Somatization in Adolescents With Persistent Symptoms After Concussion: A Retrospective Chart Review
Abstract
Objective:
After concussion, approximately 30% of adolescents experience symptoms that persist beyond 1 month postinjury. For some, these symptoms affect functioning, development, and quality of life. Somatization, where psychological distress contributes to physical symptoms, may contribute to persistent symptoms after concussion in some adolescents. Understanding how clinicians identify somatization in adolescents with persistent symptoms after concussion in practice is a critical next step in improving our understanding, identification, and subsequent treatment of somatization in this patient population. To address this, the investigators assessed and compared characteristics of adolescents with persistent symptoms after concussion with and without clinician-identified somatization.
Methods:
Participants were adolescents (N=94) referred for persistent symptoms after concussion to a specialty youth concussion clinic between January 2016 and May 2018. A retrospective chart review extracted demographic and injury characteristics, symptoms after concussion, school attendance, premorbid experiences, mental health, and medical service use. Participants with physician-identified somatization were compared with those without physician-identified somatization on these measures.
Results:
Adolescents with identified somatization had more severe and atypical neurological and psychiatric symptoms after concussion and more postinjury impairment in school attendance, were more likely to have a history of premorbid chronic pain or medically unexplained symptoms, and obtained more neuroimaging and health care after injury compared with those unaffected by somatization. They did not differ in mood or anxiety symptom self-reports.
Conclusions:
This study identified characteristic differences and similarities in adolescents with and without clinician-identified somatization after a prolonged concussion recovery. These findings have the potential to improve clinical identification of somatization in youths following a concussion and may aid in treatment among this demographic group.
Up to 30% of adolescents who sustain a concussion experience symptoms after concussion, such as headache, dizziness, fatigue, visual disturbances, and balance deficits that persist beyond one month postinjury and fail to improve with appropriate therapies (1). These symptoms can last for months to years, leading to decreased physical, emotional, and social functioning and lower overall quality of life (2, 3).
A history of mental health problems, as measured by both self-report scales and clinical diagnoses, is one of the most robust predictors of symptom persistence after a concussion in both pediatric and adult populations (4, 5). Research efforts have mostly addressed mood and anxiety as common mental health risks in this population. However, somatization, the process whereby psychological stress is experienced in the form of unintentionally produced physical symptoms (6), may be another mental health risk factor for symptom persistence following a concussion (7–10).
The role of somatization in adolescents with persistent symptoms after concussion and how to identify it in this population is poorly understood. In a prospective study of high school athletes, higher preinjury scores on self-report somatization symptom inventories, measures that assessed the number and severity of diffuse bodily symptoms, were the strongest premorbid predictor of length of concussion recovery when compared with other preinjury emotional, behavioral, and demographic factors (7). Because underlying psychological processes were not assessed, the authors could only hypothesize that somatization indirectly lengthened recovery from concussion. In pediatric populations, immediate postinjury scores on similar types of somatization symptom inventories were related to delayed symptom resolution following concussion (8, 9). These findings suggest that somatization could play a role in the severity and duration of persistent symptoms after concussion for some youths.
However, although some somatization scales have shown adequate to good diagnostic accuracy (11), these symptom inventories do not take into account the biopsychosocial complexities that make up a diagnosis of somatization in clinical practice. Because of this, our understanding of how clinicians identify somatization in adolescents with persistent symptoms after concussion in a clinical setting is poor and must be further explored using data from physicians in clinical practice.
Certain factors known to play a role in somatization in the general adolescent population that have yet to be investigated in adolescents with persistent symptoms after concussion may hold the key to a better understanding of how clinicians identify somatization in this population. In the general adolescent somatization literature, clinically significant somatization affects girls more than boys, with prevalence in adolescence increasing in step with puberty (12). Somatization is associated with anxiety and depression (13), high rates of medical service use (14), poorer academic and social functioning (15), and reduced quality of life (15), as well as other psychosocial and temperamental characteristics (16). Somatization is also an established contributing factor to symptoms in chronic pain, medically unexplained symptoms, chronic fatigue syndrome, irritable bowel syndrome, and fibromyalgia (17–22).
By focusing on these characteristics, we aimed to describe a clinical cohort of adolescents with persistent symptoms after concussion and physician-identified somatization and to compare these characteristics with those of adolescents with persistent symptoms after concussion who were not identified as having somatization.
Methods
A review of medical records was conducted at the tertiary care adolescent complex concussion clinic at the GF Strong Rehabilitation Center in Vancouver, British Columbia, and was approved by the institutional clinical review board at the University of British Columbia. Each adolescent filled out an intake package that included demographic, concussion symptom, injury, and health history information. The adolescent attended an intake evaluation, which may have involved a consultation with a physiatrist if indicated by the complexity of the case. The adolescent may then have been referred to a psychiatrist outside of the clinic if a mental health concern such as somatization was identified by the physiatrist and the family or patient consented to a referral.
