Sexual Dimorphism in Brain Development: Influence on Affective Disorders
Sexual dimorphism refers to the differences between genetic males and females within the same species. It manifests in multiple anatomical trait differences, including morphological features and structures of the human brain (1). Sexual dimorphisms in the human brain were initially reported in the early twentieth century, beginning with the observation that, on average, male brains are heavier and have a larger circumference than female brains (2). In the intervening years, sexual dimorphism in total brain volume have been broadly described in the literature (3–10). On average, total brain size in males is about 8%–15% larger than in females (uncorrected for body size) and this sexual dimorphism has been observed from early development studies, childhood, adolescence and adulthood (3–8, 10–13). Even after correcting for brain volume differences, sexual dimorphism in size-by-age trajectories of brain development (as distinct from group averages across broad age ranges) are observed in frontal, temporal, and parietal gray matter, among other structures (5). Sexual dimorphisms in the human brain also are associated with differences in behaviors, including courtship, mate seeking, and even aggression (14). These types of behaviors are instinctual and do not require any specific prior training; instead, they are manifestations of sexual dimorphism in brain development, differentiation, and network function (14).
Sex steroids (i.e., androgens, estrogens and progestins) are intimately related to the development and further regulation of sexually dimorphic structures, somatic functions, and varied behaviors throughout life, including brain structure and function (15). Brain exposure to the sex steroids during critical periods of development not only results in structural (anatomical) changes in the brain but also potentiates behavioral changes such as aggression and mating. These hormones program neural circuits to elicit different behavioral responses during adolescence and adulthood upon reinduction from the sex steroids (16–18). Additionally, sex differences in stress responses follow upon exposure to sex hormones. Those sex hormones, along with their active metabolites, modulate sex differences in stress responses (19–21).
In humans and other mammals, the brain is wired in a female-like fashion during early stages of neural development. Male-like traits (structurally and functionally) are acquired as a result of in utero exposure to androgens (17), including fetal testosterone (22). Synaptic pruning of the medial amygdala is also regulated by sex steroids (23). Studies indicate that in humans, the third trimester (in utero) is a time of rapid brain growth and development with the total brain size increasing five times its volume (5, 6). The progression of physiological events during the prenatal period serves to establish the fundamental structure for the development of the nervous system. Intrinsic inputs from within the organism, such as molecular signaling and cross-regional activity, are important as well. Neonatal estradiol (E2) exposure influences microglial cells in the preoptic area of the hypothalamus, thereby regulating the process of masculinization and subsequent male-like behaviors (i.e., copulation, aggression) (24). The brain continues to grow, while the nervous tissue differentiates after childbirth until age 2. At this age, the child’s brain volume is estimated to be 80% of the typical adult size (25, 26). Thereafter, the brain further increases its volume during the preschool period, and by age 6 it is approximately 90% of adult size (8, 26). Further changes in both, gray and white matter tissues will continue during childhood and adolescence. In addition, functional modifications (e.g., cortical, thalamic) occur later (26).
These processes continue during childhood and adolescence when the entire body and brain undergo important neurophysiological changes in response to the elevated secretion of gonadal hormones during puberty (27). Additionally, during adolescence there is significant rewiring of the neuronal networks leading to adult cognition acquisition, social behaviors and decision-making mechanisms (27).These take place subsequent to the remodeling of cortical and limbic system neuroanatomical structures (27–29).
There are two additional and distinctive ways steroid hormones interact with the brain and effect behaviors: organizational and activational (28).These principles were first described several decades ago and have been further investigated since that time (17, 27, 30). Organizational processes potentiate permanent changes within brain structures resulting from the action of steroid hormones during development. These changes persist long after the initial hormonal induction, thereby generating programing codes for important activational mechanisms for steroids during adulthood (17, 28). Activational processes involve the transient actions of sex steroids on the brain cells in a manner that alter behaviors in relation to certain social stimuli/contexts. The activation is temporary depending primarily on hormonal fluctuations, but influences steroids’ function during later stages of adulthood (27).
