Loss of Consciousness and Altered Mental State as Predictors of Functional Recovery Within 6 Months Following Mild Traumatic Brain Injury
Abstract
Objective:
The authors tested the hypothesis that a combination of loss of consciousness (LOC) and altered mental state (AMS) predicts the highest risk of incomplete functional recovery within 6 months after mild traumatic brain injury (mTBI), compared with either condition alone, and that LOC alone is more strongly associated with incomplete recovery, compared with AMS alone.
Methods:
Data were analyzed from 407 patients with mTBI from Head injury Serum Markers for Assessing Response to Trauma (HeadSMART), a prospective cohort study of TBI patients presenting to two urban emergency departments. Four patient subgroups were constructed based on information documented at the time of injury: neither LOC nor AMS, LOC only, AMS only, and both. Logistic regression models assessed LOC and AMS as predictors of functional recovery at 1, 3, and 6 months.
Results:
A gradient of risk of incomplete functional recovery at 1, 3, and 6 months postinjury was noted, moving from neither LOC nor AMS, to LOC or AMS alone, to both. LOC was associated with incomplete functional recovery at 1 and 3 months (odds ratio=2.17, SE=0.46, p<0.001; and odds ratio=1.80, SE=0.40, p=0.008, respectively). AMS was associated with incomplete functional recovery at 1 month only (odds ratio=1.77, SE=0.37 p=0.007). No association was found between AMS and functional recovery in patients with no LOC. Neither LOC nor AMS was predictive of functional recovery at later times.
Conclusions:
These findings highlight the need to include symptom-focused clinical variables that pertain to the injury itself when assessing who might be at highest risk of incomplete functional recovery post-mTBI.
Incomplete functional recovery within the first 6 months after traumatic brain injury (TBI) is a common cause of disability and can have devastating consequences on rehabilitation outcomes (1, 2). Functional recovery refers to an individual’s ability to perform activities of daily living and instrumental activities of daily living; it also refers to social and vocational functioning postinjury (3). Mild TBI (mTBI) is the most common severity, affecting about 85% of TBI cases (4–7). Patients with mTBI are a heterogeneous population for a variety of reasons, including variability of severity or persistence of post-injury symptoms (6, 7). Therefore, examination of clinical injury variables at the time of mTBI as predictors of post-TBI functional recovery is critical in understanding who is at greatest risk of worse outcomes within the first 6 months after injury and thus who warrants closer follow-up. Across studies in the literature, there has been significant heterogeneity of functional outcomes after mTBI for a multitude of reasons. These include lack of standardization in defining mTBI, ambiguous descriptions of source populations and samples, selection and attrition bias, confounding factors, and use of outcome measures with insufficient validity, reliability and responsiveness (8–10).
Incomplete functional recovery has been reported to occur in 18%−32% of individuals 1 month after mTBI (8) and in approximately 33% at 3 and 6 months, declining to 22% at 1 year postinjury (11). Predictors of functional recovery after mTBI have been investigated in large prospective cohort studies; however, these studies focused on demographic and pre-TBI clinical variables, with limited assessment of how injury-related variables affected outcomes. Findings from these studies indicate that neck pain, pre-TBI mental illness, post-TBI emotional distress, older age, and less education are all predictors of incomplete functional recovery within the first 6 months (12–17). Clinical symptoms present at the time of mTBI and caused by the TBI as predictors of functional recovery have been studied, but findings have yielded mixed results.
Loss of consciousness (LOC) and altered mental state (AMS) are often used to make the diagnosis of TBI and to define TBI severity (18, 19). Little is known about the relationship between these injury variables and functional recovery after mTBI. Given that LOC and AMS are critical data assessed routinely at the time of injury, they have the potential to set prognostic standards if they predict development of incomplete functional recovery. A small number of studies have examined the association between LOC and post-mTBI neuropsychiatric symptoms, but none have included AMS as an injury variable nor have they focused on the role these variables play in functional recovery in the 6 months after mTBI (20–23). This study aimed to assess whether time-of-injury LOC or AMS is associated with incomplete functional recovery within the first 6 months after mTBI. We hypothesized that the presence of both LOC and AMS predict the highest risk of incomplete functional recovery within the first 6 months postinjury, and that LOC is a stronger predictor of incomplete functional recovery within the first 6 months after injury, compared with AMS alone.