Records of all 199 patients referred to the adolescent complex concussion clinic between January 2016 and May 2018 were reviewed (Figure 1). To be referred to the adolescent complex concussion clinic, patients had to be between the ages of 11 and 19, have reported symptoms after concussion lasting beyond 4 weeks after a physician-diagnosed concussion, or have at least one risk factor for prolonged recovery after a physician-diagnosed concussion: prior concussion(s), history of a learning disability or attention-deficit hyperactivity disorder or a developmental disability, history of depression or anxiety, history of migraines/headaches, or a sleep disorder.
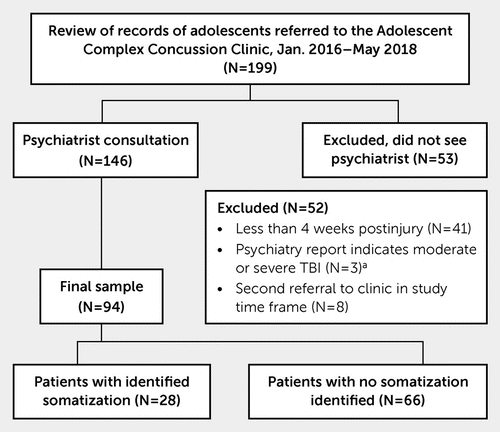
FIGURE 1. Flow diagram of the study inclusion and exclusion criteria and group assignment based on Strengthening the Reporting of Observational Studies in Epidemiology guidelines
a TBI=traumatic brain injury
Records were excluded if the adolescent was less than 1 month postinjury at the time of referral to clinic (N=41), if the adolescent was not seen by the physiatrist (N=53), or if the physiatry consultation report described the adolescent’s head injury as a moderate or severe traumatic brain injury instead of a concussion (N=3). If an adolescent had been referred to the clinic more than once in the study time frame, data from the first referral where the adolescent was seen by a physiatrist were used to avoid duplicate records in the sample (excluded as duplicates, N=8). A total of 94 records remained in the sample. Records were then assigned to the study groups based on the presence or absence of clinicians’ wording in the patient chart that explicitly indicated somatization as defined by one or more of the criteria listed below.
Identified Somatization (N=28)
The physiatry report included any of the following words to describe the child’s condition: “somatization,” “somatic symptom disorder,” “psychosomatic,” “somatic component,” or “somatoform.”
The adolescent was referred to a child psychiatrist for persistent symptoms after concussion, and the diagnosis, impression, or formulation section of the psychiatrist’s report included any of the following words to describe the child’s condition: “somatization,” “somatic symptom disorder,” “psychosomatic,” “somatic component,” or “somatoform.”
In the treatment recommendations section of the physiatrist’s report, the adolescent was referred to an intervention program specifically targeting somatization.
In the treatment recommendations section of the psychiatrist’s report the adolescent was referred to an intervention program specifically targeting somatization.
No Identified Somatization (N=66)
All records in the sample that did not meet the criteria for the identified somatization group.
Data were sourced from adolescent complex concussion clinic charts accessed via the health records department at the GF Strong Rehabilitation Center. A detailed data extraction manual with guidelines and operationalized definitions was developed to reduce bias and used for standardized data extraction by a single reviewer (K.G.), in step with accepted guidelines (23).
Demographic information, injury details, medical and mental health history, medical service use, and school attendance were extracted from the physiatry report (for further details, see Table S1 in the online supplement). Post-concussive, depression, and anxiety symptoms were extracted from self-report scales administered by the adolescent complex concussion clinic to all incoming patients at the time of initial consultation.
Symptoms after concussion were assessed with the Rivermead Post-Concussion Symptoms Questionnaire (RPQ), a 16-item self-report questionnaire that inventories physical (e.g., headache), emotional (e.g., feeling irritable), and cognitive symptoms (e.g., poor concentration) of concussion scored on a Likert-type scale from 0 (not experienced) to 4 (severe problem) (24). The RPQ has a maximum total score of 64; item ratings of 1 were not counted toward total scores as they denote “no more of a problem” since injury, in accordance with King et al. (24) The RPQ demonstrates high reliability in populations with persistent symptoms after concussion and has been validated in adolescent populations (24, 25). In this sample, the RPQ was internally consistent (α=0.88) and showed a one-factor solution, using principal-axis factoring (for further details, see Figure S2 in the online supplement).
