Outcome Measures for Functional Neurological Disorder: A Review of the Theoretical Complexities
Abstract
The development and selection of optimal outcome measures is increasingly recognized as a key component of evidence-based medicine, particularly the need for the development of a standardized set of measures for use in clinical trials. This process is particularly complex for functional neurological disorder (FND) for several reasons. FND can present with a wide range of symptoms that resemble the full spectrum of other neurological disorders. Additional physical (e.g., pain, fatigue) and psychological (e.g., depression, anxiety) symptoms are commonly associated with FND, which also can be highly disabling with implications for prognosis, and warrant concurrent assessment, despite an unclear etiological relationship with FND. Furthermore, several unique clinical aspects of FND make it likely that the usual prioritization of “objective” (or clinician-rated) over “subjective” (or patient-rated) measures might not be appropriate. Self-report measures may be more clinically meaningful in this patient population. Despite being a common and disabling disorder, there has been little research into outcome measures in FND, and to date trials have largely used measures designed for the assessment of other disorders. An international FND Core Outcome Measure group (FND-COM) has been established to develop a consensus battery of outcomes for FND: a “core outcome set.” In this perspective article, the authors reviewed the process of outcome measure development and selection before considering the specific features of FND affecting the development of a core outcome set, as well as a research agenda to optimize outcome measurement in this complex neuropsychiatric disorder.
Despite being a common and disabling condition, functional neurological disorder (FND), also known as conversion disorder, has been relatively neglected by the research community (1). Until recently, there were few clinical trials of treatment, and pathophysiological and etiological investigations were limited (2), even though FND is the second most common reason for a new outpatient referral to neurology (3) and individuals with FND have high rates of disability and consequent health care utilization (4).
Fortunately, this situation has started to change (5). Over the past decade, patient groups and an international society, the Functional Neurological Disorder Society (FNDS) (6), have been established—two landmark developments in the history of the disorder. There has also been increased research attention and funding, resulting in accumulating evidence for the efficacy of specific interventions for FND. In particular, research has documented the effectiveness of cognitive-behavioral therapy (CBT)—both conventional and CBT-informed psychotherapy—for the seizure variant of FND, also known as dissociative or psychogenic nonepileptic seizures (PNES) (7, 8), and specialized intensive outpatient physiotherapy for patients with FND who have movement disorders or limb weakness (motor symptoms) (9). The first large-scale multicenter treatment trial for any FND symptom, which is evaluating conventional CBT for dissociative seizures, will be reporting results shortly (10), and a similarly sized trial of intensive outpatient physiotherapy for motor symptoms is under way (11).
These and other planned trials have highlighted the lack of specific outcome measures for FND and the urgent need for the development of a consensus on which outcome measures are optimal for FND. This is particularly important to allow specific treatment modalities to be studied across countries, cultures, and health care settings, where clinical presentations, resources, and values regarding aspects of recovery may vary, as well as to facilitate the eventual pooling of data for meta-analyses. Furthermore, specialized FND services are emerging in many countries and cultural contexts, which will increasingly be expected to formally monitor patient progress and demonstrate cost-effectiveness.
This need for FND research arises against the background of a general recognition across medical, neurological, psychiatric, and psychological specialties of the importance of research into outcome measures, particularly with regard to the development and application of a core outcome set or battery of outcome measures that are disorder appropriate. A core outcome set establishes a minimum standardized list of data points, allowing results to be compared, contrasted, and collated across trials. This standardization is being driven by international collaborations, such as the COMET (Core Outcome Measures in Effectiveness Trials) initiative in Europe, which provides development guidance and a searchable database for completed core outcome sets (12–14). In the United States, a similar initiative is focusing on the development of a common data element (CDE) resource portal, coordinated by the National Institute of Neurological Disorders and Stroke (NINDS) (15), addressing a variety of conditions, such as epilepsy and traumatic brain injury.
Although these initiatives have included work on some neurological conditions, there has been no specific focus on FND, which led to the establishment of the FND-COM (FND Core Outcome Measure) group within the COMET framework (16). The FND-COM group now has 45 members from 13 countries and includes the full range of relevant clinical and academic disciplines, as well as patient group representation. Following two international consensus meetings, it became clear that there are several challenges in the development and selection of outcome measures for FND that are specific to this disorder, over and above the usual tasks inherent in the development of an outcome measure set.
This perspective article reviews key concepts in outcome measure design and selection for any disorder, such as choosing what to measure and how to measure it. Regarding what to measure, the core symptoms of the disorder itself are clearly important, but it is also possible that associated symptoms, such as other physical symptoms (e.g., pain and fatigue) or psychological symptoms (e.g., depression and anxiety), may be of particular significance. However, these associated symptoms are not a focus of this article, because they are the subject of other ongoing projects. We illustrate the key features of FND that are pertinent to the design and selection of outcome measures and detail the specific challenges and complexities that FND poses, focusing on adults. Outcome measure options from related disorders are then discussed as examples that could inform approaches and choices in FND. We conclude with recommendations for how we might move forward as a clinical and scientific community in the development of a core outcome set for FND.
Key Concepts In Outcome Measure Design And Selection
When designing or selecting outcomes measures, it is important to initially consider what the optimal features of a measure might be for all disorders, and these have been variously defined—for example, by the COMET initiative (12). It is critical that measures have high content validity (including face validity)—that is, that they are representative of the disorder—to patients as well as to other key stakeholders, such as caregivers, relevant health care professionals, health care commissioners, and funders. Other key features are that measures should be reliable (have low measurement error), replicable over time and between raters, responsive (sensitive to change), culturally sensitive and relevant, and internally consistent (items are interrelated). Finally, they should be practical and easy to administer to minimize patient and clinician burden and to keep costs low.
