Anemia and Cognitive Performance in the ELSA-Brasil Cohort Baseline
Abstract
Objective:
The association between cognitive performance and hemoglobin concentration has long been a topic of debate, but few data for middle-aged persons have been explored. The authors examined the association between anemia and cognitive performance at baseline assessment in the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil), a multicenter cohort study of individuals from six Brazilian cities.
Methods:
A total of 13,624 participants (mean age=51.6 years [SD=9.0]) were included in this cross-sectional study. Cognitive performance was evaluated by using standardized scores for verbal learning, late recall, word recognition, a semantic verbal fluency test, and the Trail-Making Test, Part B (TMT-B). The association between anemia and cognitive performance was examined by using linear regression models adjusted for sociodemographic characteristics and cardiovascular risk factors.
Results:
Anemia was diagnosed in 713 (5.2%) participants. No association was found between anemia and worse cognitive performance for the main models. Global cognitive scores were similar between participants with and without anemia in adjusted models for the entire sample (β=–0.004; 95% CI=–0.052, 0.044) or for men (β=0.047; 95% CI=–0.053, 0.146) and women (β=–0.015; 95% CI=–0.070, 0.040) separately. In addition, hemoglobin levels (in quintile groups) were not associated with global cognitive scores. Similarly, no significant associations with anemia or hemoglobin levels were observed when each cognitive performance test was evaluated separately.
Conclusions:
Anemia and hemoglobin levels were not associated with worse cognitive performance in this large cohort.
Anemia and cognitive disorders are common conditions in clinical practice and often neglected. According to data from the Global Burden of Disease Study (1), both conditions are important causes of disability-adjusted life years worldwide.
Deficiency in oxygen supply to the brain may result in cognitive dysfunction, which may explain the putative association between low hemoglobin levels and cognitive performance (2). Thereby, the association between anemia and cognitive performance has long been a topic of discussion.
Some epidemiological studies have found anemia to be associated with cognitive impairment. In a cross-sectional analysis of 793 participants (mean age=81 years [SD=7.2 years]) in the Rush Memory and Aging Project, both low and high hemoglobin concentrations were associated with worse global cognition performance, analyzed with a set of 21 tests (3). Deal et al. (4) examined 374 women aged 70–80 years in the Women’s Health and Aging Study II and found that anemia was associated with poorer performance on tests of executive function at baseline and with a steeper decline in scores on memory tests during the first 3 years of follow-up (4). In 1,744 participants aged ≥71 years in the National Institute on Aging Study, anemia at baseline was associated, after 4 years of follow-up, with performance decline on the Short Portable Mental Status Questionnaire, a test measuring orientation, personal history, remote memory, and calculations (5).
However, other large studies have reported negative or mixed results. In cross-sectional data from 19,701 participants in the Renal REasons for Geographic And Racial Differences in Stroke (REGARDS) study (6), no association was found between anemia and performance on the six-item screener, a validated test of global cognitive function that includes recall and orientation items. In the Atherosclerosis Risk in Communities (ARIC) study (7), 13,133 participants with a mean age of 57 years at baseline were evaluated at two study visits (with a 6-year interval between visits). In this study, anemia was associated with lower global scores and lower performance on the digit symbol substitution test at baseline but was not associated with decline in performance during follow-up on any test or in global scores.
In the present study, we aimed to analyze the cross-sectional association between anemia and performance on cognitive tests in the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil) baseline assessment (8), a large multicenter cohort of 15,105 civil servant participants in Brazil.
Methods
Participants
The ELSA-Brasil study is described in detail elsewhere (8, 9). The study enrolled 15,105 civil servant participants from six Brazilian cities (São Paulo, Belo Horizonte, Porto Alegre, Salvador, Rio de Janeiro, and Vitória) between August 2008 and December 2010. Validated questionnaires, cognitive tests, and other clinical evaluations, as well as laboratory examinations, were used in the baseline assessment. Exclusion criteria were current or recent pregnancy, intention to discontinue working, severe cognitive or communication impairment, and, if retired, residence outside of the metropolitan area associated with the study center.