The collective effects of sex hormones on these aspects of neurodevelopment therefore stem from their influences on multiple dimensions of neuronal and glial structure and function. This includes processes such as signaling (intracellular), transcription, modification of transcription (epigenetic), and proteins translation (31). Accordingly, the effects of sex hormone exposure on human affective behavior are substantial. The individual’s unique experiences (i.e., environment) play an essential role in establishing the mature organization of the neural system (26). The anatomical and physiological differences of brain development may underlie important risk factors for specific psychiatric conditions for both sexes (9). Accordingly, the Institute of Medicine (IOM) held a workshop on sex differences and their implications for research in translational neuroscience; they stated: “In the current era of translational research and personalized medicine, it is increasingly important to take sex differences into account, so that the potential effects of products and therapies can be more fully understood” (32). Later in 2015, the National Institutes of Health (NIH) publicized their expectations regarding “sex” inclusion, as a biological variable, into future research efforts (33).
Consistent with these calls for research in this area, a growing body of evidence suggests that sex differences in brain development may be one of several factors influencing sex-based differences in the development, age-of-onset, expression, chronicity and course, and treatment response of neuropsychiatric conditions during childhood, adolescence, and adulthood (4, 5, 9, 12, 31, 34–39). For example, males exhibit higher prevalence of attention-deficit and hyperactivity, autism, and Tourette’s (5, 40, 41); whereas females exhibit greater prevalence of anxiety, depression and eating disorders (5, 42–44). In addition, females with mood disorders, such as premenstrual dysphoric disorder, perimenopausal depression and postpartum depression pose outstanding examples of the paramount effects of sex hormones in the brain’s affective regulation. Reports from rodent studies also support this notion (45, 46). Sex differences in stress‐related anxiety responses also have been reported (47, 48).
Central to the neural networks involved in these neuropsychiatric conditions is the amygdala, which also demonstrates sexual dimorphism. The amygdala (commonly referred as amygdaloid complex) is a bulky cluster of morphologically diverse nuclei located superior and rostral to the temporal horn of the lateral ventricle. It lies anterior to the tail of the caudate nucleus at the inferior pole of the brain (49). There are three main regional divisions of the amygdala based on its complex cytoarchitecture and function: centromedial (central and medial nuclei), superficial (ventral nuclei) and laterobasal (basolateral nuclei) (Figure 1) (50). The centromedial component is involved with autonomic, endocrine and emotional responses. The superficial group gets input from the olfactory pathways and processes affection. The laterobasal cluster process affective memory and regulates the motor responses associated with fear stimuli (50, 51). The activation of the basolateral, central, and medial subdivisions of the amygdala elicits anxiogenic effects, while their inhibition produces anxiolytic effects (49, 52).
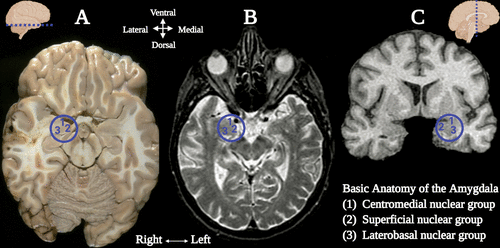
Figure 1 and COVER. Basic anatomy of the amygdala. (A) Transverse dissection at the level if the amygdala and the hippocampal formation (1). The amygdala (commonly referred to as amygdaloid complex) is a bulky cluster of morphologically diverse nuclei located superior and rostral to the temporal horn of the lateral ventricle. It lies anterior to the tail of the caudate nucleus at the inferior pole of the brain (2). The circled area shows the region of the amygdala and its three main nuclei: 1) centromedial (central and medial nuclei), 2) superficial (ventral nuclei), and 3) laterobasal (basolateral nuclei). (B) MRI of the brain, axial T2-weighed section (3). The circled region depicts the amygdaloid complex nuclei. (C) MRI of the brain, coronal T2-weighed section (3). The circled region depicts the region of the right amygdala and its three main nuclei. Created under the terms of the Creative Commons Attribution License. Created with BioRender.com
Embryologically, the appearance of the amygdaloid primordium is discernible at stage 15 (5 weeks). Whereas the ventral amygdaloid component, the superficial complex and the basolateral complex appear almost simultaneously around stage 16 (five and one-half weeks) (53). Single nuclear components are noticeable at later steps of development approximately around stages 17–21 (6–7 weeks), and the central nucleus by stage 23 (8 weeks) (53). During the fetal period, the growth of the amygdaloid complex proceeds with the cellular migration from the ventricular eminences (54). Subsequent fetal and postnatal topographic changes are attributed to the development and increased growth of the temporal lobe (53).