It is then useful to separate this task into two key decisions: what to measure and how best to measure it. Although both aspects may differ significantly between disorders, there are key principles that apply to all.
What to Measure
Symptoms of the disorder are usually the most intuitive aspect (domain) of disorders to measure; however, several other domains may be as or more meaningful. At the mechanistic level, biomarkers of disorders, if known, can be measured—e.g., acetylcholine receptor antibody levels in myasthenia gravis. Many “downstream” effects can also be measured in terms of how the disorder affects physical function (e.g., ability to do daily tasks), social function, and quality of life.
Since the inception of the biopsychosocial approach, popularized by Engel’s classic paper in 1977 (17), there have been several attempts to classify outcomes in this manner. Wilson and Cleary’s “taxonomy” (18) identified five domains: biological/physiological, symptom, function, health perception, and quality of life. The World Health Organization (WHO) has developed the International Classification of Functioning, Disability and Health, which is known as the ICF, to highlight its intended focus on function (rather than disability) (19). The ICF describes three domains similar to those of Wilson and Cleary: body function/structure (corresponding to biological/physiological), impairment (corresponding to symptoms), and activity limitation (corresponding to function). It also describes the additional domain of participation restriction, referring to the inability to be involved in life situations—for which both neurological and psychiatric examples are helpfully given (20). Paralysis associated with spinal injury can limit activities, such as using public transportation, and thus participation in religious activities. Panic disorder can limit activities, such as being able to go out and thus to participate in social relationships, as a result of symptoms themselves and also to people’s reactions to them—thereby accounting for more complex social factors and interactions.
The COMET initiative recently reviewed existing outcome measure systems, with a focus on use in clinical trials (21), and proposed a new taxonomy with five domains, two of which overlap with the other classification systems (Wilson and Cleary’s taxonomy and the WHO ICF). The first is physiological or clinical, which is a combination of the first two domains of the other classifications and is subdivided into 23 categories of physiological or anatomical systems (cardiac, endocrine, neurological, psychiatric, etc.). The second is life impact, which is equivalent to the function, quality-of-life, and health perception domains of Wilson and Cleary’s taxonomy but also includes other concepts, such as patient adherence and satisfaction. Three other domains are not included in the other classifications. These are resource use, adverse events, and mortality, which are often measured in clinical trials. A summary of how these different outcome domain taxonomies map onto each other in terms of terminology and concepts is presented in Table 1.
| Wilson and Cleary (18) | WHO ICF (20) | COMET (21) | Concepts |
|---|---|---|---|
| Biological/physiological | Body function/structure | Physiological or clinical | Physiological/biological |
| Symptom | Impairment | Physical, psychological, and other symptoms | |
| Function | Activity limitation | Life impact | Physical, psychological, and social impacts |
| Quality of life | — | Summary measure of quality of life | |
| Health perception | — | Subjective rating of general health | |
| — | Participation restriction | — | Involvement in life situations |
| — | — | Resource use | Health/social costs |
| — | — | Adverse events | Treatment side effects |
| — | — | Mortality | Death |
TABLE 1. Comparison of outcome domain classificationsa
In the past few decades, interest has also been increasing in patient-generated outcome measures, also known as individualized or idiographic outcome measures, as opposed to the traditional generalized or “normothetic” measures validated against population norms. Such individualized measures might better capture issues associated with personal relationships and employment difficulties and as such can be seen as an alternative or complementary type of measure (22). Examples used across disorders include the Canadian Occupational Performance Measure (23) and the Psychological Outcome Profiles measure (24). An individualized measure has also been developed specifically for assessing physiotherapy in Parkinson’s disease—the Patient Specific Index for Physiotherapy in Parkinson’s Disease (PSI-PD) (25), which has been used in a large trial (26).
How to Measure
There are often many different ways to measure the same outcome. For example, grip strength can be measured by using a variety of methods and from several perspectives that are of variable objectivity: an instrument measuring grip force (a dynamometer), routine neurological examination (clinician grading of power), impression of strength on observing function (clinician or caregiver rated), or patient self-report of his or her strength (by impression or activities). Similarly, seizure frequency can theoretically be measured either objectively, usually during continuous EEG and video monitoring (telemetry), or potentially by other physiological monitoring (e.g., wearable devices). Seizure frequency can also be measured subjectively, by patient recall of seizure episodes (e.g., a seizure log).
Objective measures are usually considered more reliable (having lower measurement error) and are thus generally prioritized in clinical trials. Objective measures are usually measured by clinicians, but it is important to clarify that the converse does not necessarily apply, in that some clinician-rated measurements are not truly objective—for example, the clinician grading of strength during neurological examination has a degree of subjectivity to the grade allocation and has corresponding poor interrater reliability (27), as does grading based on observation rather than clinical examination. Some measurements are inherently subjective, such as quality of life, but it can be argued that such measures may be at least as important as objective data points, because they may be more meaningful to patients and may correlate more closely with other important factors, such as socioeconomic outcomes.
Key Features Of FND Influencing Outcome Measurement
Several features of FND are particularly important in guiding outcome measure design and selection. We review how a diagnosis of FND is made before focusing on the wide variety of presenting symptoms and how specific “positive clinical signs” not only allow its differentiation from other disorders but also explain high rates of symptom variability, why patients’ reports of symptoms can differ from those of clinicians, and the implications of these features.