For the present study, we excluded 54 (0.4%) individuals who did not have a valid hemoglobin level determination and 1,427 (9.4%) individuals with missing information on cognitive tests. Therefore, the sample for our main analyses comprised 13,624 (90.2%) participants.
Informed consent was obtained from all study participants.
Cognitive Tests
The cognitive tests conducted in the ELSA-Brasil baseline assessment have been described previously (10, 11). We used the Brazilian version of the Consortium to Establish a Registry for Alzheimer’s Disease (12, 13), which included a three-phase word memory test. The first phase was word-list learning, in which participants were requested to read and memorize 10 words. After looking at the list of words three times, they were asked to remember and list them, with the final score obtained by calculating the sum of the words recalled in the three trials. The second phase was late recall, in which participants were asked to remember the previous list of words (as presented in the first phase) after about 5 minutes of performing other activities. The third phase was word recognition, in which a card was presented containing the 10 previous words and 10 distractor words, and the participant had to choose 10 words that made up the initial word list, with the final score obtained by calculating the number of words that were recalled correctly minus the number of words that were wrongly recalled. The second assessment involved the semantic verbal fluency test (14), in which the participant was asked to name animals within a 60-second time frame. The verbal fluency test evaluates language, semantic memory, and executive function. The last cognitive test applied was the Trail-Making Test, Part B (TMT-B) (15), a test of executive function, processing speed, concentration, and attention, in which the participants were instructed to draw lines connecting letters and numbers in ascending (alphabetical) order and alternating between them (e.g., 1–A−2–B–3). The time taken to perform the task was recorded (in seconds) as the measure of performance. For the TMT-B, higher scores indicate poorer performance.
Scores for each cognitive test are reported as crude, nonstandardized values only for the entire study sample. For our main analyses, participants were classified in 32 groups by sex, age (with 10-year intervals), and education level (<8, 8–10, 11–14, and >14 years of education completed) in a procedure similar to the one adopted in a previous study (16). Within each group, scores for each cognitive test were standardized to a mean of 0 and a standard deviation of 1. For the TMT-B, positive standardized scores indicate time taken to perform the task below the group mean. The global cognitive score equals the mean of the five standardized test scores rescaled to a mean of 0 and a standard deviation of 1.
Hemoglobin Evaluation
Blood collection was performed after a 12-hour fasting period. Except for blood cell counts, which were performed locally for technical reasons, all laboratory tests were performed at the ELSA-Brasil central laboratory in São Paulo. The blood cell counts in ELSA-Brasil were performed with automated equipment, in laboratories with internal and external quality controls (17). Anemia was defined by current World Health Organization criteria (hemoglobin concentration, <12.0 g/dl for women and <13.0 g/dl for men).
Other Study Variables
The glomerular filtration rate was estimated by using the Chronic Kidney Disease–Epidemiology Collaboration equation (18), as described previously (19). Mean monthly family income, medications that could alter cognitive function, and smoking status (never, past, or current) were self-reported. Excessive alcohol use was defined as ≥210 g of alcohol per week for men and ≥140 g per week for women. Race was self-defined in accordance with questions used in the Brazilian census. Asians and individuals of indigenous ancestry were grouped as “other,” because the number of individuals in each of these two categories was small. Body mass index (BMI) was obtained by anthropometric measures of weight and height and was expressed in kg/m2. Thyroid function was classified as normal (thyroid-stimulating hormone [TSH] levels between 0.4 and 4.0 IU/l without the use of thyroid hormone replacement or thyroid-suppressing medications), hypothyroidism (TSH levels >4.0 IU/l or use of thyroid hormone replacement), or hyperthyroidism (TSH levels <0.4 IU/l or use of thyroid-suppressing medications). Hypertension was defined as the use of medications to treat hypertension, systolic blood pressure ≥140 mmHg, or diastolic blood pressure ≥90 mmHg. Diabetes was defined as a medical history of diabetes, use of medications to treat diabetes, a fasting glucose level ≥126 mg/dl, glycated hemoglobin (HbA1C) levels ≥6.5%, or a 2-hour oral glucose tolerance test level ≥200 mg/dl. Dyslipidemia was defined as the use of lipid-lowering treatment or a low-density lipoprotein cholesterol level ≥130 mg/dl.