Given the potential importance of sexual dimorphism in amygdalar structure and function on our understanding of brain health and disease, the nature and extent of amygdalar sexual dimorphism has been the subject of considerable study using neuroimaging (5, 55–59). Studies on regional sex differences in the volume of the amygdala have reported mixed findings, some indicating larger volumes in females, while others showed larger volumes in males (3, 9, 60–63). Other groups have shown decreased or no sex differences when statistically correcting for brain volume (13, 64, 65). Moreover, a meta-analysis indicated that the amygdala should not be denoted as a sexually dimorphic structure of the human brain (65). Despite the conflicting results, a number of imaging studies have reported sex differences in the volume of the amygdala even after correcting for brain size (5, 51, 63, 66–69). An elegant study by Kim and colleagues (51) evaluated the effects of sex and age within the subregions of the amygdala. They reported that the superficial nuclei showed sexual dimorphism even after correcting for intracranial brain volume, with males exhibiting a larger radius than females (51). An imaging report (MRI and voxel-based morphometry) from varied brain structures including the amygdala of developing children (37 males and 41 females) between ages 10–14.9 years revealed larger amygdala in males compared with females (68). A comprehensive study analyzed MRI scans from a sample of 442 developing individuals between ages 8–30 years and reported larger amygdala in males compared with females (66). Another study of sex differences in brain structure volumes including 643 males and 591 females ages 3–21 years, showed larger amygdalar volumes in males compared with females after correcting for brain intracranial volume (67). Similar to other brain structures, the overall volume of the amygdala is affected by age. Significant reductions in the centromedial nucleus have been observed in postmenopausal females (51). Another study by Goddings and colleagues (69), evaluated MRI scans from 275 individuals studied longitudinally. They reported that males and females have similar overall volume changes in the amygdala as they age, but with different progression paths. The volume of the amygdala rapidly increases in females early in puberty before peaking and decreasing later on. However, in males its volume increases throughout the course of puberty (5). During adulthood, the amygdala undergoes important structural and morphological sexually dimorphic changes. Its volume decreases more substantively in females as compared with males (51).
The amygdala is associated with the emotion of fear, among other important functions (49, 59, 70). Essentially, it is activated during the processing of visual stimuli that transfers emotional meaning during social circumstances, such as the ones conveyed by facial expressions (Figure 2) (49, 70). If the amygdala is disrupted, the individual loses the ability to identify the affective meaning of facial expressions. Therefore, the person is unable to recognize threatening facial gestures as presented by others (49, 70). In addition, patients tend to lose the ability to interpret emotional prosody or the affective contents associated with speech, such as pitch contour, intensity and duration (49). For example, facial expressions such as staring eyes and furrowed eyebrows, a vocalization using a bold pitch, or the sustained duration of an expressed sentence can lead to fear or anxiety as interpreted by the amygdala (Figure 2) (70, 71). Neural activity in response to ambiguous facial gestures of surprise have also been reported to activate the amygdala (59). However, this activation depends on the individual’s interpretation of such stimuli. If interpreted negatively, the amygdala is activated more. Therefore, higher negative interpretations result in greater neural activity in the amygdala (59).