Methods
We analyzed data accrued from TBI participants enrolled between April 2014 and April 2016 in a prospective observational cohort (the Head Injury Serum Markers for Assessing Response to Trauma, or HeadSMART, study) (24, 25). The study was approved by the Johns Hopkins University Institutional Review Board.
Participants
Participants were recruited from two academic emergency departments located in Baltimore: Johns Hopkins Bayview Medical Center, a level 2 trauma center, and Johns Hopkins Hospital, a level 1 trauma center. Eligible TBI participants were age 18 or older and presented to the emergency department within 24 hours of blunt head injury, met the American College of Emergency Physicians (ACEP) criteria for evaluation with a cranial computerized tomography (CT) scan, and consented to research blood draws (26). Patients were excluded for the following reasons: brain tumor, severe dementia, prior history of intracranial hemorrhage or intracranial surgery, seizure-induced head injury, pregnancy, no working telephone number, inability to communicate in English, and blood transfusion before initial research blood draw was obtained. A detailed description of this cohort has previously been published (24, 25).
To identify individuals who had experienced an mTBI secondary to blunt head trauma, we used the Department of Veterans Affairs and Department of Defense (VA/DoD) criteria (27), which are comparable to the criteria developed by the Demographics and Clinical Assessment Working Group of the International and Interagency Initiative toward Common Data Elements for Research on Traumatic Brain Injury and Psychological Health, known as the American College of Rehabilitation Medicine (ACRM) criteria (28, 29). VA/DoD criteria differ in that they consider TBI to be present if an intracranial lesion is present (e.g., intracranial abnormalities or skull fracture on head CT), whereas the ACRM criteria do not explicitly comment on this. Participants were eligible for study enrollment only if the presence or absence of LOC and AMS was confirmed from documentation in the electronic medical record or the emergency medical service report, on physical exam, or from a bystander report. Patient report of LOC and AMS was corroborated by one or all of these measures. This documentation, in conjunction with confirmation that the participant met ACEP criteria for TBI, then required a final confirmation of eligibility from the principal investigator (F.K.) of the study.
All individuals who met VA/DoD criteria for mild TBI in this cohort had normal brain imaging based on head CT. Strict VA/DoD criteria were used because we intended to exclude patients with abnormal neuroimaging from the category of mTBI. Because VA/DoD criteria were used to define mTBI, for participants who did not have LOC or AMS, a diagnosis was made based on posttraumatic amnesia (PTA). PTA was defined as failure to recall hitting one’s head or the events before or after the accident. Deficits in short-term memory were confirmed via clinical documentation in the electronic medical record.
Procedures
Demographic and clinical data were collected by trained research coordinators with structured data collection tools recommended by the National Institute of Neurological Disorders and Stroke Common Data Elements (28, 29). Outcome data were collected after enrollment (time of injury) either via telephone interview or in-person assessment. Data were managed using REDCap electronic data capture tools hosted at the Johns Hopkins School of Public Health. Cranial CT images were read by a single board-certified neuroradiologist (H.S.). mTBI patients were categorized into four groups based on presence of LOC or AMS: neither LOC nor AMS, LOC only, AMS only, and both. Figure 1 presents a flowchart describing the sample. LOC was defined as complete or near-complete lack of responsiveness to people and other environmental stimuli at the time of injury, and AMS was defined as being dazed, confused, or disoriented within 24 hours of the injury. The presence or absence of LOC or AMS was based on participant’s report. If this was not possible, then witness, bystander, or emergency medical service report was used.