Depressive symptoms were assessed with the Kutcher Adolescent Depression Scale (KADS), a six-item self-report measure scored on a Likert-type scale from 0 (hardly ever) to 3 (all of the time), which inventories core physical (e.g., feeling fatigued) and emotional (e.g., feeling worthless) symptoms of depression (26). The Patient-Reported Outcomes Measurement Information System (PROMIS) Pediatric Anxiety Symptom Scale was used to evaluate symptoms of anxiety. The measure is an eight-item self-report scale assessing fear, hyperarousal, worry, and physical symptoms of anxiety over the past week, scored on a Likert-type scale from 0 (almost never to) to 4 (almost always) (27). Both the PROMIS Pediatric Anxiety Symptom Scale and KADS have been validated in pediatric and adolescent samples and show good reliability and consistency (28, 29). In the current sample, both the PROMIS and KADS were internally consistent (α=0.88 and 0.86, respectively), and each showed a one-factor solution using principal-axis factoring (for further details, see Figures S2 and S3 in the online supplement).
Descriptive information pertaining to central tendency and intercorrelations were calculated. Univariate differences between groups (somatization and no somatization) were assessed using chi-square tests for categorical variables and Mann-Whitney U tests for ordinal variables. Bivariate relationships among variables of interest were analyzed using Spearman’s rank-order tests. At the multivariate level, binary logistic regression was used to determine the estimated odds of an adolescent falling into either the somatization or no somatization group, given a specific independent variable, and adjusting for other variables of interest. Data analysis was conducted using SPSS 25. Management of missing data is described in detail in the online supplement.
Results
Descriptive characteristics of adolescents with and without somatization are reported in Table 1. The groups did not differ in age, sex, or any injury characteristics, with the exception that adolescents with identified somatization were more likely to have sustained an injury from falling down. Adolescents with identified somatization had higher RPQ total scores and were more likely to have atypical symptoms after concussion than those without, specifically atypical neurological symptoms (e.g., limb numbness, loss of color vision) and other atypical symptoms (e.g., out-of-body experiences), but not atypical pain symptoms. The two groups did not differ in medical, social, or mental health histories, with the exception that adolescents with identified somatization were more likely to have a history of chronic pain or medically unexplained symptoms.
| Complete sample | No identified somatization | Identified somatization | Group differences | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Categorical variable | Frequency | % | N | Frequency | % | N | Frequency | % | N | Pearson’s χ2 | p |
| Sex (female) | 63.0 | 67.0 | 94 | 43.0 | 65.3 | 66 | 20.0 | 71.4 | 28 | 0.35 | 0.55 |
| Injury factor | |||||||||||
| Loss of consciousness | 7.0 | 7.4 | 94 | 4.0 | 6.1 | 66 | 3.0 | 10.7 | 28 | 0.62 | 0.43 |
| Visited emergency department | 42.0 | 44.7 | 94 | 26.0 | 39.4 | 66 | 16.0 | 57.1 | 28 | 2.5 | 0.11 |
| Mechanism of injury | |||||||||||
| Sport | 59.0 | 62.8 | 94 | 42.0 | 63.6 | 66 | 17.0 | 60.7 | 28 | 0.07 | 0.79 |
| Motor vehicle accident | 17.0 | 18.1 | 94 | 14.0 | 21.2 | 66 | 3.0 | 10.7 | 28 | 1.46 | 0.23 |
| Fall | 8.0 | 8.5 | 94 | 3.0 | 4.5 | 66 | 5.0 | 17.9 | 28 | 4.47 | 0.03 |
