Clin Neurosci 2000; 12:226–232. doi:
24 : Antidepressant pharmacotherapy and the treatment of depression in patients with severe traumatic brain injury: a controlled, prospective study. J Clin Psychiatry 1996; 57:582–587. doi:
25 : Mood and behavioural disorders following traumatic brain injury: clinical evaluation and pharmacological management. Brain Inj 2001; 15:139–148. doi:
26 : An open-label study of citalopram for major depression following traumatic brain injury. J Psychopharmacol 2008; 22:860–864. doi:
27 : Therapeutic effects of milnacipran (serotonin noradrenalin reuptake inhibitor) on depression following mild and moderate traumatic brain injury. J Nippon Med Sch 2003; 70:313–320Crossref, Medline, Google Scholar
28 : Pharmacotherapy for depression posttraumatic brain injury: a meta-analysis. J Head Trauma Rehabil 2016; 31:E21–E32. doi:
29 : The effect of antidepressants on depression after traumatic brain injury: a meta-analysis. J Head Trauma Rehabil 2018; (Epub ahead of print, Aug 30, 2018). doi:
30 : Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet 2018; 391:1357–1366. doi:
31 : Antidepressants for the treatment of depression in neurological disorders: a systematic review and meta-analysis of randomised controlled trials. J Neurol Neurosurg Psychiatry 2011; 82:914–923. doi:
32 : Neuropsychological characteristics as predictors of SSRI treatment response in depressed subjects. J Neural Transm (Vienna) 2008; 115:1213–1219. doi:
33 : Role of sertraline in posttraumatic brain injury depression and quality-of-life in TBI. Asian J Neurosurg 2014; 9:182–188Crossref, Medline, Google Scholar
34 : A randomized controlled trial of sertraline for the treatment of depression in persons with traumatic brain injury. Arch Phys Med Rehabil 2009; 90:733–740Crossref, Medline, Google Scholar
35 : Comparing effects of methylphenidate, sertraline and placebo on neuropsychiatric sequelae in patients with traumatic brain injury. Hum Psychopharmacol 2005; 20:97–104Crossref, Medline, Google Scholar