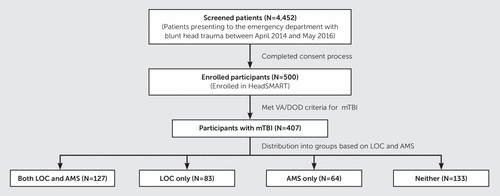
FIGURE 1. Flowchart of recruitment of participants with mild traumatic brain injury (mTBI) in four study groupsa
a AMS=altered mental state, HeadSMART=Head injury Serum Markers for Assessing Response to Trauma, LOS=loss of consciousness, mTBI=mild traumatic brain injury, VA/DoD=Department of Veterans Affairs and Department of Defense.
Measurements
Outcome data from participants were collected at 1, 3, and 6 months after time of injury, either via telephone interview or in-person assessment. Functional recovery (return to daily life activities, such as working, community activities, and social activities) was ascertained by using the Glasgow Outcome Scale–Extended (GOSE) (30, 31), which characterizes recovery on a scale of 1 (dead) to 8 (upper good recovery). In this study, a score ≤7 is defined as incomplete functional recovery, whereas a score of 8 is defined as complete functional recovery.
Analyses
Demographic, clinical, and injury characteristics of the four mTBI groups (neither LOC nor AMS, LOC only, AMS only, and both) were summarized and compared by using descriptive statistics: exact chi-square test for proportions and the Kruskal-Wallis test for continuous variables. Logistic regression models were estimated to assess whether LOC and AMS predict functional recovery based on a dichotomized GOSE variable (complete functional recovery versus incomplete functional recovery). Complete functional recovery (GOSE score of 8) was the most common GOSE score and represents normal functioning, and thus we chose to compare individuals in that group with those who had a score ≤7. A two-tailed p value <0.05 was considered statistically significant. Statistical analyses were performed using STATA/MP statistical software, version 11.2.
Results
Demographic and Injury Characteristics by LOC and AMS
A total of 407 patients met criteria for mTBI (Table 1). The group with neither LOC nor AMS was the largest (N=133), followed by both LOC and AMS (N=127), LOC only (N=83), and AMS only (N=64). The group with neither LOC nor AMS had the highest median age. The group with both LOC and AMS had the highest proportion of male participants. Table 2 presents the injury descriptors for the four groups and shows whether differences in symptoms at time of presentation were significant. The models were adjusted for age, sex, and intoxication at the time of injury because these variables were potential confounders, with statistically significant differences between groups (age, p=0.015; sex, p=0.011; and intoxication, p=0.002).
| All mTBI (N=407) | Both LOC and AMS (N=127) | LOC only (N=83) | AMS only (N=64) | Neither LOC nor AMS (N=133) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | N | % | N | % | N | % | N | % | N | % | Test statistica | p |
| Age (years) (median, IQR)b | 43 | 27–61 | 42 | 25–57 | 38 | 24–55 | 43 | 28–56 | 46 | 32–70 | 10.45 | 0.015 |
| Male | 229 | 56.3 | 84 | 66.1 | 46 | 55.4 | 38 | 59.4 | 61 | 45.9 | 11.16 | 0.011 |
| Race | 9.01 | 0.177 | ||||||||||
| Black | 174 | 42.8 | 63 | 49.6 | 39 | 47.0 | 26 | 40.6 | 46 | 34.6 | ||
| White | 206 | 50.6 | 57 | 44.9 | 40 | 48.2 | 31 | 48.4 | 78 | 58.6 | ||
| Other | 27 | 6.6 | 7 | 5.5 | 4 | 4.8 | 7 | 11.0 | 9 | 6.8 | ||
| Highest level of education | 10.91 | 0.099 | ||||||||||