Statistical Analyses
Categorical variables were expressed as absolute counts and proportions and compared among groups by using chi-square tests. Continuous variables were expressed as means and standard deviations and compared by using one-way analysis of variance. In our main analyses, we built linear regression models to examine the association between age, sex, and education-standardized global cognitive scores (dependent variable) and anemia. We report crude and adjusted models. We adjusted the models for race, family income, smoking status, hypertension, diabetes, dyslipidemia, thyroid function, and excessive alcohol use (adjusted model 1). In addition, we adjusted for variables in the adjusted model 1 plus age, sex, years of formal education (to reduce residual confounding), BMI, and estimated glomerular filtration rate as well as use of antipsychotic medications, antiparkinsonian agents, or anticonvulsants (adjusted model 2). We also considered models stratified by sex and models with each cognitive test score separately as the dependent variable. To reduce the influence of residual confounding, we built post hoc models with further adjustment for age, sex, and years of formal education. We also ran sensitivity analyses excluding other potential confounders for the association between anemia and cognitive performance. In these analyses, we restricted our sample to 13,016 participants without previous stroke, with a BMI ≥18.5 kg/m2, with an estimated glomerular filtration rate ≥30 ml/minute/1.73 m2, and who did not use medications that could alter cognitive function (e.g., antipsychotic medications, antiparkinsonian agents, and anticonvulsants).
To evaluate a putative nonlinear association between hemoglobin levels and cognitive test performance, we repeated the main analyses considering hemoglobin levels according to their classification by sex-specific quintile groups in order to evaluate a possible effect of different distributions of hemoglobin levels associated with sex. In addition, we report on these analyses considering individuals with anemia as a separate group and defining hemoglobin quintiles by using the distribution within the normal hemoglobin range. Furthermore, we verified whether hemoglobin levels, as a continuous variable, were associated with cognitive performance in fully adjusted models in two steps: 1) analyzing hemoglobin levels linearly, utilizing the entire study sample and stratified by sex, and 2) further including a quadratic term for hemoglobin levels in these models. A significance level was set at a p value of 0.05. We used R software, version 3.2.0, for all analyses.
Results
Table 1 shows the study sample characteristics according to the presence of anemia. Anemia was present in 158 of 6,001 (2.6%) men and 555 of 6,910 (8.0%) women. The distribution of hemoglobin levels, for all samples and by sex, is shown in Figure S1 in the online supplement. No individuals in the sample (with valid hemoglobin level determinations and cognitive test performance scores) reported previous stroke. Participants with anemia were more prone to self-identify as black, to report low socioeconomic status, to have a lower estimated glomerular filtration rate, to be slightly younger, and to have more thyroid dysfunction compared with participants without anemia. In bivariate analyses, individuals with anemia had poorer performance on the semantic verbal test (p=0.006) and the TMT-B (p<0.001).