| Assault | 1.0 | 1.1 | 94 | 1.0 | 1.5 | 66 | 0.0 | 0.0 | 28 | 0.43 | 0.51 |
| Other | 9.0 | 9.6 | 94 | 6.0 | 9.1 | 66 | 3.0 | 10.7 | 28 | 0.25 | 0.62 |
| History | |||||||||||
| Prior concussion(s) | 45.0 | 47.9 | 94 | 31.0 | 47.0 | 66 | 14.0 | 50.0 | 28 | 0.07 | 0.79 |
| ADHD | 9.0 | 9.6 | 94 | 7.0 | 10.6 | 66 | 2.0 | 7.1 | 28 | 0.27 | 0.60 |
| Depression and/or anxiety | 19.0 | 20.2 | 94 | 12.0 | 18.2 | 66 | 7.0 | 25.0 | 28 | 0.57 | 0.45 |
| Chronic pain or medically unexplained symptoms | 5.0 | 5.3 | 94 | 1.0 | 1.5 | 66 | 4.0 | 14.3 | 28 | 6.37 | 0.01 |
| Persistent symptoms after concussion | |||||||||||
| Atypical persistent symptoms | 44.0 | 46.8 | 94 | 24.0 | 36.4 | 66 | 20.0 | 71.4 | 28 | 9.71 | 0.00 |
| Neurological | 19.0 | 20.2 | 94 | 5.0 | 7.6 | 66 | 14.0 | 50.0 | 28 | 21.94 | 0.00 |
| Pain | 21.0 | 22.3 | 94 | 15.0 | 22.7 | 66 | 6.0 | 21.4 | 28 | 0.02 | 0.89 |
| Other | 16.0 | 17.0 | 94 | 8.0 | 12.1 | 66 | 8.0 | 28.6 | 28 | 3.77 | 0.05 |
| Medical service use | |||||||||||
| Neuroimaging | 30.0 | 31.9 | 93 | 13.0 | 19.7 | 65 | 17.0 | 60.7 | 28 | 15.22 | 0.00 |
| MRI | 13.0 | 13.8 | 93 | 3.0 | 4.5 | 65 | 10.0 | 35.7 | 28 | 15.72 | 0.00 |
| CT | 23.0 | 24.5 | 93 | 11.0 | 16.7 | 65 | 12.0 | 42.9 | 28 | 7.07 | 0.01 |
| Referring physician | |||||||||||
| General practitioner | 56.0 | 59.6 | 82 | 49.0 | 75.4 | 56 | 7.0 | 25.0 | 26 | 19.79 | 0.00 |
| Pediatrician | 15.0 | 16.0 | 82 | 6.0 | 9.2 | 56 | 9.0 | 32.1 | 26 | 7.79 | 0.01 |
| Neurologist | 8.0 | 8.5 | 82 | 1.0 | 1.5 | 56 | 7.0 | 25.0 | 26 | 13.93 | 0.00 |
| Other | 5.0 | 5.3 | 82 | 2.0 | 3.1 | 56 | 3.0 | 10.7 | 26 | 2.31 | 0.13 |
| School attendance | |||||||||||
| At preinjury level | 13.0 | 13.8 | 93 | 13.0 | 20.0 | 65 | 0.0 | 0.0 | 28 | 6.40 | 0.01 |
| With accommodations | 52.0 | 55.9 | 93 | 38.0 | 58.5 | 65 | 14.0 | 50.0 | 28 | 0.46 | 0.50 |
| Not attending school | 14.0 | 14.9 | 93 | 7.0 | 10.8 | 65 | 8.0 | 28.6 | 28 | 4.73 | 0.03 |
| Not mentioned | 13.0 | 13.8 | 93 | 7.0 | 10.8 | 65 | 6.0 | 21.4 | 28 | 1.93 | 0.17 |
| Continuous variables (mean±SD) | |||||||||||
| Age in years (mean±SD) | 15.26 | 1.78 | 94 | 15.42 | 1.61 | 66 | 14.86 | 2.10 | 28 | 777.00b | 0.22 |
| RPQ total score (mean±SD) | 33.07 | 12.87 | 87 | 31.09 | 12.25 | 62 | 37.97 | 13.30 | 25 | 535.00b | 0.02 |
| PROMIS total score (mean±SD) | 14.38 | 7.14 | 84 | 13.75 | 7.11 | 60 | 15.96 | 7.14 | 24 | 599.00b | 0.23 |
| KADS total score (mean±SD) | 5.86 | 4.21 | 85 | 5.66 | 4.18 | 62 | 6.39 | 4.33 | 23 | 626.00b | 0.39 |
| Number of health care providers | 2.68 | 2.49 | 94 | 1.92 | 1.76 | 66 | 4.46 | 3.05 | 28 | 407.00b | 0.00 |
| Number of prescription medications | 0.53 | 1.02 | 94 | 0.36 | 0.69 | 66 | 0.93 | 1.50 | 28 | 774.50b | 0.13 |
| Months since injury at consult | 6.62 | 4.39 | 93 | 6.36 | 4.21 | 66 | 7.24 | 4.84 | 27 | 842.50b | 0.68 |
TABLE 1. Descriptive statistics of the complete sample, no identified somatization group, and identified somatization groupa
Adolescents identified with somatization were more likely to have been referred to the clinic by a specialist than those without somatization; the majority of adolescents without identified somatization were referred by their general practitioner. Furthermore, adolescents with somatization had seen more health care providers and were more likely to have had neuroimaging after their injury than those without. Adolescents without somatization were more likely to be attending school at a preinjury level than those with somatization, who were more likely to not be attending school.
