A Systematic Review of Fragile X–Associated Neuropsychiatric Disorders
Abstract
Objective:
Fragile X premutation carriers are reported to have increased neuropsychiatric problems, and thus the term fragile X–associated neuropsychiatric disorders (FXAND) has been proposed. Unfortunately, published prevalence estimates of these phenomena are inconsistent. This systematic review clarified this issue by reviewing both fragile X premutation prevalence in patients with neurodevelopmental disorders and psychiatric disorder prevalence in premutation carriers without fragile X–associated tremor/ataxia syndrome (FXTAS). Average prevalence was derived from studies that used semistructured clinical interviews, diagnostic criteria, and validated rating scales.
Methods:
Forty-six studies were reviewed. The rate of fragile X premutation in neurodevelopmental disorders was assessed from five studies. Probands with neurodevelopmental disorders were more likely than those in the general population to be premutation carriers. The rate of psychiatric disorders in premutation carriers was assessed from five studies for neurodevelopmental, 13 studies for mood, 12 studies for anxiety, and two studies for psychotic disorders. The phenotype and sex distribution among premutation carriers were similar to those with fragile X syndrome.
Results:
Compared to control group and general population estimates, the most prevalent psychiatric disorders were neurodevelopmental disorders, anxiety disorders, and bipolar II disorder. Psychiatric disorders were also more common in males. Most studies relied only on past medical history to define the prevalence of psychiatric disorders, yielding variability in results.
Conclusions:
Future studies are needed to avoid bias by identifying cohorts from population-based sampling, to describe cohort demographic characteristics to elucidate differences in age and sex, and to prioritize the use of validated psychiatric assessment methods.
Fragile X syndrome is due to a trinucleotide expansion of more than 200 CGG repeats in the fragile X messenger ribonucleoprotein 1 gene (FMR1) on the X chromosome. It is the most common inherited cause of intellectual disability (ID) (1) and has a well-established association with psychiatric phenomena, such as anxiety disorders (2). Fragile X premutation, defined as 55–200 CGG repeats, is associated with conditions such as primary ovarian insufficiency (FXPOI) and a tremor/ataxia syndrome (FXTAS) (3). FXTAS is a neurodegenerative disease that manifests mostly in men over 50 years of age with an intention tremor, cerebellar gait ataxia, parkinsonism, and neuropathy (4). Symptomatic FXTAS is also associated with deterioration in cognition, often leading to dementia, and an increase in neuropsychiatric symptoms such as apathy (5). Of the premutation carriers who do not have FXTAS (predominantly women and younger men), there remains an increased prevalence of psychiatric disorders (3, 6). There is debate whether psychiatric conditions in premutation carriers are prodromal to FXTAS or independent of FXTAS pathogenesis (7). Accordingly, the term fragile X–associated neuropsychiatric disorders (FXANDs) was proposed in 2018 to recognize these neuropsychiatric manifestations among fragile X premutation carriers without FXTAS (6). The term fragile X-associated neuropsychiatric conditions (FXANCs) has also been proposed to distinguish neuropsychiatric conditions in premutation carriers that do not meet the threshold of a disorder and to distinguish these neuropsychiatric phenomena from other fragile X–associated conditions, such as FXTAS and FXPOI (8).
The neuropathology of fragile X syndrome is characterized by a deficiency of fragile X protein (FMRP), which is required for normal brain development (9). As the FMR1 gene is not completely silenced with less than 200 CGG trinucleotide repeats, it was originally proposed that fragile X premutation was not associated with the fragile X phenotype (10). It is now known that fragile X premutation carriers have increased FMR1 mRNA, decreased FMRP, and polyglycine-containing protein (FMRpolyG) and ubiquitin-positive intranuclear inclusions. Toxic polypeptides are produced from FMR1 mRNA, and protein sequestration occurs due to interactions with the expanded CGG-repeat RNA. These changes are associated with neuronal cell death and dysfunction (11). The neuropathology of premutation carriers with or without FXTAS is similar in that both have mitochondrial dysfunction; those without FXTAS, however, have fewer intranuclear inclusions in astrocytes and lack white matter disease (11, 12). Structural neuroimaging of premutation carriers without FXTAS has shown generalized cerebral atrophy, increased ventricular size, and regional changes such as decreased grey matter in the dorsomedial frontal regions. These neuroimaging changes in premutation carriers are milder and less frequent than in those with FXTAS (13).
Despite these established neuropathological and radiological abnormalities in premutation carriers, the neuropsychiatric phenotype of premutation carriers without FXTAS is not clearly defined. The rate of psychiatric disorders in premutation carriers without FXTAS reported in previous reviews varies significantly. For example, rates of developmental delay have been reported to range from 6% to 32% (3), rates of depression from 20% to 43% (14), and rates of anxiety from 12% to 41% (15). In contrast, the prevalence of neuropsychiatric phenomena in premutation carriers with FXTAS has been described in prior reviews with a clear phenotype of apathy, irritability, depression, anxiety, and a neurodegenerative cognitive impairment that progresses to dementia (4, 16, 17). Accordingly, there is a consensus on the clinical presentation of FXTAS with established diagnostic criteria (4). This systematic review thus aimed to define a consensus on the rate of psychiatric disorders in premutation carriers without FXTAS. Also, as fragile X syndrome is predominately associated with neurodevelopmental disorders, the prevalence of the premutation in neurodevelopmental disorders was also reviewed to strengthen the association between the FMR1 gene premutation and FXAND phenotype.
Methods
Search Strategy
This review was completed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (18). Search methods, selection criteria, and data collection were prospectively planned. Keywords included “fragile X–associated neuropsychiatric disorder,” “fragile X–associated neuropsychiatric condition,” “FXAND,” “fragile X premutation,” and “FMR1 premutation.” For each study, the title and abstract were reviewed for reference to psychiatric disorders in patients with fragile X premutation and fragile X genetic testing in probands with neurodevelopmental disorders. The full text was then reviewed for eligibility criteria. Finally, the reference lists from the included studies were searched for further citations.
Information Sources
Literature was reviewed from two main medical databases: PubMed and Web of Science. There was no time limitation set for the published literature. The databases were accessed on July 31, 2021.
Eligibility Criteria
Studies were included if they met all of the following criteria:
Observational studies were published in a peer-reviewed journal and in English.
Studies reported the prevalence of either psychiatric disorders in patients with fragile X premutation without FXTAS or fragile X premutation in probands diagnosed with a neurodevelopmental disorder.
Studies reported psychiatric disorders recognized by standardized diagnostic criteria (e.g., the Diagnostic Statistical Manual [DSM] criteria, International Classification of Diseases [ICD] criteria, or Research Diagnostic Criteria [RDC]).
For studies that assessed for ID, studies used an assessment measure of both intellect and adaptive functioning (e.g., studies that just used an assessment of intellect to diagnose ID were not included).
