Panic Attack as the Sole Manifestation of Epilepsy Localized to the Nondominant Temporal Region
Temporal lobe epilepsy represents a complex, neuropsychiatric illness in which neurological presentation may be complicated by a varied assortment of emotional, behavioral, or personality abnormalities. When panic is a prominent symptom or the sole manifestation of epilepsy, diagnosis and effective management may elude health care providers for years, with significant worsening of morbidity both medically and psychosocially. We present two cases of isolated ictal panic episodes that were misdiagnosed by psychiatric services for many years and were ultimately evaluated by a neurologist following serial treatment failure and development of salient convulsive features.
Case 1
A 40-year-old right-handed man with a 6-year history of crippling, medically refractory panic disorder presented to our university hospital neurology clinic after the development of atypical staring episodes. On presentation, the patient reported an insidious and progressive history of panic attacks described as recurrent, unprovoked episodes of anxiety and hyperventilation typically lasting <1 minute. Historically, these episodes were stereotyped beginning with the sensation of pins and needles on his face, which spread to his limbs, and his breathing would become rapid, and he would develop dryness in his mouth along with mild nausea followed by a sensation of unease. These symptoms would develop and recede rapidly. On his presentation at the neurology clinic, the family reported new symptoms of mild confusion during these panic attacks as well as daily episodes of amnestic staring. Additionally, the family stated that the patient’s anxiety and short-term memory deficits became so invasive in his daily life that he was unable to maintain gainful employment. The patient reported no personal or family history of panic attacks, and multiple prior trials of anxiolytics and antidepressants, including lorazepam, clonazepam, lamotrigine, and citalopram, were unsuccessful.
Noninvasive continuous video-EEG monitoring was performed with a 10–20 international system with sphenoidal electrodes captured multiple stereotyped panic episodes associated with abnormal electrographic activity. The preictal activity demonstrated rhythmic 8-Hz wave complexes across the bilateral anterior temporal regions, followed by evolution into an ictal spike-wave discharge in the bilateral anterior temporal region with continuous activity in the left anterior temporal area. Clinical symptoms of a panic attack and the symptomatogenic zone were associated with activity in the medial dorsal edge of the nondominant-right anterior temporal region (Figure 1A).
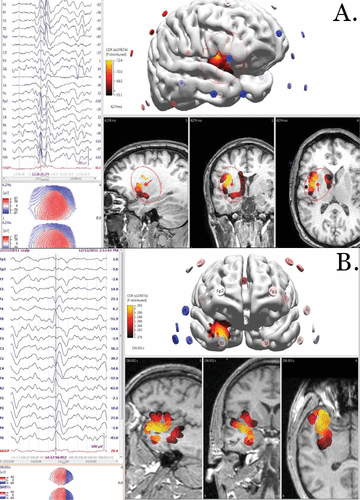
FIGURE 1. Ictal activity, clinical symptoms of panic, and the symptomatogenic zone in two case patientsa
a In case 1 (panel A), preictal activity demonstrated rhythmic 8-Hz wave complexes across the bilateral anterior temporal regions, followed by evolution into an ictal spike-wave discharge in the bilateral anterior temporal region with clinical symptoms of panic and the symptomatogenic zone associated with activity in the medial-dorsal edge of the nondominant/right anterior temporal region. In case 2 (panel B), ictal activity began in the left temporal region with clinical panic and the symptomatogenic zone associated with propagation to the medial nondominant insular cortex approximately 10 seconds after the onset of epileptiform activity. s=seconds.
A 3-T MRI brain scan with and without contrast revealed mild bilateral mesial temporal sclerosis. Following a diagnosis of localization-related complex partial epilepsy, trials of many antiepileptic medications (zonisamide, lacosamide, carbamazepine, and lamotrigine) were unsuccessful, and the patient subsequently developed generalized convulsions within 2 years. He was eventually considered as a candidate for surgical resection.
The patient later agreed to an elective surgical procedure. Following a bilateral craniotomy, two strip electrodes were placed anterolaterally, mesially, and posterolaterally over the temporal lobe on each side. Intracranial video-EEG monitoring with high-frequency oscillation (>80 Hz) analysis (source analysis with Curry version 7 Neuroimaging software) localized the most consistent epileptic foci or epileptogenic zone to the left anterior temporal pole with rapid propagation to right anterior temporal epilepsy. The episodes of amnesia and confusion were consistent with left anterior temporal lobe activity, while episodes of anxiety and hyperventilation were associated with rapid propagation to the right temporal region. Following stereotactic lobectomy of the left anterior temporal pole, the patient has remained clinically free of panic attacks, and oxcarbazepine monotherapy has controlled his convulsions.
Case 2
A 39-year-old right-handed woman with a relevant medical history of panic attacks, generalized anxiety disorder, and unexplained gastrointestinal dysmotility and a remote history of unspecified seizures presented to our university hospital epilepsy monitoring unit following an isolated, unprovoked generalized convulsion.
The patient came to our neurology clinic upon her referral following an episode of isolated convulsion. She reported a generalized tonic-clonic episode, which was witnessed by her coworkers, and that occurred after a period of insomnia and “stressful” work. The episode was without known localizing semiology and lasted <2 minutes, followed by mild postictal confusion for a duration <20 minutes. The patient conveyed a remote history of childhood epilepsy that occurred between the ages of 12 and 14; she described these childhood episodes as staring spells and was transiently treated with valproic acid. For the past 20 years before presentation to our clinic, she had remained seizure-free and off all antiepileptic medication.