Spearman rank intercorrelations among variables for the complete sample are reported in Table 2. Significant positive associations were found among the scores on the RPQ, KADS Depression, and PROMIS Anxiety scales. Higher PROMIS Anxiety scores were also associated with being female, and higher PROMIS Anxiety and KADS Depression scores were both associated with older age. Seeing more health care providers was correlated with neuroimaging done after injury, a greater number of months since injury, and a greater number of prescription medications at the time of the physiatry consult. More prescriptions were also correlated with not attending school at a preinjury level.
| Variable | Sex | Loss of consciousness | Months since injury | RPQ | Atypical symptoms | PROMIS | KADS | History of pain or MUS | Number of health care providers | Number of prescription medications | Neuroimaging | School attendanceb | Identified somatization group |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | 0.07 | –0.19 | 0.11 | 0.16 | –0.12 | 0.23* | 0.23* | –0.03 | 0.12 | 0.20* | –0.15 | –0.15 | –0.13 |
| Sex (female, N=1) | — | –0.06 | –0.03 | 0.27* | 0.08 | 0.24* | 0.16 | 0.07 | –0.06 | –0.10 | –0.10 | 0.14 | 0.06 |
| Loss of consciousness | — | — | –0.03 | 0.18 | –0.23* | 0.14 | 0.11 | –0.07 | –0.00 | 0.06 | 0.15 | 0.03 | 0.08 |
| Months since injury | — | — | — | 0.01 | 0.15 | –0.09 | 0.08 | –0.02 | 0.34** | 0.01 | 0.24** | 0.25* | 0.04 |
| RPQ total | — | — | — | — | –0.00 | 0.57** | 0.63** | 0.03 | 0.12 | 0.17 | 0.04 | 0.01 | 0.24* |
| Presence of atypical persistent symptoms after concussion | — | — | — | — | — | 0.09 | –0.09 | 0.16 | 0.20 | 0.03 | 0.23* | 0.01 | 0.32** |
| PROMIS total | — | — | — | — | — | — | 0.66** | –0.13 | –0.03 | 0.13 | –0.07 | –0.19 | 0.13 |
| KADS total | — | — | — | — | — | — | — | –0.10 | –0.03 | 0.16 | –0.05 | 0.01 | 0.09 |
| History of chronic pain or medically unexplained symptoms | — | — | — | — | — | — | — | — | 0.01 | –0.16 | 0.14 | –0.10 | 0.26* |
| Number of health care providers | — | — | — | — | — | — | — | — | — | 0.27** | 0.37** | –0.01 | 0.45** |
| Number of prescription medications | — | — | — | — | — | — | — | — | — | — | 0.11 | –0.23* | 0.16 |
| Neuroimaging | — | — | — | — | — | — | — | — | — | — | — | 0.10 | 0.40** |
| Attending school at a preinjury level | — | — | — | — | — | — | — | — | — | — | — | — | –0.27* |
TABLE 2. Bivariate correlations between independent variables and outcome groupa
A binary logistic regression was then conducted to clarify the relationship between somatization and each demographic or clinical feature, while accounting for the other features as covariates. The regression was conducted using the following covariates: RPQ total score, presence of atypical symptoms after concussion, PROMIS Anxiety total score, KADS Depression total score, presence of a history of chronic pain or medically unexplained symptoms, number of health care providers seen after injury, having neuroimaging done after injury, number of prescription medications, and attending school at a preinjury level. Outcome was the presence versus absence of identified somatization. The analysis also accounted for age, sex, months since injury, and loss of consciousness. History of chronic pain or medically unexplained symptoms as well as school attendance were dropped from the analysis due to small cell count.
The results of the logistic regression are reported in Table 3 (see Figure S4 in the online supplement). The presence of atypical symptoms after concussion, having neuroimaging done after injury, and a greater number of health care providers seen for the injury were uniquely associated with somatization, when adjusting for all other covariates (overall model p<0.001 level, Nagelkerke pseudo-R2=0.64) (30). Notably, there were no differences between groups on the mental health indicators of depression and anxiety.