Intervention studies (e.g., studies that assessed anxiety associated with genetic testing), studies that duplicated the cohort from another study (if this occurred, studies with the smaller sample size, studies other than the original if the cohorts were identical, studies with the least information, or studies with a less comprehensive psychiatric assessment method were excluded), and studies that skewed the prevalence of the psychiatric disorder(s) due to selection bias were excluded.
Data Extraction
The following data were extracted from each study: study characteristics, sample characteristics, and prevalence. Study characteristics included study design, psychiatric assessment method, inclusion of a control group, and genetic testing. Sample characteristics included the sample size, sex, age, and CGG repeat length (either as mean±SD or the study’s definition of a premutation range). All data were rounded to one decimal place.
Psychiatric Assessment Methods
Psychiatric disorders were separated into four categories based on the following assessment methods: diagnostic criteria, rating scales, subscales within larger instruments, and medical history.
Diagnostic criteria.
Studies that used diagnostic criteria were documented as those that used a semistructured or nonstructured clinical interview. The use of a semistructured clinical interview, in combination with the assessment of diagnostic criteria, is considered the gold standard to diagnose psychiatric disorders (19). With the exception of studies assessing autism, all studies that used a semistructured interview used it in combination with an assessment of diagnostic criteria. The semistructured clinical interviews included the Structured Clinical Interview for DSM Disorders (SCID) (20), Anxiety Disorders Interview Schedule (ADIS) (21), Schedule for Affective Disorders and Schizophrenia (SADS) (22), Diagnostic Interview for Genetic Studies (DIGS) (23), Family Informant Schedule Criteria (FISC) (24), Autism Diagnostic Observation Scale (25), and Autistic Diagnostic Interview Revised (26). Diagnostic criteria included the DSM criteria, ICD criteria, and RDC. The RDC was used in combination with the SADS, and the DSM criteria were used in combination with the SCID, ADIS, FISC, and DIGS.
Rating scales.
Studies that used rating scales or subscales were only included if they specified the name of the instrument used and the instrument mapped to ICD, DSM, or RDC criteria. For studies that used rating scales, the cutoff scores used to define the presence of the psychiatric disorder were documented. The rating scales included the Center for Epidemiologic Studies Depression Scale (CES-D) (27), Depression Anxiety Stress Scales (28), Hospital Anxiety and Depression Scale (HADS) (29), Social Phobia and Anxiety Inventory (30), Liebowitz Social Anxiety Scale (31), Brown Attention-Deficit Disorder Scale for Adults (32), Conners’ Parent Rating Scales–Revised: Short Form (CPRS-R:S) (33), Autism Spectrum Quotient (AQ) (34), Mullen Scales of Early Learning (35), Vineland Adaptive Behavior Scale (36), and Griffiths Mental Developmental Scale (37).
Subscales.
The included subscales were all subsections of larger instruments. They included the anxiety section of the Developmental Behavior Checklist (38), the anxiety section of the Profile of Mood States (POMS) (39), and the anxiety, depression, and obsessive-compulsive disorder (OCD) sections of the Symptom Checklist–90–Revised (40).
Medical history.
The studies that reported psychiatric disorder based on medical history were separated into those that used medical records or self-reported history (e.g., psychiatric history was disclosed by the patient but not validated by their medical records).
Psychiatric Disorders
As the majority of studies used DSM-IV diagnostic criteria, psychiatric disorders were grouped into categories from the DSM-IV. This included neurodevelopmental, depressive, bipolar, anxiety, and psychotic disorders. Neurodevelopmental disorders included developmental delay (DD), ID, attention-deficit hyperactivity disorder (ADHD), and autism spectrum disorder (ASD). Depressive and bipolar disorders (mood disorders) included major depressive disorder, persistent depressive disorder (dysthymia), and bipolar disorders I and II. Anxiety disorders included generalized anxiety disorder (GAD), panic disorder, social phobia, specific phobia, agoraphobia, OCD, and posttraumatic stress disorder (PTSD). Psychotic disorders included schizophrenia, schizoaffective disorder, and psychotic disorder not otherwise specified. Studies that used differing terminologies for the same psychiatric disorder were grouped together under the same DSM diagnostic label (e.g., learning disability was grouped with ID).
Estimation of Prevalence
Overall current (point prevalence) and lifetime prevalence estimates of psychiatric disorders were calculated as a weighted average percentage.
Quality Appraisal
The quality of the studies was assessed using the Joanna Briggs Institute Prevalence Critical Appraisal (JBIPCA) Checklist for prevalence studies (41). Each study was assessed by nine criteria. They were graded as one for meeting each criterion or zero for not meeting the criterion. A total score ranging from zero to nine was assigned to each study, with nine representing the highest quality. These data are available in the online supplement accompanying the online version of this article.
Results
The systematic literature search generated 3,100 articles, of which 1,827 articles remained after duplicate removal. One hundred fifty-one articles were reviewed in full, with 34 meeting eligibility criteria. An additional 12 articles were identified after reference list review (Figure 1). The final 46 articles assessed neurodevelopmental (N=29) (42–70), mood (N=17) (45–47, 51, 71–83), anxiety (N=21) (45–48, 51, 71–74, 76–87), and psychotic (N=3) (48, 77, 83) disorders.
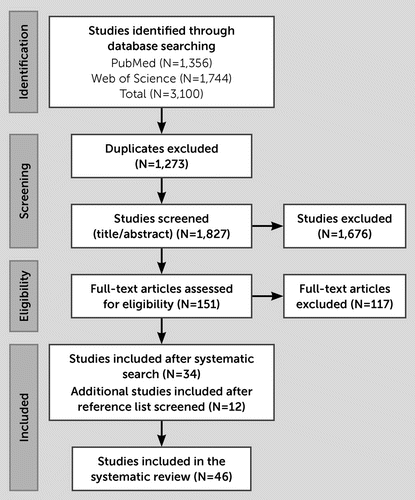
FIGURE 1. PRISMA flowchart of studies assessing FXAND
Prevalence of Fragile X Premutation in Those With Neurodevelopmental Disorders
Five studies were used to assess the prevalence of fragile X premutation in probands with neurodevelopmental disorders (52–56). Three studies used diagnostic criteria (52–54), one used a rating scale (56), one used both a semistructured interview and rating scale (55), none used subscales, and 15 used medical history (56–70). Three out of the five studies used for the prevalence estimates separated sexes (53–55), and no studies had a control group (Table 1).
| Total | Male | Female | ||||
|---|---|---|---|---|---|---|
| Neurodevelopmental disorder | Prevalence (%) | N | Prevalence (%) | N | Prevalence (%) | N |
| Developmental delay/intellectual disability | 5.0 | 239 | — | — | — | — |
| Attention-deficit hyperactivity disorder | 6.3 | 16 | — | — | — | — |
| Autism spectrum disorder | 2.6 | 691 | 1.5 | 589 | 0.0 | 69 |
TABLE 1. Weighted mean prevalence of fragile X premutation in neurodevelopmental disorders (probands)a
The average premutation prevalence in probands with DD/ID was 5.0% (52, 55, 56); with ADHD, 6.3% (52); and with ASD, 2.6% (52–55). Sex differences could only be assessed in ASD, with the fragile X premutation being more prevalent in males (1.5%) than females (0.0%) (53–55). However, these data were skewed with two studies reporting a prevalence of 0.0% for both sexes (54, 55) and one study reporting a prevalence of 9.4% for males (53).