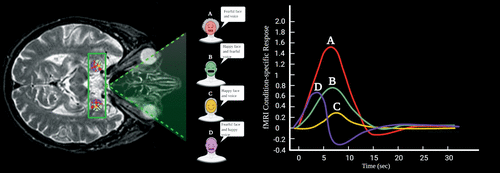
Figure 2 and COVER. Axial illustration depicting a qualitative functional MRI (fMRI) representation of bilateral activation of the amygdala in a subject seeing varied faces accompanied by different voice tones (2, 3). In the green square area, the color-coded circles within the amygdaloid region represent the activation of the amygdala in response to visual (faces) and auditory stimuli (voice tones). An fMRI mapping study showed bilateral activation of the amygdala in the subject’s scan while observing fearful facial expression pictures along with fearful voice tones (2). The color-coded lines in the graph represent A) fearful face and voice tone, B) happy face and fearful voice tone, C) happy face and voice tone, and D) fearful face and happy voice tone. Note the increased activity of the amygdala with a fearful face and intimidating voice tone (A). On contrary, the activity of the amygdala in the presence of a happy face and pleasant voice tone is decreased (C). Created under the terms of the Creative Commons Attribution License. Created with BioRender.com
When a subject is presented with images depicting aversive facial expressions and strong emotional gestures, regional neural activation in the amygdala and related structures is followed immediately thereafter by an increase in blood flow to those structures (Figure 2) (72, 73). Functional MRI (fMRI) and positron emission tomography techniques have been extremely informative in eliciting the functional connection of the amygdala to emotional disorders (74–77). Such studies have revealed sex differences in the activity of the amygdala, with males demonstrating higher activation in response to sexual stimulus (visual) compared with females (78, 79). Pleasant emotional stimuli seem to have a sex-specific effect in the amygdala as well. Positive visual stimuli generate greater activity in males, whereas females show higher activation to negative inductions (80, 81). In addition, there is an age-related decline in the amygdalar activity affecting females more than males. The perception of unpleasant (negative) gestures in elderly females seems to deteriorate more than in male counterparts (51). This is not unexpected, as the volume of the amygdala is also reduced more in aging females than in aging males (50).
Sex differences in affective disorders involving the amygdala are consistently reported (5, 40, 42–44, 82). The literature suggests that males and females experience the same level in the intensity of stress and anxiety, but with some differences that are sexually dimorphic (48). In general, males are less likely to verbally express signs of distress and anxiety, rather the manifestation of anxiety is internalized physiologically. Males participate more in action‐oriented stress coping strategies compared with females (48, 83, 84). Seo and colleagues (48) performed an interesting study using fMRI to examine trait anxiety differences in males and females. The two sexes exhibited opposite paradigms of brain stimulation in response to stress. Females experiencing anxiety during acute stress demonstrated difficulty regulating the increased levels of brain activity. Males manifested anxiety in response to acute stress with a lack of activation or perhaps total inhibition of brain activity during the stressful imagery task (48).
In summary, sexual dimorphisms in the human brain have neurodevelopmental origins, are modulated by sex hormones, and are further influenced by genetic, environmental, and sociocultural factors in the expression of sex differences in neuropsychiatric health and disease. Among the sexually dimorphic brain structures of greatest relevance to neuropsychiatric disorders is the amygdala, particularly given its role emotional processing and disorders involving disturbances in emotional generation and regulation. However, the consistency and neurobehavioral relevance of sexual dimorphisms in the human brain remain subjects of active study and debate in the clinical and social sciences. Accordingly, further research incorporating methods that explicitly address and meet the calls of the IOM and NIH for study of sex differences and their implications for research in translational neuroscience are needed.