Diagnostic Features
Key developments occurred in the update from the DSM-IV to the current DSM-5 diagnostic criteria for FND (28). A major advance is that the diagnosis is now a “rule in” diagnosis, based on the requirement that the neurological symptoms are associated with “clinical evidence of incompatibility between symptoms and recognized neurological or medical conditions” (29), by using clinical examination signs (30) that allow FND to be positively differentiated from other disorders, rather than defined by the absence of another condition, and that have high rates of interrater reliability (31). Another key change introduced in DSM-5 was moving the requirement of identifying preceding psychological stressors to a clinical note for the condition, such that this feature is still retained as a specifier. The recently published ICD-11 criteria have endorsed similar changes (32). Box 1 lists the DSM-5 diagnostic criteria for FND.
BOX 1. DSM-5 Criteria for Functional Neurological Symptom Disorder (28)a
A. One or more symptoms of altered voluntary motor or sensory function.
B. Clinical findings provide evidence of incompatibility between the symptom and recognized neurological or medical conditions.
C. The symptom or deficit is not better explained by another medical or mental disorder.
D. The symptom or deficit causes clinically significant distress or impairment in social, occupational, or other important areas of functioning or warrants medical evaluation.
Specify symptom type:
With weakness or paralysis
With abnormal movement (e.g., tremor, dystonic movement, myoclonus, gait disorder)
With swallowing symptoms
With speech symptom (e.g., dysphonia, slurred speech)
With attacks or seizures
With anesthesia or sensory loss
With special sensory symptom (e.g., visual, olfactory, or hearing disturbance)
With mixed symptoms
Specify if:
Acute episode: Symptoms present for less than 6 months.
Persistent: Symptoms occurring for 6 months or more.
Specify if:
With psychological stressor(specify stressor)
Without psychological stressor
a Copyright © 2013, American Psychiatric Association. Reprinted with permission.
There are positive clinical signs for the various symptom presentations, each with varying degrees of usefulness. Hoover’s sign of functional leg weakness is a good example of a clinically useful sign with good reliability and specificity (31) and describes hip extension weakness that normalizes when it is triggered (automatically) by contralateral hip flexion. A functional tremor sign is elicited in response to an externally cued rhythmic movement, which may lead to tremor entrainment or cessation. Patterns of PNES can also be differentiated from epileptic seizures on the basis of semiological features (33, 34). Functional sensory deficits are typically of a non-neuroanatomic pattern that cannot be explained by anything other than FND (e.g., a tubular visual field that is incongruous with the laws of optics). Crucially, the presence of these signs allows FND to be diagnosed, even in the presence of another neurological condition, such as multiple sclerosis or epilepsy. These positive clinical signs are dealt with in more detail below, because they and other etiological factors have particular implications for outcome measure selection and development.
Core Symptom Heterogeneity
As detailed in Box 1, there are symptom specifiers covering the “core” symptom types, including seizures (also referred to as attacks), weakness or paralysis, other movement disorders (e.g., tremor, dystonia, gait abnormalities, and myoclonus), and sensory dysfunction (including somatosensory, visual, and auditory impairment). ICD-11 has similar symptom subtypes, and the most notable difference is the inclusion of a cognitive symptom subtype that is not part of the DSM-5 criteria (35, 36). Many patients with FND have several core symptoms, either simultaneously or over time, for which the “mixed” category in DSM-5 is appropriate. As such, FND can present with a wide variety of neurological symptoms in many different combinations (37) that are likely to make varying contributions to loss of function, disability, and reduced quality of life. It is also hypothesized that symptom replacement (or substitution) can occur, in that the resolution of one symptom can be followed by the appearance of another, although there is not convincing data to support this occurrence (38).
Such symptom heterogeneity raises the question of what symptoms, or combination of symptoms, should be measured in a given individual, as well as in groups, for both research studies and clinical practice. In answering these questions, it would be important to consider which symptoms are most troubling for the patient; if multiple symptoms are to be measured, whether it is possible to create a weighted global score for comparison between individuals and groups; and whether it is possible to create a valid single measure of FND symptom severity.
Physical and Psychological Comorbidities
Beyond these core symptoms, many other physical and psychological symptoms are commonly reported in FND that have been shown to negatively affect health-related quality of life (39, 40) and clinical outcomes (41, 42). These symptoms include pain, fatigue, sleep disturbance, poor concentration, bladder and bowel dysfunction, and psychological symptoms, such as depression, anxiety, panic, dissociative phenomena (e.g., depersonalization), and posttraumatic stress symptoms (43, 44). These concurrent symptoms can be and often are, among the most severe and disabling symptoms reported by patients—sometimes more so than the presenting FND symptom itself. For example, in one study of functional movement disorder, patients’ pain, anxiety, and cognitive symptoms (45) correlated strongly with quality of life in a way that the movement disorder symptoms did not, and in another study fatigue and depression were similarly strongly correlated with quality of life (46). Similarly, for adults with PNES, depression and a range of other characteristics, such as dissociation, escape-avoidance coping, and aspects of family dysfunction, were important factors associated with quality of life, although this may at least in part have been due to the use of particular quality-of-life scales with items that ask about anxiety, depression, and pain (39).
It is unclear whether comorbid symptoms are an intrinsic part of the disorder or simply common comorbidities. In the case of depression and anxiety, they can clearly be a secondary consequence of having the disorder, a predisposing vulnerability, or both. Posttraumatic stress disorder (PTSD) potentially shares common pathophysiological elements with FND, given that adverse life events are a shared risk factor (47). The same could also apply to many other symptoms experienced by patients with FND, such as dissociative symptoms, due to multiple overlapping risk factors, and reflect different disorder subtypes. Therefore, it may be helpful to consider symptoms as occurring in core FND, other physical, and psychological domains (Figure 1).