Prevalence of Psychiatric Disorders in Fragile X Premutation Carriers
Neurodevelopmental disorders.
Five studies were used to assess the prevalence of neurodevelopmental disorders in premutation carriers (42–46). Three studies used a semistructured interview or diagnostic criteria (42–44), two studies used rating scales (45, 46), no studies used subscales, and seven studies used medical history (43, 45, 47–51). Four of the five included studies separated sexes (42, 43, 45, 46) (Table 2). Three studies had a control group (44–46) (Table 3).
| Psychiatric disorder | Point prevalence | Lifetime prevalence | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Male | Female | Total | Male | Female | |||||||
| Prevalence (%) | N | Prevalence (%) | N | Prevalence (%) | N | Prevalence (%) | N | Prevalence (%) | N | Prevalence (%) | N | |
| Neurodevelopmental | ||||||||||||
| ADHD | 29.0 | 48 | 38.0 | 13 | 25.7 | 35 | — | — | — | — | — | — |
| ASD | — | — | — | — | — | — | 4.7 | 151 | 7.7 | 13 | 2.4 | 130 |
| Mood | ||||||||||||
| Any mood disorder | 5.4 | 93 | — | — | 5.4 | 93 | 48.3 | 207 | — | — | 49.7 | 171 |
| Any depressive disorder | 24.5 | 449 | 26.9 | 26 | 24.4 | 423 | 40.7 | 184 | — | — | 40.7 | 184 |
| Major depressive disorder | 4.3 | 93 | — | — | 4.3 | 93 | 38.4 | 389 | — | — | 40.2 | 252 |
| PDD | 0.0 | 93 | — | — | 0.0 | 93 | 12.5 | 401 | 33.3 | 24 | 11.2 | 341 |
| Any bipolar disorder | 1.1 | 93 | — | — | 1.1 | 93 | 8.7 | 241 | — | — | 8.8 | 205 |
| Bipolar I | — | — | — | — | — | — | 0.0 | 78 | — | — | 0.0 | 78 |
| Bipolar II | — | — | — | — | — | — | 11.3 | 124 | — | — | 11.3 | 124 |
| Anxiety | ||||||||||||
| Any anxiety disorder | 33.4 | 159 | — | — | 32.7 | 144 | 40.0 | 315 | — | — | 38.9 | 277 |
| GAD | 7.4 | 108 | — | — | 4.3 | 93 | 7.2 | 379 | — | — | 7.7 | 341 |
| Panic disorder | 5.9 | 389 | 11.5 | 26 | 5.8 | 348 | 13.5 | 305 | 12.5 | 24 | 13.6 | 243 |
| Agoraphobia | 2.8 | 108 | — | — | 3.2 | 92 | 3.6 | 197 | — | — | 4.1 | 171 |
| Specific phobia | 4.6 | 108 | — | — | 4.3 | 93 | 14.8 | 203 | — | — | 12.7 | 165 |
| Social phobia | 18.7 | 424 | 19.2 | 26 | 18.9 | 383 | 21.1 | 357 | 8.3 | 24 | 20.4 | 295 |
| OCD | 4.7 | 108 | — | — | 2.2 | 93 | 5.3 | 305 | 25.0 | 24 | 4.1 | 243 |
| PTSD | 0.0 | 108 | — | — | 0.0 | 93 | 8.9 | 203 | — | — | 10.3 | 165 |
| Psychotic | ||||||||||||
| Any psychotic disorder | — | — | — | — | — | — | 0.9 | 112 | — | — | 0.9 | 112 |
| Schizophrenia | — | — | — | — | — | — | 0.0 | 112 | — | — | 0.0 | 112 |
| Schizoaffective disorder | — | — | — | — | — | — | 0.0 | 112 | — | — | 0.0 | 112 |
| Psychotic disorder NOS | — | — | — | — | — | — | 0.9 | 112 | — | — | 0.9 | 112 |
TABLE 2. Weighted mean prevalence of psychiatric disorders in fragile X premutation carriers (nonprobands)a
| Psychiatric disorder | Point prevalence | Lifetime prevalence | ||||||
|---|---|---|---|---|---|---|---|---|
| FX PM | Control | FX PM | Control | |||||
| Prevalence (%) | N | Prevalence (%) | N | Prevalence (%) | N | Prevalence (%) | N | |
| ADHD | 25.7 | 35 | 8.6 | 35 | — | — | — | — |
| ASD | — | — | — | — | 10.3 | 59 | 0.0 | — |
| Any mood disorder | — | — | — | — | 50.4 | 78 | 30.0 | 60 |
| Any depressive disorder | 24.3 | 367 | 13.7 | 237 | 38.8 | 138 | 34.7 | 124 |
| PDD | — | — | — | — | 19.1 | 226 | 15.5 | 181 |
| Major depressive disorder | — | — | — | — | 34.1 | 202 | 30.1 | 149 |
| Any bipolar disorder | — | — | — | — | 12.5 | 112 | 7.0 | 100 |
| Bipolar I | — | — | — | — | 0.0 | 78 | 0.0 | 60 |
| Bipolar II | — | — | — | — | 11.6 | 78 | 3.4 | 60 |
| Any anxiety disorder | 57.8 | 66 | 29.2 | 75 | 38.9 | 138 | 29.0 | 124 |
| GAD | 26.7 | 15 | 3.2 | 31 | 8.4 | 202 | 3.3 | 149 |
| Panic disorder | 7.1 | 296 | 1.6 | 189 | 13.3 | 128 | 6.9 | 116 |
| Agoraphobia | 0.0 | 15 | 0.0 | 31 | 3.9 | 104 | 3.6 | 84 |
| Specific phobia | 6.7 | 15 | 12.9 | 31 | 30.8 | 26 | 41.7 | 24 |
| Social phobia | 21.8 | 331 | 8.5 | 224 | 24.5 | 226 | 8.8 | 181 |
| OCD | 20.0 | 15 | 3.2 | 31 | 7.8 | 128 | 4.3 | 116 |
| PTSD | 0.0 | 15 | 6.5 | 31 | 11.5 | 26 | 8.3 | 24 |
| Any psychotic disorder | — | — | — | — | 0.9 | 112 | 2.0 | 100 |
TABLE 3. Studies with a control group: prevalence of psychiatric disorders in fragile X premutation carriers compared to the prevalence among those in a control groupa
DD/ID: No studies met criteria to evaluate the prevalence of DD or ID in premutation carriers.