| Unadjusted modelb | Adjusted modelc | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Independent variable | Odds ratiod | 95% CI | p | B | SE | Wald’s χ2 | p | Odds ratiod | 95% CI |
| Persistent symptoms after concussion | |||||||||
| RPQ total score | 1.05 | 1.01, 1.09 | 0.03 | 0.09 | 0.05 | 3.71 | 0.05 | 1.09 | 1.00, 1.20 |
| Atypical symptoms after concussion | 4.38 | 1.67, 11.44 | 0.00 | 2.88 | 1.28 | 5.03 | 0.03 | 17.83 | 1.44, 221.05 |
| Mental health | |||||||||
| PROMIS total score | 1.05 | 0.98, 1.12 | 0.20 | –0.02 | 0.07 | 0.06 | 0.81 | 0.98 | 0.85, 1.14 |
| KADS total score | 1.04 | 0.93, 1.17 | 0.48 | 0.22 | 0.17 | 1.67 | 0.20 | 1.24 | 0.90, 1.72 |
| Medical service use | |||||||||
| Number of health care providers | 1.65 | 1.29, 2.11 | 0.00 | 0.98 | 0.37 | 7.09 | 0.01 | 2.67 | 1.30, 5.50 |
| Number of prescription medications | 1.69 | 1.06, 2.68 | 0.03 | –0.20 | 0.45 | 0.20 | 0.65 | 0.82 | 0.34, 1.96 |
| Neuroimaging | 6.30 | 2.39, 16.64 | 0.00 | 2.07 | 0.93 | 4.94 | 0.03 | 7.93 | 1.28, 49.29 |
| Controls | |||||||||
| Age (years) | 0.84 | 0.65, 1.07 | 0.16 | –0.54 | 0.30 | 3.40 | 0.07 | 0.58 | 0.33, 1.04 |
| Sex | 1.34 | 0.51, 3.51 | 0.55 | –0.64 | 1.08 | 0.36 | 0.55 | 0.53 | 0.06, 4.33 |
| Months since injury | 1.05 | 0.95, 1.15 | 0.38 | –0.30 | 0.19 | 2.54 | 0.11 | 0.74 | 0.51, 1.07 |
| Loss of consciousness | 1.86 | 0.39, 8.92 | 0.44 | 1.73 | 1.61 | 1.16 | 0.28 | 5.65 | 0.24, 132.29 |
TABLE 3. Summary of logistic regression for variables predicting identified somatizationa
Discussion
We described characteristics of adolescents with persistent symptoms after concussion with and without physician-identified somatization in order to take the first step toward elucidating how clinicians identify somatization in this population. Key differences in health characteristics were observed between adolescents that physicians did and did not identify with somatization. Adolescents with identified somatization were more likely to have a history of premorbid chronic pain or medically unexplained symptoms prior to their injury. Postinjury, they reported more severe and atypical neurological and psychiatric symptoms after concussion, had poorer school attendance, saw more medical specialists, and obtained more neuroimaging and health services compared with those without identified somatization.
When somatization occurs in the context of a medical condition, although the symptoms may be consistent with the condition, the severity of the symptoms, along with the associated thoughts, feelings and behaviors are in excess of what is typically expected (31). The findings of the present study suggest that clinicians might apply the same principle to the identification of somatization in adolescents with persistent symptoms after concussion: Patients with identified somatization had more severe self-reported symptoms after concussion. This is also consistent with previous adult studies using self-report somatization inventories in which preinjury and immediate postinjury somatization inventory scores were related to more persistent symptoms after concussion in the months following the injury (7–10). Our results expand upon these findings by demonstrating these same patterns in a sample using a more clinically applicable and nuanced method of assessing somatization that, unlike symptom inventories, could also be capturing the biopsychosocial complexities at play in an adolescent’s experience of somatization and physical symptoms.
Adolescents with identified somatization were also more likely to experience atypical symptoms after concussion spanning multiple body systems. Of these, the most striking difference between those with and without identified somatization was in the experience of atypical neurological symptoms. These included sensory and motor (e.g., limb numbness or weakness), visual (e.g., tunnel vision, loss of color vision) and cognitive symptoms (e.g., aphasia, inability to recognize family members), and are not consistent with a concussive injury (24). Instead, they are characteristic of functional neurological (conversion) disorder (31). The greater burden of these atypical neurological symptoms in the identified somatization group suggests that clinicians are using these symptoms, which are inconsistent with a concussive injury, to aid in their identification of somatization. This is the first study to identify and describe these atypical symptoms in a postconcussive population, which may represent an uncommon but specific marker that clinicians can use to identify somatization after concussion.
Although adolescents with identified somatization reported more severe and atypical physical symptoms, the two groups did not differ in their self-report anxiety and depression symptoms, despite consistent associations between anxiety and depression and somatization in the general somatization literature (13). This may be due to elevated symptoms of anxiety and depression in the sample as a whole: Mean scores on the depression inventory came close to reaching cut-offs for a diagnosis of depression in both groups in this sample, and mood disturbances are common after concussion (3). However, this finding could also reflect that emotional distress may be experienced as physical symptoms instead of explicit emotional symptoms in adolescents with somatization, as per Lipowski’s theory of somatization (6). In any case, in this sample mood symptoms do not appear to be a factor used by clinicians to differentiate between adolescents with and without somatization.