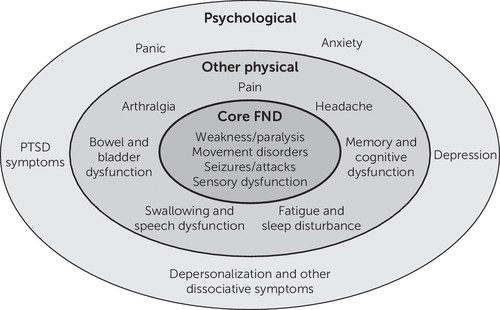
FIGURE 1. Schematic diagram of symptom domains in functional neurological disorder (FND)
Such a wide range of core symptoms and comorbidities clearly presents challenges in choosing which aspects of the disorder to focus on for measuring outcomes. It is also a reminder of the importance of consulting patients individually and as a group about which symptoms are of most importance to them when selecting the outcomes collected, including the choice of a primary outcome measure for trials. Therefore, measurement of the severity of the core symptom on which the diagnosis is based may not always best reflect the most important or meaningful change for a patient (48).
Variability of Symptoms and Signs
The clinical signs described above illustrate how clinical features can be highly variable in FND in that there can be an instantaneous improvement, albeit briefly, with a clinical maneuver. It is sometimes helpful to explain this observed feature to patients, demonstrated on the FND examination. Contemporary theories for why these signs occur in FND propose that they arise from an abnormality in the way that the brain predicts motor and sensory activity, mediated in large part by dysfunctional attention to bodily symptoms (49, 50). Such models also integrate dysfunction of interoception, self-agency, and motor control into their mechanistic account of FND (2, 51), as well as aberrant stress response (52) and emotion processing (53).
As such, high levels of symptom variability are a hallmark of the disorder. This feature has important implications for outcome measurement. For example, when is the ideal time to measure function? That is, should leg weakness or tremor be measured during voluntary movement, when it could be highly impaired, or when the patient is distracted, when it could potentially normalize? A related issue is the variability of symptoms from one day to the next, and sometimes over shorter time periods, in people with FND, which has implications for time frames for assessing symptoms. “In the moment” questions may not, therefore, accurately reflect the general (overall) state of the disorder, as might assessments covering short time periods, such as the last few hours or days.
Thus it is more likely that assessments based over the last week (or longer unit of time) might give a more stable and reliable index of change, despite potential trade-offs, such as increased recall bias. This temporal variability is a particularly important issue for objective snapshot tests of motor symptoms, such as power (e.g., hand dynamometer), gait speed (e.g., 10-meter walk test), and endurance (e.g., 2-minute walk test). It is also noted that attention (54), and therefore physical examination, tends to exacerbate such symptoms, so that routine clinical assessment may not accurately reflect the burden of a given symptom outside the context of the examination. There is also very wide variation in the severity of symptoms experienced in FND, ranging from patients with mild transient symptoms to among the most disabled and distressed patients seen in medicine, which is likely to produce floor and ceiling effects in symptom measurement.
Differences Between Patient-Rated and Objective Symptom Measures
There can also be marked discrepancies between an FND patient’s perception of his or her own symptom or function and that observed by clinicians or objectively measured. The most compelling evidence of this comes from a study comparing objectively measured tremor using an actigraphy watch with patient self-report among FND patients, compared with patients with tremor as a result of Parkinson’s disease, essential tremor, and dystonic tremors (54). Patients with FND reported tremor 84% of the time, but the actigraphy watch recorded tremor for only 4% of the day—a nearly 20-fold overestimation. Patients in the neurological control group estimated that they had a tremor 58% of the time, just over twice the amount that they objectively had a tremor. Thus, although both groups significantly overestimated their tremor, it was an order of magnitude greater among FND patients. Importantly, the disability reported by people with functional tremor in this study was greater than that reported by the other patient cohorts.
In support of this difference, a study of PNES patients found that they catastrophized their experiences, whereas those with epilepsy normalized their experiences (55). However, a recent similar study found less clear-cut differences between objective tremor in FND patients and organic controls (56). Ultimately, it is possible that patients’ own reports are as clinically meaningful as objectively rated measures, which, depending on how they are performed, might conclude that a leg is not weak or that a tremor is not frequently present. Further research is needed to investigate the relationship between these different measures and their associations with other key outcomes, such as function, quality of life, and socioeconomic outcomes.
Other Relevant Features
Qualitative research has also highlighted a number of common perceptions and experiences among patients with FND that contribute to the burden of illness and impaired quality of life (57–60). Capturing the distress associated with these experiences may be important to accurately measure the benefit of appropriate treatment. Relevant qualitative findings include a lack of understanding of one’s symptoms (and related illness beliefs), which leads the patient to feel powerless and to fear a future with deteriorating health; a perceived or experienced lack of appropriate support, which leaves many patients feeling abandoned and isolated; an associated perception or experience that the diagnosis does not allow the patient access to appropriate levels of social support, such as financial benefits and allowances for disability; and the potential importance of certain patient resources, such as patients’ level of self-compassion and mastery, which has been described by both patients and therapists. Domains of measurement that can capture these important issues may include understanding, symptom threat, perceived support, and self-efficacy, as well as patient satisfaction.
Examples From Related Disorders
When considering outcome measure design and selection in FND, it is potentially helpful to look at what has been designed and used in related disorders, although it is not clear which disorders are the best models for FND. Given that FND is the paradigmatic functional disorder at the interface of medicine, neurology, psychiatry, and psychology, a case for model disorders from these specialties can be made. There is also a clear rationale for considering other functional disorders, given the often significant overlap in clinical features between these disorders and FND.