ADHD: The average point prevalence of ADHD was 29.0% (42, 45). The prevalence of ADHD was higher in males (38.0%) than females (25.7%) (42, 45). ADHD was also more prevalent in premutation carriers (25.7%) than in those in the control group (8.6%) (45).
ASD: The average prevalence of ASD was 4.7% (42–44, 46). The prevalence of ASD was higher in males (7.7%) than females (2.4%) (42, 43, 46). ASD was also more prevalent among premutation carriers (10.3%) than among those in the control group (0.0%) in the studies that included a control group (44, 46).
Mood disorders.
Thirteen studies were used to assess the prevalence of mood disorders (45, 46, 71–80, 83). Nine studies used a semistructured interview with diagnostic criteria (71–78, 83), four studies used a rating scale (45, 46, 79, 80), one study used a subscale from a larger instrument (81), and three studies used medical history (47, 51, 82). Twelve of the 13 studies separated sexes (45, 46, 72–80, 83) (Table 2). Eight studies had a control group (45, 46, 73, 76–79, 83) (Table 3).
The average lifetime and point prevalence of any mood disorder was 48.3% and 5.4%, respectively (71, 72, 77). All mood disorders were more common in premutation carriers than in those in the control group, with the exception of bipolar I disorder, which was 0.0% in both control and premutation carrier groups (77).
Depressive disorders: The average lifetime and point prevalence of all depressive disorders was 40.7% and 24.5%, respectively (45, 46, 73, 74, 77, 79, 80, 83). The average lifetime and point prevalence of major depressive disorder was 38.4% and 4.3%, respectively (71–77, 83). The average lifetime and point prevalence of dysthymia was 12.5% and 0.0%, respectively (71–74, 76–78, 83). The point prevalence of depressive disorders was similar between males and females (26.9% vs. 24.4%); however, males had an almost threefold increase in lifetime dysthymia (33.3% vs. 11.2%) (45, 46, 72–74, 76, 78–80, 83). No other sex differences could be assessed. Studies that included control groups reported only slightly higher lifetime prevalence (38.8% vs. 34.7%) but a clearly higher point prevalence (24.3% vs. 13.7%) of depressive disorders among those in the premutation carrier group than those in the control group (45, 46, 73, 77, 79, 83).
Bipolar disorders: The average lifetime and point prevalence of any bipolar disorder was 8.7% and 1.1%, respectively (71, 72, 74, 77, 83). There was variability in definition: one study (83) included bipolar I, bipolar II, and subthreshold bipolar disorders (cyclothymic disorder); two studies (72, 77) only included bipolar I and II; the final study (71) did not specify the types of bipolar disorders included. The lifetime prevalence of bipolar I was 0.0% (77), and lifetime prevalence of bipolar II was 11.3% (74, 77). No evaluation of sex differences could be made. The lifetime prevalence of any bipolar disorder was slightly higher in premutation carriers than in those in the control groups (12.5% vs. 7.0%) (77, 83).
Anxiety disorders.
Twelve studies were used to assessed the prevalence of anxiety disorders (45, 46, 71–74, 76–79, 83, 84). Nine studies used a semistructured interview with diagnostic criteria (71–74, 76–78, 83, 84), three studies used a rating scale (45, 46, 79), four studies used a subscale (80–82, 85), and six studies used medical history (45, 47, 48, 51, 86, 87). Ten out of the 12 studies separated sexes (45, 46, 72–74, 76–79, 83) (Table 2). Nine studies had a control group (45, 46, 73, 76–79, 83, 84) (Table 3).
The average lifetime and point prevalence of any anxiety disorder was 40.0% and 33.4%, respectively (46, 71–74, 77, 83, 84). The average lifetime prevalence of anxiety disorders was higher among premutation carriers than among those in the control group (38.9% vs. 29.0%) as was the point prevalence (57.8% vs. 29.2%) (46, 73, 77, 83, 84). Exceptions to this general tendency were that the point prevalence of PTSD was lower among premutation carriers than among those in the control groups (0.0% vs. 6.5%), as was that of specific phobia (6.7% vs. 12.9%) (84).
GAD: The average lifetime and point prevalence of GAD was 7.2% and 7.4%, respectively (71–74, 76, 77, 83, 84). No evaluation of sex differences could be made.
Panic disorder: The average lifetime and point prevalence of panic disorder was 13.5% and 5.9%, respectively (71–74, 77–79, 84). The lifetime prevalence of panic disorder was similar in males and females (12.5% vs. 13.6%); however, the point prevalence of panic disorder was higher in males than females (11.5% vs. 5.8%) (72–74, 77–79).
Phobias: The average lifetime and point prevalence of social phobia was 21.1% and 18.7%, respectively (45, 71–73, 76–79, 83, 84). The average lifetime and point prevalence of specific phobia was 14.8% and 4.6% respectively (71–74, 84). The average lifetime and point prevalence of agoraphobia was 3.6% and 2.8%, respectively (72, 73, 77, 84). The lifetime prevalence of social phobia was lower in males than females (8.3% vs. 20.4%); however, the point prevalence of social phobia was similar in males and females (19.2% vs. 18.9%) (45, 72, 73, 76–79, 83).
OCD: The average lifetime and point prevalence of OCD was 5.3% and 4.7%, respectively (71–74, 77, 78, 84). The lifetime prevalence of OCD was higher in males than females (25.0% vs. 4.1%) (72–74, 77, 78).
PTSD: The average lifetime and point prevalence of PTSD was 8.9% and 0.0%, respectively (71–74, 84). No evaluation of sex differences could be made.
Psychotic disorders.
Two studies were used to assess the prevalence of psychotic disorders (77, 83). Two studies used a semistructured interview with diagnostic criteria (77, 83), no studies used a rating scale or a subscale, and one study used medical history (48). Both studies used to assess the prevalence of psychotic disorders included only females (77, 83) (Table 2). Both studies had a control group (77, 83) (Table 3).
The average lifetime prevalence of any psychotic disorder was 0.9% (77, 83). The lifetime prevalence of both schizophrenia and schizoaffective disorder was 0.0% (77, 83). No evaluation of sex differences could be made. The lifetime prevalence of psychotic disorders was lower among premutation carriers than among those in control groups (0.9% vs. 2.0%) (77, 83).
Discussion
Summary of Results
This systematic review included 46 studies (42–87). For the prevalence of fragile X premutation in neurodevelopmental disorder probands, five studies were used (52–56). There was a prevalence of 5.0% for DD/ID, 6.3% for ADHD, and 2.6% for ASD. From these studies, probands with ASD were the least likely to have the fragile X premutation (2.6% vs. 5.0%–6.3%). The only sex difference assessed was for ASD, and the analysis revealed that males were more likely than females to have the fragile X premutation (1.5% vs. 0.0%).