| Characteristic | Without anemia (N=12,911) | With anemia (N=713) | Total sample (N=13,624) | |||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| Categorical variables | ||||||
| Female | 6,910 | 53.5 | 555 | 77.8 | 7,465 | 54.8 |
| Education (years) | ||||||
| <8 | 496 | 3.8 | 25 | 3.5 | 521 | 3.8 |
| 8–10 | 739 | 5.7 | 46 | 6.5 | 785 | 5.8 |
| 11–14 | 4,412 | 34.2 | 325 | 45.6 | 4,737 | 34.8 |
| >14 | 7,264 | 56.3 | 317 | 44.5 | 7,581 | 55.6 |
| Racea | ||||||
| White | 6,964 | 54.6 | 234 | 33.1 | 7,198 | 53.4 |
| Brown | 3,552 | 27.8 | 224 | 31.7 | 3,776 | 28.0 |
| Black | 1,791 | 14.0 | 236 | 33.4 | 2,027 | 15.0 |
| Other | 455 | 3.6 | 13 | 1.8 | 468 | 3.5 |
| Monthly family income | ||||||
| <$1,245 (U.S.) | 3,089 | 24.0 | 219 | 30.8 | 3,308 | 24.4 |
| $1,245–$3,319 (U.S.) | 5,729 | 44.5 | 357 | 50.2 | 6,086 | 44.8 |
| ≥$3,320 (U.S.) | 4,052 | 31.5 | 135 | 19.0 | 4,187 | 30.8 |
| Excessive alcohol use | 975 | 7.6 | 57 | 8.0 | 1,032 | 7.6 |
| Thyroid function | ||||||
| Normal | 11,034 | 85.7 | 587 | 82.8 | 11,621 | 85.5 |
| Hypothyroidism | 1,604 | 12.5 | 99 | 14.0 | 1,703 | 12.5 |
| Hyperthyroidism | 237 | 1.8 | 23 | 3.2 | 260 | 1.9 |
| Smoking status | ||||||
| Never smoked | 7,385 | 57.2 | 433 | 60.7 | 7,818 | 57.4 |
| Past smoker | 3,850 | 29.8 | 219 | 30.7 | 4,069 | 29.9 |
| Current smoker | 1,676 | 13.0 | 61 | 8.6 | 1,737 | 12.7 |
| Hypertension | 4,387 | 34.0 | 266 | 37.3 | 4,653 | 34.2 |
| Diabetes | 2375 | 18.4 | 151 | 21.2 | 2,526 | 18.5 |
| Dyslipidemia | 7,551 | 58.5 | 319 | 44.7 | 7,870 | 57.8 |
| Antipsychotic, antiparkinsonian, or anticonvulsant drug use | 414 | 3.2 | 28 | 3.9 | 442 | 3.2 |
| Mean | SD | Mean | SD | Mean | SD | |
| Quantitative variables | ||||||
| Age (years) | 51.7 | 9.0 | 50.7 | 9.0 | 51.6 | 9.0 |
| Body mass index (kg/m2) | 27.0 | 4.7 | 26.9 | 5.2 | 27.0 | 4.7 |
| Glomerular filtration rate (mL/minute/1.73 m2) | 104.9 | 13.0 | 98.4 | 10.9 | 104.5 | 13.0 |
| Cognition tests | ||||||
| Global cognitive standardized score | 0.00 | 0.63 | –0.03 | 0.63 | 0.00 | 0.63 |
| Word list learningb | 21.29 | 3.84 | 21.53 | 3.67 | 21.30 | 3.83 |
| Late recallb | 7.03 | 1.96 | 7.11 | 1.95 | 7.04 | 1.96 |
| Word list recognitionb | 9.59 | 0.85 | 9.59 | 0.91 | 9.59 | 0.85 |
| Animal semantic fluency testc | 18.80 | 5.18 | 18.25 | 4.93 | 18.77 | 5.17 |
| Trail-Making Test, Part Bd | 121.60 | 84.49 | 134.12 | 94.34 | 122.25 | 85.08 |
TABLE 1. Demographic and clinical characteristics of the entire study sample according to the presence of anemia
Table 2 shows the beta coefficients for the association between anemia and cognitive test performance. Global cognitive scores were similar for individuals with and without anemia in crude and adjusted models. In addition, adjusted models did not show associations when performances on word list learning, late recall, word recognition, animal semantic fluency assessment, and the TMT-B were analyzed as separate dependent variables. Analyses stratified by sex led to similar findings. Additionally, exclusion of individuals with low BMI, with a low glomerular filtration rate, or using antipsychotic, antiparkinsonian, or anticonvulsant medications did not materially change the results (for further details, see Table S1 in the online supplement).