| Less than high school | 76 | 18.7 | 32 | 25.2 | 11 | 13.3 | 7 | 10.9 | 26 | 19.5 | ||
| High school graduate | 239 | 58.7 | 70 | 55.1 | 56 | 67.4 | 42 | 65.6 | 71 | 53.4 | ||
| College graduate | 92 | 22.6 | 25 | 19.7 | 16 | 19.3 | 15 | 23.5 | 36 | 27.1 | ||
| Married | 121 | 29.7 | 35 | 27.6 | 25 | 30.1 | 16 | 25.0 | 45 | 33.8 | 2.05 | 0.575 |
| Employed | 202 | 49.6 | 62 | 48.8 | 42 | 50.6 | 37 | 57.8 | 61 | 45.9 | 2.53 | 0.469 |
| Prior concussion | 126 | 31.0 | 41 | 32.3 | 20 | 24.1 | 28 | 43.8 | 37 | 27.8 | 7.45 | 0.064 |
| Mood disorder | 123 | 30.2 | 46 | 36.2 | 23 | 27.7 | 17 | 26.6 | 37 | 27.8 | 5.57 | 0.416 |
| Nonmood psychiatric disorder | 85 | 20.9 | 31 | 24.4 | 20 | 24.1 | 12 | 18.8 | 22 | 16.5 | 5.47 | 0.413 |
TABLE 1. Baseline demographic and clinical characteristics of 407 participants with mild traumatic brain injury (mTBI), by whether they experienced loss of consciousness (LOC) or altered mental state (AMS) at the time of injury
| All mTBI (N=407) | Both LOC and AMS (N=127) | LOC only (N=83) | AMS only (N=64) | Neither LOC nor AMS (N=133) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | N | % | N | % | N | % | N | % | N | % | Test statistica | p |
| Mode of injury | 22.03 | 0.231 | ||||||||||
| Pedestrian struck | 41 | 10.1 | 16 | 12.6 | 6 | 7.2 | 6 | 9.4 | 13 | 9.8 | ||
| Motor vehicle collision | 114 | 28.0 | 26 | 20.5 | 29 | 34.9 | 20 | 31.3 | 39 | 29.3 | ||
| Fall | 138 | 33.9 | 45 | 35.4 | 26 | 31.3 | 16 | 25.0 | 51 | 38.3 | ||
| Assault | 65 | 16.0 | 22 | 17.3 | 16 | 19.3 | 9 | 14.1 | 18 | 13.5 | ||
| Struck by or against an object | 17 | 4.2 | 7 | 5.5 | 0 | — | 5 | 7.8 | 5 | 3.8 | ||
| Bicycle collision | 30 | 7.4 | 11 | 8.7 | 5 | 6.0 | 8 | 12.4 | 6 | 4.5 | ||
| Other | 2 | 0.4 | 0 | — | 1 | 1.3 | 0 | — | 1 | 0.8 | ||
| Intoxicated by drugs or alcohol | 79 | 19.4 | 39 | 30.7 | 13 | 15.7 | 11 | 17.2 | 16 | 12.0 | 15.94 | 0.002 |
| Symptoms at time of presentation | ||||||||||||
| GCS score <15b | 44 | 10.8 | 29 | 22.8 | 2 | 2.4 | 13 | 20.3 | 0 | — | 47.23 | <0.001 |
| Posttraumatic amnesia | 202 | 49.6 | 111 | 87.4 | 64 | 77.1 | 19 | 29.7 | 8 | 6.0 | 214.04 | <0.001 |
| Deficits in short-term memory | 35 | 8.6 | 28 | 22.0 | 1 | 1.2 | 6 | 9.4 | 0 | — | 58.39 | <0.001 |
| Focal neurological deficit | 26 | 6.4 | 12 | 9.4 | 5 | 6.0 | 4 | 6.3 | 5 | 3.8 | 7.45 | 0.264 |
| Headache | 332 | 81.6 | 113 | 89.0 | 67 | 80.7 | 52 | 81.3 | 100 | 75.2 | 8.28 | 0.035 |
| Vomiting since injury | 40 | 9.8 | 17 | 13.4 | 9 | 10.8 | 4 | 6.3 | 10 | 7.5 | 3.64 | 0.323 |
| GOAT (mean total score, IQR)c | 99 | 95–100 | 95 | 89–100 | 100 | 97–100 | 99 | 88–100 | 100 | 98–100 | 43.45 | <0.001 |
TABLE 2. Injury descriptors for 407 participants with mild traumatic brain injury (mTBI), by whether they experienced loss of consciousness (LOC) or altered mental state (AMS) at the time of injury
Outcomes as a Function of AMS or LOC or Both
The observed probabilities of functional recovery at 1, 3, and 6 months postinjury across all four groups as a function of LOC or AMS or both are summarized in Table 3. A general gradient of risk of incomplete functional recovery was noted at 1, 3, and 6 months, moving from neither, to LOC only or AMS only, to both. The group with both LOC and AMS, which had the highest odds ratio of the four groups across time points, comprised 31% (N=127) of participants with mTBI (Table 3).