For the prevalence of psychiatric disorders in premutation carriers, 17 studies were used (42–46, 71–80, 83, 84). The disorders that were more prevalent in premutation carriers than in those in the control group were neurodevelopmental and anxiety disorders, specifically ADHD (25.7% vs. 8.6%), ASD (10.3% vs. 0.0%), social phobia (24.5% vs. 8.8%), and GAD (8.4% vs. 3.3%). Bipolar II disorder was also more prevalent in premutation carriers (11.6% vs. 3.4%); however, the lifetime prevalence of most other mood disorders was similar in the premutation carrier and control groups. Psychotic disorders were less prevalent in the premutation carrier group than the control group (0.9% vs. 2.0%). For the studies that differentiated between sexes, psychiatric disorders were generally more common in males or similar between sexes. The only disorder significantly more prevalent in women was lifetime social phobia, which could be explained by inconsistencies in the data.
Fragile X Premutation in Those With Neurodevelopmental Disorders Compared to Population Estimates
The rate of fragile X premutation in the general population varies from 0.39% to 0.77% in females and 0.12% to 0.4% in males (88). The rates of fragile X premutation in the prevalence estimates included in this review were universally higher than the general population (2.6%–6.3% vs. 0.1%–0.8%). In contrast to the five studies used for these prevalence estimates (52–56), there were 15 studies with much larger cohorts that used medical history (56–70). These studies had rates of fragile X premutation generally similar to or less than the general population. Though this could be explained by a selection bias in the studies that used medical history with unknown assessment methods, thus increasing the risk of misclassification, these results are nonetheless important. Most of these studies that relied only on medical history suggested probands with neurodevelopmental disorders do not have higher rates of fragile X premutation.
Psychiatric Disorders in Premutation Carriers Compared to Population Estimates
The National Comorbidity Survey Replication (NCS-R) is one of the few studies to use a semistructured interview to assess psychiatric disorders in a large population-based cohort (89). Compared to NCS-R results (90–94), the lifetime rates of all psychiatric disorders except bipolar I disorder and psychotic disorders appeared to be higher in premutation carriers than the general population. The NCS-R study did not assess for ASD; however, the prevalence of ASD was also higher in premutation carriers than other general population estimates (4.7% vs. 0.2%) (95). The lifetime rates of psychiatric disorders in the control groups included in the reviewed studies were generally consistent with the NCS-R results, with the exception of depressive disorders and specific phobias. The control groups had much higher rates of major depressive disorder (30.1% vs. 16.6%), dysthymia (15.5% vs. 2.5%), and specific phobia (41.7% vs. 12.5%) in the reviewed studies than in the NCR-R population estimates (Table 4).
| Psychiatric disorder | FX PM | NCS-R |
|---|---|---|
| Childhood ADHD | 38.0%b | 8.1% |
| Adult ADHD | 25.7%b | 4.4%b |
| ASD | 4.7% | 0.2%c |
| Any mood disorder | 48.3% | 20.8% |
| Any depressive disorder | 40.7% | — |
| PDD | 12.5% | 2.5% |
| Major depressive disorder | 38.4% | 16.6% |
| Any bipolar disorder | 8.7% | 4.5% |
| Bipolar I | 0.0% | 1.0% |
| Bipolar II | 11.3% | 1.1% |
| Any anxiety disorder | 40.0% | 28.8% |
| GAD | 7.2% | 5.7% |
| Panic disorder | 13.5% | 4.7% |
| Agoraphobia | 3.6% | 1.4% |
| Specific phobia | 14.8% | 12.5% |
| Social phobia | 21.1% | 12.1% |
| OCD | 5.3% | 1.6% |
| PTSD | 8.9% | 6.8% |
| Any psychotic disorder | 0.9% | 1.5% |
TABLE 4. Lifetime prevalence of psychiatric disorders in fragile X premutation carriers compared to general population estimatesa
Limitations
Study quality.
The majority of studies identified by this review scored below six on the JBIPCA quality appraisal checklist; only five studies scored six or more (43, 47, 51, 55, 79). The most common reasons for low quality scores were cohort selection, assessment method, and response rate. Most studies had small sample sizes; they recruited from cascade genetic testing, and their demographics were not documented or did not represent the general population. The age of the cohort was not reported in 30.4% (14/46) of studies. This is important, as fragile X is an X-linked condition, and psychiatric disorders are known to have different ages of onset (96). Thus, younger cohorts would likely underestimate the lifetime prevalence for some diagnoses. Regarding sex, 22.7% (10/46) of studies did not separate sexes; 34.8% (16/46) included only females; and 13.0% (6/46) included only males. We propose that this represents a major limitation in the literature to date. X-linked genetic mutations characteristically manifest differently in males and females, as is already established for fragile X syndrome and FXTAS. Accordingly, grouping sexes together could potentially give rise to clinically spurious conclusions; for instance, if a disorder were common in males but rare in females, reporting an overall mean would misinform risks in both sexes. To account for demographic variations, studies should have a control group. Unfortunately, 41.2% (7/17) of the studies used for the psychiatric prevalence estimates did not include a control group. Finally, 60.9% (28/46) of studies used medical history or subscales. There is thus a need for future research to clearly document demographics, include a control group, and use valid assessment methods.
Assessment methods.
Psychiatric disorders were assessed by a variety of methods, and prevalence varied among these studies. Although the gold standard to assess psychiatric disorders is a semistructured clinical interview in conjunction with the assessment of diagnostic criteria, there was variation between studies even when this method was used. Different semistructured clinical interviews were used between studies, and importantly, the diagnostic criteria used also varied. Although the majority of studies used DSM-IV diagnostic criteria, there have been significant changes in the classification and illness terminology in the DSM-5. For example, the DSM-5 reclassified anxiety disorders to anxiety, obsessive-compulsive and related, and trauma- and stressor-related disorders.
It can sometimes be impractical to use the gold-standard method to assess psychiatric disorders in research; thus, rating scales are often used instead. Rating scales generally assess only point prevalence; thus, the lifetime estimates did not include studies that used rating scales. For example, someone may not meet the cutoff score for GAD on the HADS at one point in time but may at another point. The use of different methodologies to calculate lifetime and point prevalence rates may explain inconsistencies in the results. For example, the average point prevalence of social phobia in males was much higher than the lifetime prevalence (19.2% vs. 8.3%). This is impossible, but it could be explained by the use of rating scales compared to semistructured interviews. Accordingly, the exact prevalence rates identified by this review should be interpreted with caution. Instead, the general themes of this review are likely to be more meaningful—for example, the phenotype and sex distribution of FXANDs was similar to those observed with fragile X syndrome itself.
Rating scales for anxiety and depressive disorders are normally given cutoff scores that correlate to “any depressive” or “any anxiety” disorder. For example, the CES-D has been shown to accurately predict the presence of any DSM depressive disorder at a cutoff score greater than 16 (97). Some studies identified in this review, however, did not indicate the cutoff scores they used (46, 55, 79). This is problematic as they may not have used the recommended cutoff scores, which would result in misrepresentation of the psychiatric disorder in their cohort. For instance, the only study that used an anxiety rating scale did not state a cutoff score and reported a far higher rate of anxiety (63.0%) than the pooled prevalence from the studies that used a semistructured interview (19.4%). Future studies should clarify the cutoff scores used.