| Sample and measure | Crude model | Adjusted model 1a | Adjusted model 2b | |||
|---|---|---|---|---|---|---|
| Beta | 95% CI | Beta | 95% CI | Beta | 95% CI | |
| Total sample | ||||||
| Global | –0.029 | –0.076, 0.019 | 0.004 | –0.044, 0.052 | –0.004 | –0.052, 0.044 |
| Verbal learning | 0.027 | –0.049, 0.102 | 0.025 | –0.051, 0.102 | 0.017 | –0.060, 0.094 |
| Late recall | 0.003 | –0.073, 0.078 | 0.017 | –0.059, 0.094 | 0.010 | –0.067, 0.087 |
| Word recognition | –0.031 | –0.106, 0.044 | –0.034 | –0.111, 0.042 | –0.037 | –0.114, 0.040 |
| Animal semantic fluency test | –0.050 | –0.125, 0.026 | 0.004 | –0.073, 0.080 | –0.006 | –0.082, 0.070 |
| Trail-Making Test, Part B | –0.093 | –0.168, –0.017 | 0.008 | –0.068, 0.083 | –0.005 | –0.081, 0.070 |
| Male | ||||||
| Global | 0.011 | –0.088, 0.111 | 0.036 | –0.063, 0.135 | 0.047 | –0.053, 0.146 |
| Verbal learning | 0.089 | –0.069, 0.246 | 0.089 | –0.069, 0.247 | 0.089 | –0.070, 0.247 |
| Late recall | 0.047 | –0.111, 0.205 | 0.059 | –0.099, 0.218 | 0.057 | –0.102, 0.216 |
| Word recognition | 0.018 | –0.140, 0.176 | 0.022 | –0.137, 0.180 | 0.026 | –0.133, 0.186 |
| Animal semantic fluency test | –0.032 | –0.190, 0.126 | 0.003 | –0.155, 0.161 | 0.027 | –0.131, 0.186 |
| Trail-Making Test, Part B | –0.064 | –0.222, 0.094 | 0.008 | –0.149, 0.165 | 0.033 | –0.124, 0.190 |
| Female | ||||||
| Global | –0.041 | –0.096, 0.013 | –0.005 | –0.060, 0.050 | –0.015 | –0.070, 0.040 |
| Verbal learning | 0.009 | –0.078, 0.095 | 0.011 | –0.077, 0.100 | 0.001 | –0.087, 0.090 |
| Late recall | –0.011 | –0.097, 0.076 | 0.010 | –0.079, 0.099 | 0.001 | –0.088, 0.089 |
| Word recognition | –0.046 | –0.132, 0.040 | –0.050 | –0.139, 0.038 | –0.057 | –0.145, 0.032 |
| Animal semantic fluency test | –0.056 | –0.142, 0.031 | –0.001 | –0.089, 0.087 | –0.013 | –0.101, 0.074 |
| Trail-Making Test, Part B | –0.103 | –0.189, 0.016 | 0.007 | –0.080, 0.094 | –0.010 | –0.096, 0.076 |
TABLE 2. Beta coefficients for the association between standardized scores on cognitive tests and anemia
To evaluate a putative nonlinear association between hemoglobin levels and cognitive test performance, we stratified hemoglobin levels in sex-specific quintile groups (Figure 1; see also Table S2 in the online supplement). By using the third quintile group as the reference group, there were no significant associations between hemoglobin quintile groups and global cognitive scores, for the entire sample or for men and women separately in fully adjusted models. We also performed sensitivity analyses considering individuals with anemia as a separate group and defining hemoglobin quintiles only with individuals within the normal range (see Table S3 and Figure S2 in the online supplement), with similar results. Analyzing hemoglobin levels as a continuous variable, both linearly (total sample, p=0.47; men, p=0.087; and women, p=0.62) and after the addition of a quadratic term (total sample, p=0.30; men, p=0.52; and women, p=0.68 for the quadratic terms), did not show significant associations in fully adjusted models.
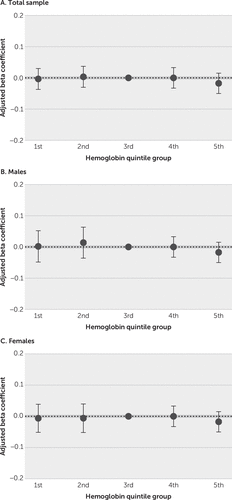
FIGURE 1. Beta coefficients (95% confidence intervals) for the association between global scores and the total sample (panel A) and sex-specific (panels B and C) hemoglobin quintiles
Discussion
In analyzing a very large sample of participants in the ELSA-Brasil baseline assessment, we did not find an association between anemia or hemoglobin levels and performance on cognitive tests. This association was not evident in our analysis of the global cognitive score or each cognition test separately, nor was there evidence for a significant association in sex-stratified analyses.