Noninvasive, continuous video-EEG monitoring was performed by using a 10–20 international system with sphenoidal electrodes. Monitoring captured multiple stereotyped panic episodes associated with electrographic activity. Ictal activity began with poorly localized focal spike-wave discharges in the left temporal region (T3), followed by rapid propagation to the bifrontal cortex and contralateral right insular cortex. Clinical symptoms of a panic attack began with the patient checking her pulse following the onset of ECG-correlated tachycardia. She then rose anxiously, with difficulty breathing and blurred vision, followed by nausea and an upset stomach. Her panic symptoms lasted <3 minutes. She reported that this episode was a typical one. Clinical symptoms of a panic attack and the symptomatogenic zone were associated with propagation to the medial nondominant insular cortex approximately 10 seconds after the onset of epileptiform activity (Figure 1B). Following the start of a clobazam medication regimen, she attained a complete resolution of her panic attacks and seizure activity by the 1-year follow-up.
Discussion
Although rare, multiple reports have documented that some patients who are initially diagnosed with a panic disorder receive a later diagnosis of epilepsy. Since 1955, approximately 200 cases of ictal panic, with preferential localization to the right hemisphere (62%−79%), have been reported in the literature; however, handedness has been reported in only 75% of these cases. Of these cases, ictal fear has been reported to be more often localized to the temporal lobe (74%) than to the frontal lobe (15.1%), suggesting a frontal-temporal network involvement rather than a specific cortical territory (1, 2). Given the similar clinical features and different treatments for these two diagnoses (panic disorder and epilepsy), it is imperative for clinicians to understand the subtle clinical features that may aid in early differentiation.
Differentiating partial seizures from panic disorder can be difficult; however, guidelines to distinguish these two conditions are provided in the literature. In a review article, Hartley and Phelps (3) demonstrated that panic attacks and epileptic auras have many fundamental differences. Ictal episodes of panic tend to last as long as common epileptic ictal spells (<10 minutes), while panic attacks generally last on average 30 minutes. Similar to typical seizures, ictal episodes of panic are defined by recurrent cortical network pathology; that is, they are more often stereotyped and demonstrate an evolution of clinical characteristics with more classic symptoms (e.g., olfactory auras, amnestic features, automatisms) than a panic disorder. Panic disorder is more likely worsened by emotional distress, associated with agoraphobia, and has a stronger familial history than ictal panic. Ictal panic is typically more associated with temporal lobe lesions on imaging (1, 4). Given both the similar clinical presentations of and diverging treatments for panic attacks and epileptic seizures, it is crucial for clinicians to understand the noninvasive imaging techniques available to assist in decision making. A full evaluation for epilepsy may require 24-hour EEG, and video monitoring can sometimes identify abnormal electrical discharges not observed in routine EEG (5–7).
Recent shifts in our perspective of neuroscience have resulted in the division of the human brain into functional networks. Functional networks are collections of brain regions with strongly correlated activity, both at rest and during cognitive tasks, and each network is believed to implement a different aspect of cognition. Anxiety disorders and high-trait anxiety are associated with a particular pattern of functional network dysfunction: increased functioning of the cingulo-opercular and ventral attention networks, as well as decreased functioning of the frontoparietal and default mode networks (3, 8). Ictal panic and panic disorder have considerable overlap concerning neuroanatomical territories. The literature suggests that both conditions are the consequence of a pathological disturbance of an intrinsic fear-regulation network, resulting in activation of the amygdala and anterior cingulate cortex that relates to an increase in primitive brainstem/hypothalamic-pituitary-adrenal-axis fear behavior. The dysfunction of the medial prefrontal cortex/nondominant temporal lobe results in decreased context-driven inhibition of amygdala activity via the hippocampus. Both cases above provide examples of these specific network dysfunctions. In these two cases, initial ictal activity began in the dominant temporal region, followed by rapid propagation to the nondominant insular and frontal cortices. Thus, we can suggest that hyperactivation of the medial temporal structures (amygdala and anterior cingulate cortex) responsible for fear behavior, with comorbid dysfunction of the nondominant frontal-temporal circuit responsible for context integration, results in sudden-onset fearful behavior (discomfort with distressing visceral) without context to make sense of feelings, hence a panic attack.
Among patients for whom ictal panic episodes are the sole manifestation of epilepsy, accurate diagnosis and effective management may elude health care providers, resulting in worsening of medical and psychosocial morbidity. Both of the cases presented here demonstrate the value of prolonged EEG in the differentiation of epileptic and nonepileptic episodes, as well as reinforce the theory that these phenomena arise from a common neuroanatomical substrate.
1 : Panic attack semiology in right temporal lobe epilepsy. Epileptic Disord 2003; 5:93–100Medline, Google Scholar
2 : Fear as the main feature of epileptic seizures. J Neurol Neurosurg Psychiatry 2001; 70:186–191Crossref, Medline, Google Scholar
3 : Changing fear: the neurocircuitry of emotion regulation. Neuropsychopharmacology 2010; 35:136–146Crossref, Medline, Google Scholar
4 : Mesial temporal lobe seizures presenting as anxiety disorders. J Neuropsychiatry Clin Neurosci 1995; 7:352–357Link, Google Scholar
5 : Panic attacks as ictal manifestations of parietal lobe seizures. Epilepsia 1995; 36:824–830Crossref, Medline, Google Scholar
6 : Temporal epileptogenesis: localizing value of scalp and subdural interictal and ictal EEG data. Epilepsia 2001; 42:508–514Crossref, Medline, Google Scholar
7 : Recurrent attacks of fear and visual hallucinations in a child. J Child Neurol 2002; 17:230–233Crossref, Medline, Google Scholar
8 : Functional network dysfunction in anxiety and anxiety disorders. Trends Neurosci 2012; 35:527–535Crossref, Medline, Google Scholar