The greater medical service use seen in adolescents with clinician-identified somatization in this sample suggests that clinicians may take medical service use into account when identifying somatization. Heavy use of medical services by adult patients with somatization is well documented and may be driven by health anxiety (14, 32, 33). High rates of medical service use among patients with somatization may also reflect the common pattern of having to see numerous physicians before finding any explanation or relief from symptoms (34), due to a dichotomous biomedical model of physical symptoms held by both patients and clinicians in which the role of emotional and psychosocial factors may not be simultaneously considered.
School attendance, which is often affected after concussion (2, 35), was poorer in adolescents with identified somatization compared with those without. This further reduction in school attendance observed in adolescents with identified somatization has not previously been described, which suggests that clinicians may take functional impairment into account when evaluating adolescents with persistent symptoms after concussion for somatization. However this finding might also reflect the greater symptom severity in the group of adolescents with identified somatization; it might also reflect psychosocial factors not evaluated in this study, such as family attitudes toward illness, care provider recommendations, and parental concern about symptoms.
Limitations
This study has several limitations. First, it was a retrospective chart review, and variables and measures used in this study were limited to what was available in patient records. While physiatry consult notes, the source of many variables of interest, generally included comparable information, these notes were not standardized and could have been missing information or could have been biased by what the clinician believed was relevant to include. Due to the variability in information provided in clinicians’ consult notes, we could not reliably apply DSM-5 criteria to the chart to determine the presence of somatization; therefore, we used clinicians’ documentation of somatization instead. Additionally, psychosocial factors were not reliably mentioned in physiatry reports, meaning that important intra- and interindividual factors related to somatization in adolescents such as emotional regulation patterns, family and social influences, and stress and trauma could not be reliably extracted from the chart (15). These psychosocial factors represent an important piece of understanding somatization in adolescents that might affect clinicians’ identification of somatization that is missing from this study. However, this study is the first step toward a more holistic and clinically relevant understanding of how somatization is identified in this population in clinical practice, which may serve as a foundation for further exploration of psychosocial factors in the development and identification of somatization in adolescents with persistent symptoms after concussion in the future. Second, this study had only one reviewer who extracted data from charts and was not blinded to study hypotheses. Although data extraction followed standardized procedures, this may be a potential additional source of bias. Third, records were sourced from a tertiary care clinic, and only patients who had seen a physiatrist were included due to practical limitations. Patients in this study are therefore likely to have more severe and persistent symptoms after concussion than the general population of adolescents recovering from a concussion. Our understanding of the relationship between somatization and persistent symptoms after concussion would be aided by the addition of a third group of adolescents who sustained a concussion but did not develop persistent symptoms.
Conclusions
Somatization is a prominent and potentially treatable clinical feature of persistent symptoms after concussion for some adolescents that can carry a high degree of functional impairment and burden of suffering if left untreated. Early identification of somatization following concussion in youths is important for early intervention and reducing the risk of somatization-related harms. The study findings offer insight into how clinicians identify somatization in adolescents with persistent symptoms after concussion in clinical practice, providing descriptions of clinical characteristics that may aid in the timely identification and treatment of somatization in this population and the prevention of long-term negative impacts of somatization on adolescent development and functioning. Our understanding of these concerns would be further aided by prospective studies using a clinician-based approach that follows structured diagnostic criteria, such as DSM-5, to identify and diagnose somatization in adolescents with persistent symptoms after concussion. This approach would more thoroughly capture the psychosocial and developmental complexities known to play important roles in adolescent somatization that cannot be captured by symptom scales or gleaned from charts. A longitudinal approach, beginning from adolescents’ time of injury and following them for an extended period of time, would provide additional insight into how somatization is related to the adolescents’ path to recovery following concussion.