Studies analyzing the association between anemia and cognitive performance among middle-aged adults are rare in the literature. Most studies of anemia and cognitive performance have been focused on children (20, 21), older individuals (22, 23), or specific clinical scenarios (24, 25). One of the largest studies to date to address this question in a less selected sample was conducted by Schneider et al. (7). The authors evaluated 13,133 participants, with a mean age of 57 years, from the ARIC cohort and found mixed results for the association between anemia and cognitive performance. In cross-sectional analyses, participants with anemia had poorer performance on the digit symbol substitution test, a test for attention and processing speed, but not on the delayed word recall test or the word fluency test. As a result, small differences were found in the global Z-score. In addition to dissimilarities between study populations and in the set of cognitive tests applied, other differences between the Schneider et al. study and our study may help to explain why these cross-sectional results were different. First, the mean age at baseline in the ARIC study was more than 5 years older than the mean age in our sample. Second, anemia prevalence for men (6% versus 2.6%) and women (14% versus 8.0%) in the ARIC study sample was higher than that in our study sample. Both characteristics (higher mean age and anemia prevalence) may have maximized their power to show a positive association. Nonetheless, no significant associations between anemia and change in cognitive test scores were found in longitudinal analyses in the ARIC study, after a follow-up of 6 years. Although participants with more significant cognitive decline and lower levels of hemoglobin levels may be more prone to be lost during follow-up, and it is arguable that 6 years may be a small time period in which to detect significant cognitive changes, this result raises further questions as to whether a significant effect of hemoglobin on cognitive performance actually exists. In addition, Kurella Tamura et al. (6) analyzed data from 19,701 REGARDS study participants aged ≥45 (mean age=63.9 years [SD=9.7 years]). The authors found no association between hemoglobin levels and cognitive performance among both men and women. Although these investigators used a less comprehensive, telephone-based tool to assess cognitive impairment, the large sample size and the high prevalence of anemia (13.8% and 16.8% in men and women, respectively) suggest that a real association in the study population was unlikely.
Studies that have focused on other age groups have also explored an association between hemoglobin levels and cognition, with mixed results. Gashu et al. (26) examined 541 Ethiopian children aged ≤5 years. In their study sample, 13.6% of children had anemia. The authors found that children with anemia had worse performance on verbal reasoning tests (but not on nonverbal reasoning or school-readiness tests) compared with children without anemia. In a study conducted in Mexico, Kordas et al. (27) analyzed data from 602 children aged 6–8 years and found that participants with hemoglobin levels <12.4 g/L had worse performance on a number-sequencing test but not on tests focusing on vocabulary skills (Peabody Picture Vocabulary Test–Revised), arithmetic, coding and memory (Wechsler Intelligence Scale for Children–Revised Form), or letter sequencing. In another study conducted in South Africa, Beard et al. (28) followed 95 women, aged 18–30 years, up to 9 months postpartum and found that the presence of anemia 6 weeks postpartum was not associated with performance on the digit symbol test.
For older adults, a significant proportion of studies evaluating hemoglobin levels and cognition have focused on dementia diagnosis, with mixed results (29–31). Some investigators, however, have analyzed how hemoglobin levels were associated with performance on cognitive tests with older individuals. In the study by Shah et al. (3), an analysis of data from the Rush Memory and Aging Project (mean age=81.0 years [SD=7.2 years]), revealed a cross-sectional U-shaped association between hemoglobin levels and global cognitive performance. This association was more pronounced for women, for whom a hemoglobin level of 13.4 g/L predicted best cognitive performance. Deal et al. (4) analyzed cross-sectional and longitudinal data from 436 women (median age=74 years) who participated in the Women’s Health and Aging Study II. At baseline, individuals with anemia had predicted standardized scores on the TMT-B that were 0.43 points lower than scores for individuals without anemia in fully adjusted models. However, differences on memory and psychomotor speed tests were not significant at baseline. During the first 3 years of follow-up, women with anemia had a steeper decline in their performance on memory tests, whereas women without anemia had a steeper decline in executive function. This last finding, however, may be partially explained by the differences in performance at baseline.