| Neither | LOC only | AMS only | Both | |
|---|---|---|---|---|
| Time point | (N=133) | (N=83) | (N=64) | (N=127) |
| 1 month | 0.45 | 0.55 | 0.62 | 0.70 |
| 3 months | 0.44 | 0.49 | 0.61 | 0.60 |
| 6 months | 0.48 | 0.52 | 0.51 | 0.57 |
TABLE 3. Observed probabilities (odds ratios) of incomplete functional recovery of 407 participants at 1, 3, and 6 months, by whether they experienced loss of consciousness (LOC) or altered mental state (AMS) at the time of injurya
Logistic regression models were estimated with AMS as a predictor (regardless of presence or absence of LOC) or with LOC as a predictor (regardless of presence or absence of AMS) (Table 4). LOC was associated with incomplete functional recovery at 1 and 3 months (odds ratio=2.17, SE=0.46, p<0.001; odds ratio=1.80, SE=0.40, p=0.008, respectively). AMS was associated with incomplete functional recovery at 1 month only (odds ratio=1.77, SE=0.37, p=0.007).
| Time point and condition | N | Odds ratio | SE | p |
|---|---|---|---|---|
| 1 month | 376 | |||
| AMS (covariate) | 1.77 | 0.37 | 0.007 | |
| LOC (covariate) | 2.17 | 0.46 | <0.001 | |
| 3 months | 345 | |||
| AMS (covariate) | 1.24 | 0.27 | 0.32 | |
| LOC (covariate) | 1.8 | 0.40 | 0.008 | |
| 6 months | 337 | |||
| AMS (covariate) | 1.29 | 0.28 | 0.248 | |
| LOC (covariate) | 1.23 | 0.27 | 0.337 |
TABLE 4. Logistic regression analysis of the entire study sample covarying for loss of consciousness (LOC) or altered mental state (AMS) at the time of injury as associates of incomplete functional recovery at 1, 3, and 6 monthsa
The odds ratios of incomplete functional recovery among those who did not have LOC are presented in Table 5. No association was found between incomplete functional recovery and AMS in this group (Table 5).
| Time point | N | Odds ratiob | SE | p |
|---|---|---|---|---|
| 1 month | 162 | 1.49 | 0.50 | 0.245 |
| 3 months | 150 | 1.21 | 0.41 | 0.579 |
| 6 months | 152 | 1.17 | 0.40 | 0.646 |
TABLE 5. Logistic regression analysis of altered mental state (AMS) among participants who did not have loss of consciousness (LOC) at the time of injury as an associate of incomplete functional recovery at 1, 3, and 6 monthsa
Discussion
This study followed a consecutive sample of patients with mTBI to assess whether LOC and AMS are alone or in combination associated with incomplete functional recovery within the first 6 months after injury. Our findings confirmed that the presence of both LOC and AMS was associated with the highest risk of incomplete functional recovery, consistent with our hypothesis. Second, LOC only was more strongly associated with incomplete functional recovery at 1 and 3 months, compared with AMS only (also consistent with our hypothesis), while AMS only was associated with incomplete functional recovery only at 1 month. Third, among those who did not experience LOC, no association was found between incomplete functional recovery and AMS at any time point.