1 : Sexual dimorphism (humans), in the International Encyclopedia of Biological Anthropology. Atlanta, American Cancer Society, 2018, pp 1–4Google Scholar
2 : Sexual dimorphism in the human brain: dispelling the myths. Dev Med Child Neurol 1989; 31:257–263Crossref, Medline, Google Scholar
3 , : Puberty influences medial temporal lobe and cortical gray matter maturation differently in boys than girls matched for sexual maturity. Cereb Cortex 2011; 21:636–646Crossref, Medline, Google Scholar
4 , : Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci 1999; 2:861–863Crossref, Medline, Google Scholar
5 : Sex differences in the developing brain: insights from multimodal neuroimaging. Neuropsychopharmacology 2019; 44:71–85Crossref, Medline, Google Scholar
6 , : Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage 2007; 36:1065–1073Crossref, Medline, Google Scholar
7 , : Mapping cortical gray matter in the young adult brain: effects of gender. Neuroimage 2005; 26:493–501Crossref, Medline, Google Scholar
8 , : Brain development, gender and IQ in children: a volumetric imaging study. Brain 1996; 119:1763–1774Crossref, Medline, Google Scholar
9 , : A meta-analysis of sex differences in human brain structure. Neurosci Biobehav Rev 2014; 39:34–50Crossref, Medline, Google Scholar
10 , : Sex differences in cortical thickness mapped in 176 healthy individuals between 7 and 87 years of age. Cereb Cortex 2007; 17:1550–1560Crossref, Medline, Google Scholar
11 , : Sex differences in brain maturation during childhood and adolescence. Cereb Cortex 2001; 11:552–557Crossref, Medline, Google Scholar
12 , : Age-related effects and sex differences in gray matter density, volume, mass, and cortical thickness from childhood to young adulthood. J Neurosci 2017; 37:5065–5073Crossref, Medline, Google Scholar
13 , : Brain size, sex, and the aging brain. Hum Brain Mapp 2015; 36:150–169Crossref, Medline, Google Scholar
14 : Molecular and neural control of sexually dimorphic social behaviors. Curr Opin Neurobiol 2016; 38:89–95Crossref, Medline, Google Scholar
15 , : Sex differences in the brain: Implications for behavioral and biomedical research. Neurosci Biobehav Rev 2018; 85:126–145Crossref, Medline, Google Scholar
16 : Evidence that the hypothalamus is responsible for androgen-induced sterility in the female rat. Endocrinology 1961; 68:68–79Crossref, Medline, Google Scholar
17 : Sexual dimorphisms of the brain. J Anim Sci 1985; 61(Suppl 3):38–61Crossref, Medline, Google Scholar
18 : Morphological changes in the brains of adult male rats after neonatal castration. J Endocrinol 1966; 36:415–416Crossref, Medline, Google Scholar
19 , : Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Horm Behav 1994; 28:464–476Crossref, Medline, Google Scholar
20 , : Estrogen promotes stress sensitivity in a prefrontal cortex-amygdala pathway. Cereb Cortex 2010; 20:2560–2567Crossref, Medline, Google Scholar
21 , : Sex differences in the hypothalamo-pituitary-adrenal axis response to inflammatory and neuroendocrine stressors. Evidence for a pituitary defect in the autoimmune disease-susceptible female Lewis rat. Neuroendocrinology 1994; 60:609–617Crossref, Medline, Google Scholar
22 , : Fetal testosterone influences sexually dimorphic gray matter in the human brain. J Neurosci 2012; 32:674–680Crossref, Medline, Google Scholar
23 , : Dendritic pruning of the medial amygdala during pubertal development of the male Syrian hamster. J Neurobiol 2006; 66:578–590Crossref, Medline, Google Scholar
24 , : Microglia are essential to masculinization of brain and behavior. J Neurosci 2013; 33:2761–2772Crossref, Medline, Google Scholar
25 , : A structural MRI study of human brain development from birth to 2 years. J Neurosci 2008; 28:12176–12182Crossref, Medline, Google Scholar
26 : The basics of brain development. Neuropsychol Rev 2010; 20:327–348Crossref, Medline, Google Scholar
27 : Pubertal hormones organize the adolescent brain and behavior. Front Neuroendocrinol 2005; 26:163–174Crossref, Medline, Google Scholar
28 : Organizational and activational effects of sex steroids on brain and behavior: a reanalysis. Horm Behav 1985; 19:469–498Crossref, Medline, Google Scholar