The studies that used subscales of larger instruments also had rates that were inconsistent and varied significantly from the other studies. The study that assessed OCD with the SCL-90R showed that 61.1% of participants had clinically significant symptoms, compared to the semistructured interviews which showed that 4.7% of participants had OCD. The four studies that assessed anxiety disorders with subscales reported prevalence rates that varied between 17.1% and 65.4% (80–82, 85). These findings could be explained by the issues associated with using subscales from larger instruments. For example, the subscales on the SCL-90 have been shown to have significant overlap and are only slightly better than chance in predicting the relevant diagnosis (98).
Although studies that used medical records were not included in the final results due to reporting bias, there were nonetheless some interesting insights. One study identified 98 premutation carriers from electronic health records who were unaware of their genetic status (51). In this study, the lifetime prevalence of major depressive disorder was reported to be 4.2%–7.7%; ADHD, 15.4%; and anxiety disorders, 15.4%. These rates were much lower than those identified by this review, suggesting underdocumentation in medical records using a retrospective methodology. Nonetheless these results offer insight into the rate of psychiatric conditions unconfounded by personal concerns for genetic status, the stress associated with being a carer for someone with fragile X syndrome (99), and the bias from clinicians assessing these patients. Future studies should either blind clinicians to genetic status or recruit premutation carriers from population-based screening.
Prevalence estimates.
This review calculated lifetime prevalence from all studies that met the inclusion criteria, yet some studies had much younger cohorts. For example, two studies that assessed mood and anxiety disorders had cohorts as young as 18 (75, 76). As lifetime estimates of psychiatric conditions increase with age, studies with younger cohorts likely underestimated the lifetime rates (90).
The number of studies that met the inclusion criteria for the prevalence estimates were not universally the same between psychiatric conditions. For instance, the prevalence of fragile X premutation in ADHD relied on only one study for each condition. In contrast, eight studies were used for the prevalence estimate of major depressive disorder in premutation carriers. The cohort sizes used for these estimates also varied. The prevalence estimates of fragile X premutation in probands with ADHD were based only on 16 people. In contrast, the estimates of psychiatric conditions in premutation carriers were based on larger cohorts, once again with the exception of ADHD which included only 48 people.
Genetic testing.
The definition of fragile X premutation range and testing methods varied. Some studies included patients with fragile X premutation without specifying the number of repeats or laboratory testing method (49, 60, 62, 72, 86). This is important as some PCR testing methods fail to detect CGG repeats in the premutation range (100). Other studies used a range that was lower than the currently established range of 55–200 CGG repeats. For example, studies included premutation carriers with CGG repeats of 50–80 (61), 50–200 (76), 52–200 (64), 50–60 (65), 52–200 (63), and 45–198 (85). Also, few studies assessed patients for genetic variables such as mosaicism.
Conclusions
This review found that the rates of fragile X premutation in those with neurodevelopmental disorders were universally higher than general population estimates. In addition, psychiatric conditions were generally more common in premutation carrier groups than in control groups and in general population estimates. The most prevalent psychiatric conditions were neurodevelopmental disorders, anxiety disorders, and bipolar II disorder. Psychiatric conditions were more prevalent in male premutation carriers. This neuropsychiatric phenotype and sex distribution are similar to those among individuals with fragile X syndrome. The studies included had significant methodical variation that could explain the variation in results. Accordingly, more detailed research is required on FXANDs moving forward; studies should identify cohorts from population-based sampling, describe the cohort demographics, include a demographically matched control group, stratify the sexes and prioritize the use of validated assessment methods.
1. : Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell 1991; 65:905–914Crossref, Medline, Google Scholar
2. : Fragile X syndrome: a psychiatric perspective. Results Probl Cell Differ 2012; 54:281–295Crossref, Medline, Google Scholar
3. : Fragile X premutation and associated health conditions: a review. Clin Genet 2021; 99:751–760Crossref, Medline, Google Scholar
4. : Fragile X-Associated Tremor/Ataxia Syndrome (FXTAS): pathophysiology and clinical implications. Int J Mol Sci 2020; 21:4391Crossref, Medline, Google Scholar
5. : Psychiatric phenotype of the fragile X-associated tremor/ataxia syndrome (FXTAS) in males: newly described fronto-subcortical dementia. J Clin Psychiatry 2006; 67:87–94Crossref, Medline, Google Scholar
6. : Fragile X-associated neuropsychiatric disorders (FXAND). Front Psychiatry 2018; 9:564Crossref, Medline, Google Scholar
7. : Fragile X-associated tremor/ataxia syndrome: another phenotype of the fragile X gene. Clin Neuropsychol 2016; 30:810–814Crossref, Medline, Google Scholar
8. : Fragile X premutation associated conditions (FXPAC). Front Pediatr 2020; 8:266Crossref, Medline, Google Scholar
9. : Fragile X syndrome: clinical presentation, pathology and treatment. Gac Med Mex 2020; 156:60–66Medline, Google Scholar
10. : Variation of the CGG repeat at the fragile X site results in genetic instability: resolution of the Sherman paradox. Cell 1991; 67:1047–1058Crossref, Medline, Google Scholar
11. : Mouse models of the fragile X premutation and fragile X-associated tremor/ataxia syndrome. J Neurodev Disord 2014; 6:25Crossref, Medline, Google Scholar
12. : Neuropathological, clinical and molecular pathology in female fragile X premutation carriers with and without FXTAS. Genes Brain Behav 2012; 11:577–585Crossref, Medline, Google Scholar
13. : Fragile X premutation carriers: a systematic review of neuroimaging findings. J Neurol Sci 2015; 352:19–28Crossref, Medline, Google Scholar
14. : A review of fragile X premutation disorders: expanding the psychiatric perspective. J Clin Psychiatry 2009; 70:852–862Crossref, Medline, Google Scholar
15. : Fragile X premutation in women: recognizing the health challenges beyond primary ovarian insufficiency. J Assist Reprod Genet 2017; 34:315–323Crossref, Medline, Google Scholar
16. : Psychiatric disorders associated with FXTAS. Curr Psychiatry Rev 2013; 9:59–64Medline, Google Scholar