Regarding neurological disorders, it may be beneficial to consider those that present with a wide range of neuropsychiatric and psychological symptoms similar to that seen in FND. Examples include multiple sclerosis and Parkinson’s disease, which, despite the historical focus on motor function, are recognized to have many nonmotor symptoms, including psychiatric and psychological symptoms (e.g., depression, cognitive symptoms, among many others). An extensive scale has been developed for Parkinson’s disease, the latest version of which is the Movement Disorder Society revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) (61). The International Consortium for Health Outcomes Measurement (ICHOM) has published a consensus outcome set for use in clinical practice (62), recommending seven domains: motor functioning, nonmotor functioning, cognitive and psychiatric functioning, falls, hospital admissions, ability to work, and health-related quality of life. The PSI-PD mentioned above (25) is also potentially an informative individualized approach for FND.
Huntington’s disease is another potential model and has a similar validated outcome measure, the Unified Huntington’s Disease Rating Scale (HDRS) (63); however, an outcome set has not yet been developed for Huntington’s disease. Both the MDS-UPDRS and the HDRS are recommended in the NINDS CDE initiative. For some subtypes of FND, there is an obvious neurological disorder to consider, such as epilepsy for PNES. Although no consensus outcome set for epilepsy in general has been published, one for childhood epilepsy has been published (64). There have also been studies in pregnant women with epilepsy (65), as well as a NINDS CDE publication (66), and research into the views of people with epilepsy and caregivers (67). Most other FND subtypes do not have a single disorder that is of particular relevance.
In terms of potentially relevant mental disorders, PTSD has a broad range of symptoms, including negative affect and dissociation that can overlap to some degree with FND. A range of outcome measures have been developed for PTSD, the latest and most comprehensive of which is the Clinician-Administered PTSD Scale (68), but there is not yet an agreed outcome set. Panic disorder has some clinical, phenomenological, and potentially mechanistic overlap with FND, especially given that some dissociative seizure cases have been compared with a state of “panic without panic” (69), and it is not uncommon for panic attacks to include focal neurological symptoms (70). There is no consensus outcome set for panic disorder, but a joint ICHOM consensus outcome set has been developed for anxiety and depression (71) that is potentially of some relevance to aspects of FND.
Although parallels to FND may not be immediately obvious, obsessive-compulsive disorder (OCD) is informative, because its key outcome measure, the Yale-Brown Obsessive Compulsive Scale (YBOCS) (72), has several different components that might be applicable to FND. The YBOCS has a checklist for past and current symptoms, as well as a current score for all obsessive symptoms and all compulsive symptoms measured over several domains (frequency of symptom, interference with social or occupational function, distress, ability to resist, and subjective feeling of control).
In related functional disorders, a European group has recently published recommendations for somatic symptom disorder, bodily distress disorder, and functional somatic syndromes (73), recommending six outcome domains: classification of disorder and comorbid mental problems, somatic symptoms (self-rated symptom intensity and interference with daily activities, both scored 0–10), psychobehavioral features (e.g., depression and anxiety), illness consequences (quality of life, disability, and health care use), patient satisfaction, and unwanted negative effects. Good progress has also been made in fibromyalgia, with the publication of a consensus core domain set (74) and a preliminary core outcome set for research (75). There has also been some progress in developing consensus outcome domains for pain treatments in general (76) and an outcome set for chronic pain (77).
It is noteworthy that patient-reported measures are prominent in the above core outcome sets and are the primary outcome for a number of neurology and psychiatry clinical trials (e.g., headache, depression, anxiety, and PTSD), underscoring the utility of self-reported symptom surveys. In conclusion, there has been variable progress in other disorders of potential relevance with respect to informing outcome measure design and selection for FND, and there are many example disorders to consider, but no single disorder stands out as uniquely relevant. Therefore, it will be of use to continue to monitor the development of outcome measure sets in most of these disorders.
Conclusions And Future Work
FND poses particular questions and challenges to the development and selection of optimal outcome measures. FND has many features that potentially affect this process. First, the wide variety of core and associated symptoms means that it should not be assumed that a single core (primary) FND symptom is the most meaningful aspect of the disorder to measure. As such, it might also be important to measure other concurrently present FND symptoms, as well as other physical and psychological symptoms (Box 2).
BOX 2. Summary Points and Recommendations
1. The range of core symptoms of functional neurological disorder (FND) and the high levels of associated other physical and psychological symptoms complicate the choice of the most important symptoms to measure. Consequently, a measure of both the primary core symptom and a general measure of all FND symptoms should be obtained, along with measures of key other physical and psychological symptoms (see Figure 1), when practicable.
2. Temporal variability in symptoms means that “snapshot” (momentary or “state”) assessments are likely to be less meaningful than assessments reflecting symptom severity over longer periods (days or weeks).
3. The discrepancy between patient report and objective measures, relating to a diagnostic feature of FND, challenges the usual preference for objective measures. A complementary set of self-reported (subjective) and objective (e.g., performance-based or some clinician-rated) measurements may provide a particularly robust core outcome measure set for this population and the basis of research on the relative benefits of each.
4. There is a need to develop an outcome measure set for FND, including research into:
a. Existing outcome measures that have been used in, or are available for use in, FND trials and clinical practice. Considering the need for the development of an FND-specific scale.
b. Personalized outcome measures that might be suitable for FND.
c. Stakeholder views on outcome measures, specifically patients, caregivers, and relevant health care professionals.
d. How patients’ culture and age and other patient characteristics might affect optimal outcome measurement.