29 : The neural basis of puberty and adolescence. Nat Neurosci 2004; 7:1040–1047Crossref, Medline, Google Scholar
30 : Effect of sex steroids on the reproductive behavior of castrated male ring doves (Streptopelia sp.). Physiol Behav 1981; 26:561–565Crossref, Medline, Google Scholar
31 : Sex differences and the neurobiology of affective disorders. Neuropsychopharmacology 2019; 44:111–128Crossref, Medline, Google Scholar
32 : Sex Differences and Implications for Translational Neuroscience Research: Workshop Summary. Washington, DC, National Academies Press, 2011Google Scholar
33 National Institutes of Health: Consideration of Sex as a Biological Variable in NIH-funded Research. Bethesda, Md., National Institutes of Health. https://grants.nih.gov/grants/guide/notice-files/not-od-15-102.htmlGoogle Scholar
34 : Sex differences in anxiety disorders: interactions between fear, stress, and gonadal hormones. Horm Behav 2015; 76:106–117Crossref, Medline, Google Scholar
35 , : Do depressed men and women respond similarly to cognitive behavior therapy? Am J Psychiatry 1994; 151:500–505Crossref, Medline, Google Scholar
36 : Delusional depressions: natural history and response to treatment. Br J Psychiatry 1977; 131:351–360Crossref, Medline, Google Scholar
37 : Forms of atypical depression and their response to antidepressant drugs. Psychiatry Res 1986; 17:87–95Crossref, Medline, Google Scholar
38 , : Gender differences in treatment response to sertraline versus imipramine in chronic depression. Am J Psychiatry 2000; 157:1445–1452Crossref, Medline, Google Scholar
39 : Sex differences in the psychopharmacological treatment of depression. Dialogues Clin Neurosci 2016; 18:447–457Crossref, Medline, Google Scholar
40 , : The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry 2007; 164:942–948Crossref, Medline, Google Scholar
41 : The influence of sex and age on prevalence rates of comorbid conditions in autism. Autism Res 2017; 10:778–789Crossref, Medline, Google Scholar
42 , : Major depression in the National Comorbidity Survey–Adolescent Supplement: prevalence, correlates, and treatment. J Am Acad Child Adolesc Psychiatry 2015; 54:37–44.e2 PubMedCrossref, Medline, Google Scholar
43 , : A systematic review of reviews on the prevalence of anxiety disorders in adult populations. Brain Behav 2016; 6:e00497Crossref, Medline, Google Scholar
44 , : Prevalence and severity of DSM-5 eating disorders in a community cohort of adolescents. Int J Eat Disord 2014; 47:610–619Crossref, Medline, Google Scholar
45 , : Allopregnanolone and mood disorders. Prog Neurobiol 2014; 113:88–94Crossref, Medline, Google Scholar
46 , : 5α-reductase inhibition prevents the luteal phase increase in plasma allopregnanolone levels and mitigates symptoms in women with premenstrual dysphoric disorder. Neuropsychopharmacology 2016; 41:1093–1102Crossref, Medline, Google Scholar
47 : Gender differences in personality: a meta-analysis. Psychol Bull 1994; 116:429–456Crossref, Medline, Google Scholar
48 , : Gender differences in neural correlates of stress-induced anxiety. J Neurosci Res 2017; 95:115–125Crossref, Medline, Google Scholar
49 : Olfactory and Limbic Systems, in Fitzgerald’s Clinical Neuroanatomy and Neuroscience, 8th ed. Philadelphia, Elsevier, 2021, pp 346–367. https://www.clinicalkey.com/#!/content/book/3-s2.0-B9780702079092000334Google Scholar
50 , : Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat Embryol (Berl) 2005; 210:343–352Crossref, Medline, Google Scholar
51 , : Sex differences in amygdala subregions: evidence from subregional shape analysis. Neuroimage 2012; 60:2054–2061Crossref, Medline, Google Scholar
52 , : The Amygdala and Anxiety. https://www.intechopen.com/books/the-amygdala-where-emotions-shape-perception-learning-and-memories/the-amygdala-and-anxietyGoogle Scholar
53 : The amygdaloid complex and the medial and lateral ventricular eminences in staged human embryos. J Anat 2006; 208:547–564Crossref, Medline, Google Scholar
54 : Transient architectonic features in the basolateral amygdala of the human fetal brain. Acta Anat (Basel) 1998; 163:99–112Crossref, Medline, Google Scholar
55 , : Developmental sex differences in resting state functional connectivity of amygdala sub-regions. Neuroimage 2015; 115:235–244Crossref, Medline, Google Scholar