These findings emphasize the importance of assessing time-of-injury variables, such as LOC and AMS, when examining clinical risk factors for incomplete functional recovery within the first 6 months post-mTBI. These findings may prompt clinicians to be more aware that the presence of both symptoms at the time of injury places patients at highest risk of incomplete functional recovery and that these patients require the most imminent monitoring from the time of presentation to the emergency department. Furthermore, the fact that LOC may have an effect on incomplete functional recovery in the early TBI period (1 and 3 months) but no effect at 6 months and the fact that AMS may have an effect only within the first month suggest that other factors might play a role for individuals who are still not recovered at 6 months.
The idea that LOC and AMS might predict functional recovery only in the early TBI period but not later has implications for return-to-work potential. It is established in the literature that among individuals who experience an mTBI, the majority experience symptom resolution within days to weeks postinjury and are able to return to their daily activities by 3 months and that functional recovery plays a role in this timeline (32, 33). It is further reported that 80%−95% of patients return to work within 3–6 months after mTBI (33, 34). Although mTBI itself is not considered a significant risk factor for delayed return to work (35), impairment in functional recovery might be the cause for this delay. Based on the findings of our study, those who experience LOC and AMS might fall into the category of those whose delayed functional recovery impedes them from immediate return to work, but future studies are needed determine this relationship.
It is important to note the limitations of our study. First, the assessment of LOC and AMS often used patient or bystander reports, which are often inaccurate. Specifically, recall of LOC can be confused with posttraumatic amnesia in that patients may believe they were unconscious during periods in which they were really awake but amnestic. Although very stringent measures were implemented to ensure reliability of patient report (i.e., participants were enrolled in the study only if there was confirmation of LOC and AMS from documentation in the electronic medical record or emergency medical service report, documentation on physical exam, or documentation in a bystander report), there was still a chance that bystander and patient report might have been inaccurate. Second, the sample included those with preinjury psychiatric illness. It is known that a history of preinjury psychiatric symptoms increases the risk of the development of postinjury psychiatric disorders (36, 37). Therefore, it was difficult to account for the fact that LOC and AMS directly influenced the development of post-TBI functional recovery, particularly in those who had psychiatric illness before the injury. Our findings showed that there were not group differences in number of participants with preinjury psychiatric conditions, and thus this factor could not have been a driver for the group difference findings.
Third, the analysis did not consider body injury that would contribute to functional recovery, such as the ability to perform activities of daily living, as well as physical and occupational capacity. This analysis, however, was done in a previous study of this cohort, focusing on the prevalence of incomplete functional and symptomatic recovery among patients who had a head injury but who may not have had brain injury (24). Fourth, a structured clinical diagnostic interview using the DSM-5 (SCID-5) was not conducted, because the in-person and telephone evaluations were semistructured psychiatric interviews. As such, psychiatric symptoms were captured with the indicated scales, but psychiatric disorders were not formally assessed.
Despite these limitations, the primary strength of this study is that it is the first to identify that the presence of both LOC and AMS increased the risk of incomplete functional recovery within the first 6 months after injury and that the presence of LOC alone was a stronger risk factor for incomplete functional recovery than AMS alone. Taken together, these findings have significant clinical implications because they provide guidance for identifying patients at highest risk of incomplete prognosis in the early TBI period.