Secondly, symptom variability across FND semiologies suggests that momentary assessment might be less reliable than longer-term assessments, particularly in cases of paroxysmal or intermittent FND. Finally, the nature of FND means that subjective report measures may be potentially as clinically meaningful as objective outcome measures. The U.S. Food and Drug Administration has also raised the profile and importance of patient-rated outcome measures in its guidance on medicinal product development (78). However, there is a clear need to continue to study the relationship between subjective and objective outcomes measures in this patient group. In the interim, a complementary set of objective (clinician-rated, performance-based) and patient-rated measures may collectively provide an adequate representation of symptom burden and associated disability.
To develop an optimal outcome set, it will be important to formally assess the views of the full spectrum of stakeholders about these issues, notably patients and caregivers, as well as all the relevant clinicians who treat the disorder, particularly neurologists, psychiatrists, psychologists, psychotherapists, physiotherapists, occupational therapists, speech and language therapists, social workers, nurses, and other allied clinicians, such as family (primary care) physicians. Such work is currently being undertaken by the FND-COM group, in the form of a qualitative interview–based study exploring the views of patients, caregivers and experienced health care professionals on outcomes, recovery, and outcome measurement in FND.
A systematic review of outcome measures in FND is under way to define outcome measures that have been developed for FND, as well as to identify which outcome measures have been selected most commonly for use in clinical trials to date. Such work will inform the development of initial consensus recommendations and eventually a formal core outcome set according to COMET guidelines (79). The potential need for the development of new outcome measures for FND should be considered, as well as the possibility of combining existing outcome measures to delineate a suggested battery of instruments. Work is also needed to study whether different outcome measures might be preferable in particular FND subgroups—for example, in children or in those with particular comorbidities, such as trauma-related conditions or learning disabilities. It is also possible that different cultures might benefit from different optimal outcome measures. Finally, it is important to note that the most useful outcome measures for research may differ from those most useful for clinical practice, where there may be less concern for measurement psychometrics and greater focus on what is important for patients and health care commissioners. For example, patients might be more concerned with performance of activities of daily living (such as walking and social activities) and commissioners more concerned with cost savings and employment status.
Medical science often searches for a singular diagnostic and treatment approach to disorders. For FND, a neuropsychiatric disorder with highly variable semiological manifestations, multiple comorbidities, and associated psychosocial elements, a single simple outcome measure may not be optimal. A concise battery of measures, both clinician scored and self-reported, monitoring physical, psychological, and social aspects of the disorder and ideally allowing comparison with other disorders, may be an approach to most meaningfully assess and monitor the whole person with FND.
1 : Current concepts in diagnosis and treatment of functional neurological disorders. JAMA Neurol 2018; 75:1132–1141Crossref, Medline, Google Scholar
2 : Functional neuroanatomy and neurophysiology of functional neurological disorders (conversion disorder). J Neuropsychiatry Clin Neurosci 2016; 28:168–190Link, Google Scholar
3 : Who is referred to neurology clinics? The diagnoses made in 3781 new patients. Clin Neurol Neurosurg 2010; 112:747–751Crossref, Medline, Google Scholar
4 : Disability, distress and unemployment in neurology outpatients with symptoms “unexplained by organic disease”. J Neurol Neurosurg Psychiatry 2011; 82:810–813Crossref, Medline, Google Scholar
5 : Functional (conversion) neurological symptoms: research since the millennium. J Neurol Neurosurg Psychiatry 2012; 83:842–850Crossref, Medline, Google Scholar
6 Functional Neurological Disorder Society. Milwaukee, Functional Neurological Disorder Society, 2019. www.fndsociety.orgGoogle Scholar
7 : Cognitive-behavioral therapy for psychogenic nonepileptic seizures: a pilot RCT. Neurology 2010; 74:1986–1994Crossref, Medline, Google Scholar
8 : Multicenter pilot treatment trial for psychogenic nonepileptic seizures: a randomized clinical trial. JAMA Psychiatry 2014; 71:997–1005Crossref, Medline, Google Scholar
9 : Randomised feasibility study of physiotherapy for patients with functional motor symptoms. J Neurol Neurosurg Psychiatry 2017; 88:484–490Crossref, Medline, Google Scholar
10 : COgnitive behavioural therapy vs standardised medical care for adults with Dissociative non-Epileptic Seizures (CODES): a multicentre randomised controlled trial protocol. BMC Neurol 2015; 15:98Crossref, Medline, Google Scholar
11 Specialist Physiotherapy for Functional Motor Disorder. (ISRCTN Registry trial no ISRCTN56136713). London, ISRCTN Registry, 2018Google Scholar
12 : How to select outcome measurement instruments for outcomes included in a “Core Outcome Set”: a practical guideline. Trials 2016; 17:449Crossref, Medline, Google Scholar
13 : The COMET Handbook: version 1.0. Trials 2017; 18(suppl 3):280Crossref, Medline, Google Scholar