56 , : The amygdala and emotional memory. Nature 1995; 377:295–296Crossref, Medline, Google Scholar
57 , : Sex differences in amygdala shape: insights from Turner syndrome. Hum Brain Mapp 2016; 37:1593–1601Crossref, Medline, Google Scholar
58 , : Sex differences in context fear generalization and recruitment of hippocampus and amygdala during retrieval. Neuropsychopharmacology 2017; 42:397–407Crossref, Medline, Google Scholar
59 : The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology 2010; 35:169–191Crossref, Medline, Google Scholar
60 , : The young adult human brain: an MRI-based morphometric analysis. Cereb Cortex 1994; 4:344–360Crossref, Medline, Google Scholar
61 , : Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4–18 years. J Comp Neurol 1996; 366:223–230Crossref, Medline, Google Scholar
62 , : Minute effects of sex on the aging brain: a multisample magnetic resonance imaging study of healthy aging and Alzheimer’s disease. J Neurosci 2009; 29:8774–8783Crossref, Medline, Google Scholar
63 , : Sex differences and the impact of steroid hormones on the developing human brain. Cereb Cortex 2009; 19:464–473Crossref, Medline, Google Scholar
64 , : Sex differences in the adult human brain: evidence from 5216 UK Biobank participants. Cereb Cortex 2018; 28:2959–2975Crossref, Medline, Google Scholar
65 : Meta-analysis reveals a lack of sexual dimorphism in human amygdala volume. Neuroimage 2017; 147:282–294Crossref, Medline, Google Scholar
66 : Sex differences and structural brain maturation from childhood to early adulthood. Dev Cogn Neurosci 2013; 5:106–118Crossref, Medline, Google Scholar
67 , :
68 , : Sex steroids and brain structure in pubertal boys and girls. Psychoneuroendocrinology 2009; 34:332–342Crossref, Medline, Google Scholar
69 , : The influence of puberty on subcortical brain development. Neuroimage 2014; 88:242–251Crossref, Medline, Google Scholar
70 , : Fear and the human amygdala. J Neurosci 1995; 15:5879–5891Crossref, Medline, Google Scholar
71 , : The amygdala response to emotional stimuli: a comparison of faces and scenes. Neuroimage 2002; 17:317–323Crossref, Medline, Google Scholar
72 , : Repeated fMRI in measuring the activation of the amygdala without habituation when viewing faces displaying negative emotions. PLoS One 2018; 13:e0198244Crossref, Medline, Google Scholar
73 : Emotional facial expressions capture attention. Neurology 2001; 56:153–158Crossref, Medline, Google Scholar
74 , : Brain systems mediating aversive conditioning: an event-related fMRI study. Neuron 1998; 20:947–957Crossref, Medline, Google Scholar
75 , : Amygdala-hippocampal involvement in human aversive trace conditioning revealed through event-related functional magnetic resonance imaging. J Neurosci 1999; 19:10869–10876Crossref, Medline, Google Scholar
76 , : Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron 1998; 20:937–945Crossref, Medline, Google Scholar
77 : Facial emotion processing in major depression: a systematic review of neuroimaging findings. Biol Mood Anxiety Disord 2011; 1:10Crossref, Medline, Google Scholar
78 : Why sex matters for neuroscience. Nat Rev Neurosci 2006; 7:477–484Crossref, Medline, Google Scholar
79 : Individual differences in emotion processing. Curr Opin Neurobiol 2004; 14:233–238Crossref, Medline, Google Scholar
80 , : The influence of gender and emotional valence of visual cues on fMRI activation in humans. Pharmacopsychiatry 2003; 36(Suppl 3):S191–S194Crossref, Medline, Google Scholar
81 , : Gender differences in the processing of standardized emotional visual stimuli in humans: a functional magnetic resonance imaging study. Neurosci Lett 2003; 348:41–45Crossref, Medline, Google Scholar
82 , : Opposite molecular signatures of depression in men and women. Biol Psychiatry 2018; 84:18–27Crossref, Medline, Google Scholar
83 : Gender differences in distress: mental health consequences of environmental stress among jail inmates. Behav Sci Law 1997; 15:503–523Crossref, Medline, Google Scholar
84 : Sex differences in coping behavior: a meta-analytic review and an examination of relative coping. Pers Soc Psychol Rev 2002; 6:2–30Crossref, Google Scholar