Conclusions
Our study found that the presence of both LOC and AMS indicated increased risk of incomplete functional recovery in the first 3 months after mTBI and that LOC alone was more closely associated with incomplete functional recovery but only up until 3 months postinjury, compared with AMS alone. These findings highlight the need to include symptom-focused clinical variables that pertain to the injury itself when assessing who might be at highest risk of experiencing incomplete functional recovery post-mTBI. This study adds to the existing literature additional risk factors for incomplete recovery post-mTBI, which will help identify patients who are in need of early psychosocial, rehabilitation, and psychiatric interventions. Further observational studies examining a wider array of injury-related variables as predictors of functional recovery should be conducted beyond 1-year postinjury to study the relationship between these symptoms and prognosis over an extended time course.
1 : Long-term outcome following severe brain injury. J Head Trauma Rehabil 2001; 16:v–viCrossref, Medline, Google Scholar
2 : Health-related quality of life of individuals with traumatic brain injury in Barranquilla, Colombia. Brain Inj 2012; 26:825–833Crossref, Medline, Google Scholar
3 : Disability after severe head injury: observations on the use of the Glasgow Outcome Scale. J Neurol Neurosurg Psychiatry 1981; 44:285–293Crossref, Medline, Google Scholar
4 Centers for Disease Control and Prevention: Report to Congress on Mild Traumatic Brain Injury in the United States: Steps to Prevent a Serious Public Health Problem. Atlanta, Centers for Disease Control and Prevention, 2003Google Scholar
5 National Hospital Discharge Survey (NHDS), National Hospital Ambulatory Medical Care Survey (NHAMCS). Atlanta, Centers for Disease Control and Prevention, 2010Google Scholar
6 : The epidemiology of traumatic brain injuries treated in emergency departments in North Carolina, 2010–2011. N C Med J 2014; 75:8–14Medline, Google Scholar
7 : Diagnosis, prognosis, and clinical management of mild traumatic brain injury. Lancet Neurol 2015; 14:506–517Crossref, Medline, Google Scholar
8 : Recovery from mild traumatic brain injury in previously healthy adults. J Neurotrauma 2016; 33:766–776Crossref, Medline, Google Scholar
9 : Systematic review of self-reported prognosis in adults after mild traumatic brain injury: results of the International Collaboration on Mild Traumatic Brain Injury Prognosis. Arch Phys Med Rehabil 2014; 95(suppl):S132–S151Crossref, Medline, Google Scholar
10 : Methodological issues and research recommendations for prognosis after mild traumatic brain injury: results of the International Collaboration on Mild Traumatic Brain Injury Prognosis. Arch Phys Med Rehabil 2014; 95(suppl 3):S266–S277Crossref, Google Scholar
11 : Trajectories and associated factors of quality of life, global outcome, and post-concussion symptoms in the first year following mild traumatic brain injury. Qual Life Res 2016; 25:2009–2019Crossref, Medline, Google Scholar
12 : Population-based, inception cohort study of the incidence, course, and prognosis of mild traumatic brain injury after motor vehicle collisions. Arch Phys Med Rehabil 2014; 95(suppl):S278–S285Crossref, Medline, Google Scholar
13 : Symptomatology and functional outcome in mild traumatic brain injury: results from the prospective TRACK-TBI study. J Neurotrauma 2014; 31:26–33Crossref, Medline, Google Scholar
14 : Mild traumatic brain injury; longitudinal study of cognition, functional status and post-traumatic symptoms. J Neurotrauma 2017; 34:1524–1530Crossref, Medline, Google Scholar
15 : A systematic review of the prognosis after mild traumatic brain injury in adults: cognitive, psychiatric, and mortality outcomes: results of the International Collaboration on Mild Traumatic Brain Injury Prognosis. Brain Inj 2014; 95(3 suppl):S152–S173Google Scholar