14 COMET Database. Rožnov pod Radhoštěm, Czech Republic, COMET System, SRO, 2019Google Scholar
15 NINDS Common Data Elements. Bethesda, Md, National Institute of Neurological Disorders and Stroke, 2019. https://commondataelements.ninds.nih.gov/Google Scholar
16 FND-COM (Functional Neurological Disorder—Core Outcome Measures) group. Liverpool, United Kingdom, COMET Initiative, 2017. http://www.comet-initiative.org/studies/details/951?result=trueGoogle Scholar
17 : The need for a new medical model: a challenge for biomedicine. Science 1977; 196:129–136Crossref, Medline, Google Scholar
18 : Linking clinical variables with health-related quality of life: a conceptual model of patient outcomes. JAMA 1995; 273:59–65Crossref, Medline, Google Scholar
19 : The International Classification of Functioning, Disability and Health and its application to cognitive disorders. Disabil Rehabil 2004; 26:235–245Crossref, Medline, Google Scholar
20 Towards a Common Language for Functioning, Disability and Health: ICF. Geneva, World Health Organization, 2002. https://www.who.int/classifications/icf/icfbeginnersguide.pdfGoogle Scholar
21 : A taxonomy has been developed for outcomes in medical research to help improve knowledge discovery. J Clin Epidemiol 2018; 96:84–92Crossref, Medline, Google Scholar
22 : Individualised or standardised outcome measures: a co-habitation? Adm Policy Ment Health Ment Health Serv Res 2019; 46:425–428Crossref, Medline, Google Scholar
23 : The Canadian occupational performance measure: an outcome measure for occupational therapy. Can J Occup Ther 1990; 57:82–87Crossref, Medline, Google Scholar
24 : Measuring mental health outcomes in primary care: the psychometric properties of a new patient-generated outcome measure, “PSYCHLOPS” (“psychological outcome profiles”). Prim Care Ment Health 2005 3:261–270 Google Scholar
25 : Evaluation of a Patient-Specific Index as an outcome measure for physiotherapy in Parkinson’s disease. Eur J Phys Rehabil Med 2009; 45:507–512Medline, Google Scholar
26 : Efficacy of community-based physiotherapy networks for patients with Parkinson’s disease: a cluster-randomised trial. Lancet Neurol 2010; 9:46–54Crossref, Medline, Google Scholar
27 : Modifying the Medical Research Council grading system through Rasch analyses. Brain 2012; 135:1639–1649Crossref, Medline, Google Scholar
28
29 : Conversion disorder: current problems and potential solutions for DSM-5. J Psychosom Res 2011; 71:369–376Crossref, Medline, Google Scholar
30 : The value of “positive” clinical signs for weakness, sensory and gait disorders in conversion disorder: a systematic and narrative review. J Neurol Neurosurg Psychiatry 2014; 85:180–190Crossref, Medline, Google Scholar
31 : Interobserver agreement and validity of bedside “positive signs” for functional weakness, sensory and gait disorders in conversion disorder: a pilot study. J Neurol Neurosurg Psychiatry 2015; 86:425–430Crossref, Medline, Google Scholar
32 International Classification of Diseases (ICD-11). Geneva, World Health Organization, 2018Google Scholar
33 : Can semiology predict psychogenic nonepileptic seizures? A prospective study. Ann Neurol 2011; 69:997–1004Crossref, Medline, Google Scholar
34 : Does the primary literature provide support for clinical signs used to distinguish psychogenic nonepileptic seizures from epileptic seizures? J Neurol Neurosurg Psychiatry 2010; 81:719–725Crossref, Medline, Google Scholar
35 : Functional cognitive disorder: a common cause of subjective cognitive symptoms. J Alzheimers Dis 2015; 48(suppl 1):S19–S24Crossref, Medline, Google Scholar
36 : Functional (psychogenic) cognitive disorders: a perspective from the neurology clinic. J Alzheimers Dis 2015; 48(suppl 1):S5–S17Crossref, Medline, Google Scholar
37 : Neuropsychiatric associations with gender, illness duration, work disability, and motor subtype in a US functional neurological disorders clinic population. J Neuropsychiatry Clin Neurosci 2017; 29:375–382Link, Google Scholar
38 : Do patients whose psychogenic non-epileptic seizures resolve, “replace” them with other medically unexplained symptoms? Medically unexplained symptoms arising after a diagnosis of psychogenic non-epileptic seizures. J Neurol Neurosurg Psychiatry 2011; 82:967–969Crossref, Medline, Google Scholar
39 : Correlates of health-related quality of life in adults with psychogenic nonepileptic seizures: a systematic review. Epilepsia 2016; 57:171–181Crossref, Medline, Google Scholar
40 : Depression and symptoms affect quality of life in psychogenic nonepileptic seizures. Neurology 2009; 73:366–371Crossref, Medline, Google Scholar
41 : Neuropsychiatric factors linked to adherence and short-term outcome in a US functional neurological disorders clinic: a retrospective cohort study. J Neuropsychiatry Clin Neurosci 2018; 30:152–159Link, Google Scholar
42 : The prognosis of functional (psychogenic) motor symptoms: a systematic review. J Neurol Neurosurg Psychiatry 2014; 85:220–226Crossref, Medline, Google Scholar
43 : Psychopathology and psychogenic movement disorders. Mov Disord 2011; 26:1844–1850Crossref, Medline, Google Scholar
44 : The symptom of functional weakness: a controlled study of 107 patients. Brain 2010; 133:1537–1551Crossref, Medline, Google Scholar
45 : The impact of non-motor symptoms on the health-related quality of life in patients with functional movement disorders. J Psychosom Res 2018; 115:32–37Crossref, Medline, Google Scholar
46 : Fatigue, not self-rated motor symptom severity, affects quality of life in functional motor disorders. J Neurol 2018; 265:1803–1809Crossref, Medline, Google Scholar