17. : Understanding the neuropsychiatric phenotype of fragile X-associated tremor ataxia syndrome: a systematic review. Neuropsychol Rev 2014; 24:491–513Crossref, Medline, Google Scholar
18. : The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372:n71Crossref, Medline, Google Scholar
19. : Assessment of clinical information: comparison of the validity of a Structured Clinical Interview (the SCID) and the Clinical Diagnostic Interview. J Nerv Ment Dis 2015; 203:459–462Crossref, Medline, Google Scholar
20. : The Structured Clinical Interview for DSM-III-R (SCID). I. History, rationale, and description. Arch Gen Psychiatry 1992; 49:624–629Crossref, Medline, Google Scholar
21. : The Anxiety Disorders Interview Schedule for Children for DSM-IV: Child and Parent Versions. San Antonio, TX, Psychological Corporation, 1996 Google Scholar
22. : A diagnostic interview: the schedule for affective disorders and schizophrenia. Arch Gen Psychiatry 1978; 35:837–844Crossref, Medline, Google Scholar
23. : Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH Genetics Initiative. Arch Gen Psychiatry 1994; 51:849–859Crossref, Medline, Google Scholar
24. : Family Informant Schedule and Criteria (FISC). New York, New York State Psychiatric Institute, 1986 Google Scholar
25. : The Autism Diagnostic Observation Schedule–Generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord 2000; 30:205–223Crossref, Medline, Google Scholar
26. : Autism Diagnostic Interview–Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord 1994; 24:659–685Crossref, Medline, Google Scholar
27. : The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1977; 1:385–401 Crossref, Google Scholar
28. : Manual for the Depression Anxiety Stress Scales. Sydney, Australia, Psychology Foundation of Australia, 1995 Google Scholar
29. : The hospital anxiety and depression scale. Acta Psychiatr Scand 1983; 67:361–370Crossref, Medline, Google Scholar
30. : Social Phobia and Anxiety Inventory: Manual. Toronto, Canada, Multi-Health Systems, Inc, 1996 Google Scholar
31. : Social phobia. Mod Probl Pharmacopsychiatry 1987; 22:141–173Crossref, Medline, Google Scholar
32. : Brown Attention-Deficit Disorder Scales Manual. San Antonio, TX, Psychological Corporation, 1996 Google Scholar
33. : Conners’ Parent Rating Scales Revised-Short Form (CPRS-R:S). North Tonawanda, NY, Multi-Health Systems, Inc, 1997 Google Scholar
34. : The autism-spectrum quotient (AQ): evidence from Asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. J Autism Dev Disord 2001; 31:5–17Crossref, Medline, Google Scholar
35. : Mullen Scales of Early Learning. Circle Pines, MN, American Guidance Service, 1995 Google Scholar
36. : Vineland Adaptive Behavior Scales: Survey Forms Manual. Circle Pines, MN, American Guidance Service, 1984 Google Scholar
37. : The Abilities of Young Children. Bucks: Association for Research in Infant and Child Development. A comprehensive system of measurement for the first eight years of life. Thames, Test Agency, 1984, pp 101–172 Google Scholar
38. : The use of factor analysis for ascertaining patterns of psychopathology in children with intellectual disability. J Intellect Disabil Res 1996; 40 (Pt 3):198–207Crossref, Medline, Google Scholar
39. : Profile of Mood States (POMS) Manual. San Diego, Educational and Industrial Testing Service, 1981 Google Scholar
40. : Symptom Checklist 90–R: Administration, Scoring, and Procedures Manual. Minneapolis, National Computer Systems, Inc, 1994 Google Scholar
41. : Joanna Briggs Institute Critical Appraisal Checklist for Studies Reporting Prevalence Data. Adelaide, Australia, Joanna Briggs Institute, 2014, pp 182–188 Google Scholar
42. : Autism spectrum disorders and attention-deficit/hyperactivity disorder in boys with the fragile X premutation. J Dev Behav Pediatr 2006; 27:S137–S144Crossref, Medline, Google Scholar
43. : Fragile X premutation carrier epidemiology and symptomatology in Israel: results from a tertiary child developmental center. Cerebellum 2016; 15:595–598Crossref, Medline, Google Scholar
44. : Developmental profiles of infants with an FMR1 premutation. J Neurodev Disord 2016; 8:40Crossref, Medline, Google Scholar
45. : Impaired response inhibition is associated with self-reported symptoms of depression, anxiety, and ADHD in female FMR1 premutation carriers. Am J Med Genet B Neuropsychiatr Genet 2014; 165b:41–51Crossref, Medline, Google Scholar
46. : Autistic traits and mental health in women with the fragile-X premutation: maternal status versus genetic risk. Br J Psychiatry 2021; 218:28–34Crossref, Medline, Google Scholar
47. : Co-occurring conditions associated with FMR1 gene variations: findings from a national parent survey. Am J Med Genet A 2008; 146a:2060–2069Crossref, Medline, Google Scholar
48. : Co-occurring diagnoses among FMR1 premutation allele carriers. Clin Genet 2010; 77:374–381Crossref, Medline, Google Scholar
49. : Prediction of mental status in carriers of the fragile X mutation using CGG repeat length. Am J Med Genet 1994; 51:497–500Crossref, Medline, Google Scholar
50. : A multicenter study on genotype-phenotype correlations in the fragile X syndrome, using direct diagnosis with probe StB12.3: the first 2, 253 cases. Am J Hum Genet 1994; 55:225–237Medline, Google Scholar
51. : Data-driven phenotype discovery of FMR1 premutation carriers in a population-based sample. Sci Adv 2019; 5:eaaw7195Crossref, Medline, Google Scholar
52. : Screening for fragile X syndrome among neurobehavioural patients from Kolkata, eastern India. J Clin Diagn Res 2009; 3:1266–1273 Google Scholar
53. : Autistic phenotype of permutation and intermediate alleles of FMR1 gene. Iran J Pediatr 2017; 27:e9445 Crossref, Google Scholar
54. : Fragile X carrier screening and FMR1 allele distribution in the Japanese population. Brain Dev 2010; 32:110–114Crossref, Medline, Google Scholar
55. : Identification of expanded alleles of the FMR1 gene in the CHildhood Autism Risks from Genes and Environment (CHARGE) study. J Autism Dev Disord 2013; 43:530–539Crossref, Medline, Google Scholar
56. : DNA diagnosis of FRAXA and FRAXE in Chinese children with neurodevelopmental disorders and fragile X syndrome. Clin Genet 1998; 53:179–183Crossref, Medline, Google Scholar
57. : Direct diagnosis by DNA analysis of the fragile X syndrome of mental retardation. N Engl J Med 1991; 325:1673–1681Crossref, Medline, Google Scholar
58. : Cytogenetic versus DNA diagnosis in routine referrals for fragile X syndrome. Lancet 1993; 342:1025–1026Crossref, Medline, Google Scholar
59. : Evaluation of school children at high risk for fragile X syndrome utilizing buccal cell FMR-1 testing. Am J Med Genet 1994; 51:474–481Crossref, Medline, Google Scholar
60. : DNA diagnosis of the fragile X syndrome in a series of 236 mentally retarded subjects and evidence for a reversal of mutation in the FMR-1 gene. Am J Med Genet 1994; 51:482–485Crossref, Medline, Google Scholar