It is important to emphasize that direct comparisons between our results in the present study and results in studies focusing on children or older adults (including those assessing dementia diagnosis) are very difficult to make. The distribution of hemoglobin levels, main causes of anemia, adequate tools to measure cognitive performance, and the mean cognitive performance within each sample vary greatly according to age group. The ELSA-Brasil sample comprised individuals with a low mean age (compared with cohorts in most studies addressing cognitive performance among adults) and who were highly educated (compared with the Brazilian population). In this scenario, ceiling effects in cognitive performance may reduce the sensitivity to detect more subtle alterations and the power to identify significant associations. In our sample, a ceiling effect occurred in the word list recognition test, in which 9,935 (72.9%) participants reached maximum performance. To a lesser extent, this also may have occurred in the late-recall test, in which 1,337 (9.8%) participants reached maximum performance. For the other cognitive tests, <0.5% of participants reached the best performance found in the sample. Although the ceiling effect may have had some influence on our negative results, we believe that the study sample was sufficiently heterogeneous to evaluate a putative association between anemia and cognitive performance.
Our study has several strengths that are noteworthy. We observed a lack of association of both anemia and hemoglobin levels with cognitive performance in a very large sample. We had enough power to detect even associations with small effect sizes. In addition, we used multiple analytical strategies, and in all scenarios, the absence of an association remained. We included a large number of middle-aged adults, an age stratum poorly explored in previous studies, for whom previous findings may not be applicable. Dementia has a long preclinical phase, with evidence of neuropathological lesions starting many years before clinically detectable symptoms (32). Compared with studies focused exclusively on older adults, analyzing samples that are similar to ours may help to disclose early preventive strategies for cognitive decline and dementia. We used multiple cognitive tests for cognition performance definition. In addition, the detailed protocol and large sample size in the ELSA-Brasil study enabled us to control for a large set of confounders.
Our study also includes several limitations. The socioeconomic status of the ELSA-Brasil sample was higher than that for the entire country of Brazil, and therefore extrapolations to the Brazilian population must be made with caution. Additionally, as discussed above, the high socioeconomic status and relatively low mean age contributed to a ceiling effect in some memory tests, which may have reduced our power to detect a significant association. It is possible that the current cutoffs for anemia are not feasible enough to identify individuals for whom hemoglobin levels influence cognition, and in our sample, most anemia cases were mild. Therefore, our findings cannot exclude that very low hemoglobin levels are not associated with poor cognitive performance. Although there were no participants with self-reported stroke in the sample, we could not rule out the presence of silent cerebrovascular disease, because neuroimaging studies were not available. The cross-sectional nature of this study precludes inferences about causation. Follow-up data from the ELSA-Brasil study may provide further insight about causality between anemia and cognition.
Conclusions
We did not find an association between anemia or hemoglobin levels and cognitive performance at the ELSA-Brasil baseline assessment. Our study extends these findings to a large, racially heterogeneous sample of middle-aged adults, not investigated in previous studies.
1 Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018; 392:1859–1922Crossref, Medline, Google Scholar
2 : Jugular bulb saturation and cognitive dysfunction after cardiopulmonary bypass. Ann Thorac Surg 1994; 58:1702–1708Crossref, Medline, Google Scholar
3 : Relation of hemoglobin to level of cognitive function in older persons. Neuroepidemiology 2009; 32:40–46Crossref, Medline, Google Scholar
4 : Anemia and 9-year domain-specific cognitive decline in community-dwelling older women: The Women’s Health and Aging Study II. J Am Geriatr Soc 2009; 57:1604–1611Crossref, Medline, Google Scholar
5 : Impact of anemia on mortality, cognition, and function in community-dwelling elderly. Am J Med 2006; 119:327–334Crossref, Medline, Google Scholar