16 : Systematic review of multivariable prognostic models for mild traumatic brain injury. J Neurotrauma 2015; 32:517–526Crossref, Medline, Google Scholar
17 : Early predictors of outcome after mild traumatic brain injury (UPFRONT): an observational cohort study. Lancet Neurol 2017; 16:532–540Crossref, Medline, Google Scholar
18 . Complications of Mild Traumatic Brain Injury in Veterans and Military Personnel: A Systematic Review. VA Evidence-based Synthesis Program Reports. Washington, DC, US Department of Veterans Affairs, 2013Google Scholar
19 : Definition of mild traumatic brain injury. J Head Trauma Rehabil 1993; 8:86–87Crossref, Google Scholar
20 : Mild traumatic brain injury in US soldiers returning from Iraq. N Engl J Med 2008; 358:453–463Crossref, Medline, Google Scholar
21 : Head injury and loss of consciousness raise the likelihood of developing and maintaining PTSD symptoms. J Trauma Stress 2013; 26:727–734Crossref, Medline, Google Scholar
22 : Does brief loss of consciousness affect cognitive functioning after mild head injury? Arch Clin Neuropsychol 2000; 15:643–648Crossref, Medline, Google Scholar
23 : Comparison of concussive symptoms, cognitive performance, and psychological symptoms between acute blast-versus nonblast-induced mild traumatic brain injury. J Int Neuropsychol Soc 2011; 17:36–45Crossref, Medline, Google Scholar
24 : Prevalence of incomplete functional and symptomatic recovery among patients with head injury but brain injury debatable. J Neurotrauma 2017; 34:1531–1538Crossref, Medline, Google Scholar
25 : Head injury serum markers for assessing response to trauma: design of the HeadSMART study. Brain Inj 2017; 31:370–378Crossref, Medline, Google Scholar
26 : Clinical policy: neuroimaging and decisionmaking in adult mild traumatic brain injury in the acute setting. Ann Emerg Med 2008; 52:714–748Crossref, Medline, Google Scholar
27 : VA/DoD clinical practice guideline for management of concussion/mild traumatic brain injury. J Rehabil Res Dev 2009; 46:CP1–CP68Crossref, Medline, Google Scholar
28 : Position statement: definition of traumatic brain injury. Arch Phys Med Rehabil 2010; 91:1637–1640Crossref, Medline, Google Scholar
29 : Common data elements for traumatic brain injury: recommendations from the biospecimens and biomarkers working group. Arch Phys Med Rehabil 2010; 91:1667–1672Crossref, Medline, Google Scholar
30 : Reliability of ratings on the Glasgow Outcome Scales from in-person and telephone structured interviews. J Head Trauma Rehabil 2003; 18:252–258Crossref, Medline, Google Scholar
31 : Structured interviews for the Glasgow Outcome Scale and the extended Glasgow Outcome Scale: guidelines for their use. J Neurotrauma 1998; 15:573–585Crossref, Medline, Google Scholar
32 : Methodological issues and research recommendations for mild traumatic brain injury: the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehab Med 2004; 36(43 suppl):113–125Crossref, Google Scholar
33 : Correlates of persistent postconcussional disorder: DSM-IV criteria versus ICD-10. J Clin Exp Neuropsychol 2008; 30:360–379Crossref, Medline, Google Scholar
34 : An integrated review of recovery after mild traumatic brain injury (MTBI): implications for clinical management. Clin Neuropsychol 2009; 23:1368–1390Crossref, Medline, Google Scholar
35 : Systematic review of return to work after mild traumatic brain injury: results of the International Collaboration on Mild Traumatic Brain Injury Prognosis. Arch Phys Med Rehabil 2014; 95(suppl):S201–S209Crossref, Medline, Google Scholar
36 : Neuropsychiatric problems after traumatic brain injury: unraveling the silent epidemic. Psychosomatics 2009; 50:198–205Crossref, Medline, Google Scholar
37 : The DSM-5 approach to the evaluation of traumatic brain injury and its neuropsychiatric sequelae. NeuroRehabilitation 2014; 34:613–623Crossref, Medline, Google Scholar