61. : FRAXA locus in fragile X diagnosis: family studies, prenatal diagnosis, and diagnosis of sporadic cases of mental retardation. Am J Med Genet 1994; 51:486–489Crossref, Medline, Google Scholar
62. : Direct mutation analysis of 495 patients for fragile X carrier status/proband diagnosis. Am J Med Genet 1994; 51:501–502Crossref, Medline, Google Scholar
63. : Comparison between the cytogenetic test for fragile X and the molecular analysis of the FMR-1 gene in Japanese mentally retarded individuals. Am J Med Genet 1994; 51:466–470Crossref, Medline, Google Scholar
64. : Expansion mutation frequency and CGG/GCC repeat polymorphism in FMR1 and FMR2 genes in an Indian population. Genet Epidemiol 2001; 20:129–144Crossref, Medline, Google Scholar
65. : Molecular screening of FRAXA and FRAXE in Indian patients with unexplained mental retardation. Genet Test 2002; 6:335–339Crossref, Medline, Google Scholar
66. : 10 years’ experience in fragile X testing among mentally retarded individuals in Greece: a molecular and epidemiological approach. In Vivo 2008; 22:451–455Medline, Google Scholar
67. : Molecular screening of fragile X syndrome in children with mental retardation in Hualien. Tzu Chi Med J 2008; 20:309–313 Crossref, Google Scholar
68. : FMR1 allele size distribution in 35,000 males and females: a comparison of developmental delay and general population cohorts. Genet Med 2018; 20:1627–1634Crossref, Medline, Google Scholar
69. : Screening for FMR1 expanded alleles in patients with autism spectrum disorders in Manaus, northern Brazil. Acad Bras Cienc 2019; 91:e20180882Crossref, Medline, Google Scholar
70. : Frequencies of “grey-zone” and premutation-size FMR1 CGG-repeat alleles in patients with developmental disability in Cyprus and Canada. Am J Med Genet 1999; 84:195–197Crossref, Medline, Google Scholar
71. : Lifetime prevalence of mood and anxiety disorders in fragile X premutation carriers. J Clin Psychiatry 2011; 72:175–182Crossref, Medline, Google Scholar
72. : Mood and anxiety disorders in females with the FMR1 premutation. Am J Med Genet B Neuropsychiatr Genet 2009; 150b:130–139Crossref, Medline, Google Scholar
73. : Psychiatric disorders among women with the fragile X premutation without children affected by fragile X syndrome. Am J Med Genet B Neuropsychiatr Genet 2016; 171:1139–1147Crossref, Medline, Google Scholar
74. : High rates of comorbid depressive and anxiety disorders among women with premutation of the FMR1 gene. Am J Med Genet B Neuropsychiatr Genet 2013; 162b:872–878Crossref, Medline, Google Scholar
75. : Neurobehavioral characteristics of CGG amplification status in fragile X females. Am J Med Genet 1994; 54:378–383Crossref, Medline, Google Scholar
76. : Emotional and neurocognitive deficits in fragile X. Am J Med Genet 1994; 51:378–385Crossref, Medline, Google Scholar
77. : Genotype-phenotype relationship in female carriers of the premutation and full mutation of FMR-1. Psychiatry Res 1998; 80:113–127Crossref, Medline, Google Scholar
78. : Behavioral and psychiatric disorders in adult male carriers of fragile X. J Am Acad Child Adolesc Psychiatry 1994; 33:256–264Crossref, Medline, Google Scholar
79. : Investigation of phenotypes associated with mood and anxiety among male and female fragile X premutation carriers. Behav Genet 2008; 38:493–502Crossref, Medline, Google Scholar
80. : Differential sensitivity to life stress in FMR1 premutation carrier mothers of children with fragile X syndrome. Health Psychol 2012; 31:612–622Crossref, Medline, Google Scholar
81. : Clinical and molecular correlates in fragile X premutation females. eNeurologicalSci 2017; 7:49–56Crossref, Medline, Google Scholar
82. : Clinical involvement in daughters of men with fragile X-associated tremor ataxia syndrome. Clin Genet 2010; 78:38–46Crossref, Medline, Google Scholar
83. : Neurobehavioral effects of the fragile X premutation in adult women: a controlled study. Am J Hum Genet 1993; 52:884–894Medline, Google Scholar
84. : Anxiety disorders in fragile X premutation carriers: preliminary characterization of probands and non-probands. Intractable Rare Dis Res 2015; 4:123–130Crossref, Medline, Google Scholar
85. : Clinical features of boys with fragile X premutations and intermediate alleles. Am J Med Genet B Neuropsychiatr Genet 2003; 121b:119–127Crossref, Medline, Google Scholar
86. : Clustering of comorbid conditions among women who carry an FMR1 premutation. Genet Med 2020; 22:758–766Crossref, Medline, Google Scholar
87. : Endocrine dysfunction in female FMR1 premutation carriers: characteristics and association with ill health. Genes 2016; 7:E101Crossref, Medline, Google Scholar
88. : FMR1 CGG allele size and prevalence ascertained through newborn screening in the United States. Genome Med 2012; 4:100Crossref, Medline, Google Scholar
89. : The national comorbidity survey replication (NCS-R): background and aims. Int J Methods Psychiatr Res 2004; 13:60–68Crossref, Medline, Google Scholar
90. : Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 2005; 62:593–602. Erratum in: Arch Gen Psychiatry 2005; 62:768. Merikangas, Kathleen RCrossref, Medline, Google Scholar
91. : The prevalence and correlates of nonaffective psychosis in the National Comorbidity Survey Replication (NCS-R). Biol Psychiatry 2005; 58:668–676Crossref, Medline, Google Scholar
92. : Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry 2007; 64:543–552Crossref, Medline, Google Scholar
93. : The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry 2006; 163:716–723Crossref, Medline, Google Scholar
94. : Patterns and predictors of attention-deficit/hyperactivity disorder persistence into adulthood: results from the national comorbidity survey replication. Biol Psychiatry 2005; 57:1442–1451Crossref, Medline, Google Scholar
95. : Systematic review of prevalence studies of autism spectrum disorders. Arch Dis Child 2006; 91:8–15Crossref, Medline, Google Scholar
96. : Age of onset of mental disorders: a review of recent literature. Curr Opin Psychiatry 2007; 20:359–364Crossref, Medline, Google Scholar
97. : Criterion validity of the Center for Epidemiological Studies Depression (CES-D) scale in a sample of rehabilitation inpatients. J Rehabil Med 2002; 34:221–225Crossref, Medline, Google Scholar
98. : Is SCL-90R helpful for the clinician in assessing DSM-IV symptom disorders? Acta Psychiatr Scand 2004; 110:215–224Crossref, Medline, Google Scholar
99. : Cortisol response to behavior problems in FMR1 premutation mothers of adolescents and adults with fragile X syndrome: a diathesis-stress model. Int J Behav Dev 2012; 36:53–61Crossref, Medline, Google Scholar
100. : Comparison between the polymerase chain reaction-based screening and the Southern blot methods for identification of fragile X syndrome. Genet Test Mol Biomarkers 2012; 16:1303–1308Crossref, Medline, Google Scholar