1 : Clinical risk score for persistent postconcussion symptoms among children with acute concussion in the ED. JAMA 2016; 315:1014–1025Crossref, Medline, Google Scholar
2 : Association of persistent postconcussion symptoms with pediatric quality of life. JAMA Pediatr 2016; 170:e162900Crossref, Medline, Google Scholar
3 : Postconcussion syndrome: a review. J Child Neurol 2016; 31:57–67Crossref, Medline, Google Scholar
4 : Systematic review of multivariable prognostic models for mild traumatic brain injury. J Neurotrauma 2015; 32:517–526Crossref, Medline, Google Scholar
5 : Predictors of clinical recovery from concussion: a systematic review. Br J Sports Med 2017; 51:941–948Crossref, Medline, Google Scholar
6 : Somatization: the concept and its clinical application. Am J Psychiatry 1988; 145:1358–1368Crossref, Medline, Google Scholar
7 : Preinjury somatization symptoms contribute to clinical recovery after sport-related concussion. Neurology 2016; 86:1856–1863 Crossref, Medline, Google Scholar
8 : History of somatization is associated with prolonged recovery from concussion. J Pediatr 2016; 174:39–44.e1Crossref, Medline, Google Scholar
9 : Psychological factors associated with delayed symptom resolution in children with concussion. J Pediatr 2016; 174:27–32.e1Crossref, Medline, Google Scholar
10 : Somatization in post-concussion syndrome: a retrospective study. Cureus 2016; 8:e743Medline, Google Scholar
11 : Screening for DSM-5 somatic symptom disorder: diagnostic accuracy of self-report measures within a population sample. Psychosom Med 2017; 79:974–981Crossref, Medline, Google Scholar
12 : Relationship of pain and symptoms to pubertal development in adolescents. Pain 2005; 118:201–209Crossref, Medline, Google Scholar
13 : Epidemiology of the association between somatoform disorders and anxiety and depressive disorders: an update. Psychosom Med 2007; 69:860–863Crossref, Medline, Google Scholar
14 : Somatization in pediatric primary care: association with psychopathology, functional impairment, and use of services. J Am Acad Child Adolesc Psychiatry 1999; 38:1093–1101Crossref, Medline, Google Scholar
15 : Relations between anxiety sensitivity, somatization, and health-related quality of life in children with chronic pain. J Pediatr Psychol 2012; 37:808–816Crossref, Medline, Google Scholar
16 : A developmental perspective on functional somatic symptoms. J Pediatr Psychol 2008; 33:547–562Crossref, Medline, Google Scholar
17 : Fibromyalgia syndrome: a somatoform disorder? Eur J Pain 2014; 18:1052–1059Crossref, Medline, Google Scholar
18 : Somatization and chronic pain. Acta Anaesthesiol Scand 2001; 45:1114–1120Crossref, Medline, Google Scholar
19 : The association of irritable bowel syndrome and somatization disorder. Ann Clin Psychiatry 2001; 13:25–30Crossref, Medline, Google Scholar
20 : Assessment and management of medically unexplained symptoms. BMJ 2008; 336:1124–1128Crossref, Medline, Google Scholar
21 : The relationship between chronic fatigue and somatization syndrome: a general population survey. J Psychosom Res 2007; 63:147–156Crossref, Medline, Google Scholar
22 : Medically unexplained symptoms in children and adolescents. Clin Psychol Rev 2007; 27:855–871Crossref, Medline, Google Scholar
23 : A methodology for conducting retrospective chart review research in child and adolescent psychiatry. J Can Acad Child Adolesc Psychiatry 2006; 15:126–134Medline, Google Scholar
24 : The Rivermead Post Concussion Symptoms Questionnaire: a measure of symptoms commonly experienced after head injury and its reliability. J Neurol 1995; 242:587–592Crossref, Medline, Google Scholar
25 : Which symptom assessments and approaches are uniquely appropriate for paediatric concussion? Br J Sports Med 2009; 43(Suppl 1):i13–i22Crossref, Medline, Google Scholar
26 : Screening for adolescent depression: comparison of the Kutcher Adolescent Depression Scale with the Beck Depression Inventory. J Child Adolesc Psychopharmacol 2002; 12:113–126Crossref, Medline, Google Scholar
27 : An item response analysis of the pediatric PROMIS Anxiety and Depressive Symptoms scales. Qual Life Res 2010; 19:595–607Crossref, Medline, Google Scholar
28 : Psychometric properties of the PROMIS pediatric scales: precision, stability, and comparison of different scoring and administration options. Qual Life Res 2014; 23:1233–1243Crossref, Medline, Google Scholar
29 : Contribution to the validation of the Kutcher Adolescent Depression Scale (KADS-6) in a Portuguese population. Psicol Reflex Crit 2015; 28:313–321Crossref, Google Scholar
30 : A note on a general definition of the coefficient of determination. Biometrika 1991; 78:691–692Crossref, Google Scholar
31 : Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC, American Psychiatric Publishing, 2013Crossref, Google Scholar
32 : Somatization increases medical utilization and costs independent of psychiatric and medical comorbidity. Arch Gen Psychiatry 2005; 62:903–910Crossref, Medline, Google Scholar
33 : Somatization and health anxiety as predictors of health care use. Psychosom Med 2012; 74:656–664Crossref, Medline, Google Scholar
34 : The challenge of diagnosing non-specific, functional, and somatoform disorders: a systematic review of barriers to diagnosis in primary care. J Psychosom Res 2016; 80:1–10Crossref, Medline, Google Scholar
35 : Academic effects of concussion in children and adolescents. Pediatrics 2015; 135:1043–1050Crossref, Medline, Google Scholar