6 : Hemoglobin concentration and cognitive impairment in the renal REasons for Geographic And Racial Differences in Stroke (REGARDS) study. J Gerontol A Biol Sci Med Sci 2010; 65:1380–1386Crossref, Medline, Google Scholar
7 : Hemoglobin, anemia, and cognitive function: the Atherosclerosis Risk in Communities study. J Gerontol A Biol Sci Med Sci 2016; 71:772–779Crossref, Medline, Google Scholar
8 : Brazilian Longitudinal Study of Adult Health (ELSA-Brasil): objectives and design. Am J Epidemiol 2012; 175:315–324Crossref, Medline, Google Scholar
9 : Cohort profile: Longitudinal Study of Adult Health (ELSA-Brasil). Int J Epidemiol 2015; 44:68–75Crossref, Medline, Google Scholar
10 : Methods of cognitive function investigation in the Longitudinal Study on Adult Health (ELSA-Brasil). Sao Paulo Med J 2014; 132:170–177Crossref, Medline, Google Scholar
11 : Subclinical carotid artery atherosclerosis and performance on cognitive tests in middle-aged adults: baseline results from the ELSA-Brasil. Atherosclerosis 2015; 243:510–515Crossref, Medline, Google Scholar
12 : The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD), part I: clinical and neuropsychological assessment of Alzheimer’s disease. Neurology 1989; 39:1159–1165Crossref, Medline, Google Scholar
13 : Performance of Brazilian population in neuropsychological battery of Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Rev Psiquiatr Clín (São Paulo) 1998; 25(2):80–83Google Scholar
14 : Differential verbal fluency deficits in the preclinical stages of Alzheimer’s disease and vascular dementia. Cortex 2006; 42:347–355Crossref, Medline, Google Scholar
15 : Application of the Trail Making Test in differentiating neuropsychological impairment of elderly persons. Percept Mot Skills 1985; 61:1283–1289Crossref, Medline, Google Scholar
16 : Education plays a greater role than age in cognitive test performance among participants of the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). BMC Neurol 2015; 15:191Crossref, Medline, Google Scholar
17 : Logistics of collection and transportation of biological samples and the organization of the central laboratory in the ELSA-Brasil. Rev Saude Publica 2013; 47(Suppl 2):63–71Crossref, Medline, Google Scholar
18 : A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150:604–612Crossref, Medline, Google Scholar
19 : Chronic kidney disease among adult participants of the ELSA-Brasil cohort: association with race and socioeconomic position. J Epidemiol Community Health 2016; 70:380–389Crossref, Medline, Google Scholar
20 : Iron deficiency anemia and cognitive function in infancy. Pediatrics 2010; 126:e427–e434Crossref, Medline, Google Scholar
21 : Iron-deficiency anemia in infancy and poorer cognitive inhibitory control at age 10 years. Dev Med Child Neurol 2013; 55:453–458Crossref, Medline, Google Scholar
22 : Albumin, hemoglobin, and the trajectory of cognitive function in community-dwelling older Japanese: a 13-year longitudinal study. J Prev Alzheimers Dis 2017; 4:93–99Medline, Google Scholar
23 : Low hemoglobin levels and the onset of cognitive impairment in older people: the PRO.V.A. Study. Rejuvenation Res 2016; 19:447–455Crossref, Medline, Google Scholar
24 : Anemia and cognitive performance in hospitalized older patients: results from the GIFA study. Int J Geriatr Psychiatry 2006; 21:529–534Crossref, Medline, Google Scholar
25 : Anemia and risk for cognitive decline in chronic kidney disease. BMC Nephrol 2016; 17:13Crossref, Medline, Google Scholar
26 : Stunting, selenium deficiency and anemia are associated with poor cognitive performance in preschool children from rural Ethiopia. Nutr J 2016; 15:38Crossref, Medline, Google Scholar
27 : Blood lead, anemia, and short stature are independently associated with cognitive performance in Mexican school children. J Nutr 2004; 134:363–371Crossref, Medline, Google Scholar
28 : Maternal iron deficiency anemia affects postpartum emotions and cognition. J Nutr 2005; 135:267–272Crossref, Medline, Google Scholar
29 : Risk of Alzheimer’s disease among elderly patients with anemia: population-based investigations in Olmsted County, Minnesota. Ann Epidemiol 1997; 7:219–224Crossref, Medline, Google Scholar
30 : Anemia and dementia among the elderly: the São Paulo Ageing & Health Study. Int Psychogeriatr 2012; 24:74–81Crossref, Medline, Google Scholar
31 : Anaemia increases the risk of dementia in cognitively intact elderly. Neurobiol Aging 2006; 27:278–284Crossref, Medline, Google Scholar
32 : The evolution of preclinical Alzheimer’s disease: implications for prevention trials. Neuron 2014; 84:608–622Crossref, Medline, Google Scholar