47 : Stressful life events and maltreatment in conversion (functional neurological) disorder: systematic review and meta-analysis of case-control studies. Lancet Psychiatry 2018; 5:307–320Crossref, Medline, Google Scholar
48 : Measuring outcome in psychogenic nonepileptic seizures: how relevant is seizure remission? Epilepsia 2005; 46:1788–1795Crossref, Medline, Google Scholar
49 : A Bayesian account of “hysteria”. Brain 2012; 135:3495–3512Crossref, Medline, Google Scholar
50 : Symptoms and the body: taking the inferential leap. Neurosci Biobehav Rev 2017; 74(pt A):185–203Crossref, Medline, Google Scholar
51 : Pathogenesis and pathophysiology of functional (psychogenic) movement disorders. Neurobiol Dis 2019; 127:32–44Crossref, Medline, Google Scholar
52 : Stress and functional neurological disorders: mechanistic insights. J Neurol Neurosurg Psychiatry 2019; 90:813–821Crossref, Medline, Google Scholar
53 : Emotional processing in functional neurological disorder: a review, biopsychosocial model and research agenda. J Neurol Neurosurg Psychiatry 2019; 90:704–711Crossref, Medline, Google Scholar
54 : Believing is perceiving: mismatch between self-report and actigraphy in psychogenic tremor. Brain 2012; 135:117–123Crossref, Medline, Google Scholar
55 : Catastrophising and normalising in patients’ accounts of their seizure experiences. Seizure 2012; 21:795–801Crossref, Medline, Google Scholar
56 : Similar association between objective and subjective symptoms in functional and organic tremor. Parkinsonism Relat Disord 2019; 64:2–7Crossref, Medline, Google Scholar
57 : A qualitative study of the experiences and perceptions of patients with functional motor disorder. Disabil Rehabil 2019; 1–6Crossref, Google Scholar
58 : What patients say about living with psychogenic nonepileptic seizures: a systematic synthesis of qualitative studies. Seizure 2016; 41:100–111Crossref, Medline, Google Scholar
59 : Understanding the narratives of people who live with medically unexplained illness. Patient Educ Couns 2005; 56:205–210Crossref, Medline, Google Scholar
60 : “Seizures have become a means of somehow learning things about myself”: a qualitative study of the development of self-efficacy and mastery during a psychotherapeutic intervention for people with epilepsy. Epilepsy Behav 2018; 84:152–161Crossref, Medline, Google Scholar
61 : Movement Disorder Society–sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord 2008; 23:2129–2170Crossref, Medline, Google Scholar
62 : A consensus set of outcomes for Parkinson’s disease from the International Consortium for Health Outcomes Measurement. J Parkinsons Dis 2017; 7:533–543Crossref, Medline, Google Scholar
63 : Unified Huntington’s Disease Rating Scale: reliability and consistency. Neurology 2001; 11:136–142Google Scholar
64 : Core Health Outcomes in Childhood Epilepsy (CHOICE): development of a core outcome set using systematic review methods and a Delphi survey consensus. Epilepsia 2019; 60:857–871Medline, Google Scholar
65 : Development of a core outcome set for epilepsy in pregnancy (E-CORE): a national multi-stakeholder modified Delphi consensus study. BJOG 2017 124:661–667Crossref, Medline, Google Scholar
66 : Common data elements in epilepsy research: development and implementation of the NINDS epilepsy CDE project. Epilepsia 2011; 52:1186–1191Crossref, Medline, Google Scholar
67 : Which outcomes should we measure in adult epilepsy trials? The views of people with epilepsy and informal carers. Epilepsy Behav 2016; 59:105–110Crossref, Medline, Google Scholar
68 : The Clinician-Administered PTSD Scale for DSM-5 (CAPS-5): development and initial psychometric evaluation in military veterans. Psychol Assess 2018; 30:383–395Crossref, Medline, Google Scholar
69 : Ictal symptoms of anxiety, avoidance behaviour, and dissociation in patients with dissociative seizures. J Neurol Neurosurg Psychiatry 2006; 77:616–621Crossref, Medline, Google Scholar
70 : Focal neurologic symptoms in panic attacks. Am J Psychiatry 1986; 143:648–649Crossref, Medline, Google Scholar
71 : Standardization of health outcomes assessment for depression and anxiety: recommendations from the ICHOM Depression and Anxiety Working Group. Qual Life Res 2017; 26:3211–3225Crossref, Medline, Google Scholar
72 : The Yale-Brown Obsessive Compulsive Scale: I. development, use, and reliability. Arch Gen Psychiatry 1989; 46:1006–1011Crossref, Medline, Google Scholar
73 : Core outcome domains for clinical trials on somatic symptom disorder, bodily distress disorder, and functional somatic syndromes: European Network on Somatic Symptom Disorders recommendations. Psychosom Med 2017; 79:1008–1015Crossref, Medline, Google Scholar
74 : Fibromyalgia syndrome module at OMERACT 9: domain construct. J Rheumatol 2009; 36:2318–2329Crossref, Medline, Google Scholar
75 : Content and criterion validity of the preliminary core dataset for clinical trials in fibromyalgia syndrome. J Rheumatol 2009; 36:2330–2334Crossref, Medline, Google Scholar
76 : Developing a core outcome domain set to assessing effectiveness of interdisciplinary multimodal pain therapy: the VAPAIN consensus statement on core outcome domains. Pain 2018; 159:673–683Crossref, Medline, Google Scholar
77 : Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain 2005; 113:9–19Crossref, Medline, Google Scholar
78 Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims: Guidance for Industry. Washington, DC, US Food and Drug Administration, 2009. www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM193282.pdfGoogle Scholar
79 : Core Outcome Set–STAndards for Development: the COS-STAD recommendations. PLoS Med 2017; 14:e1002447Crossref, Medline, Google Scholar



