The Use of Herbal Alternative Medicines in Neuropsychiatry
Abstract
Growing numbers of people throughout the United States (40% in 1998) are using various forms of alternative therapies. A MEDLINE literature search of journals from the past three decades and an Internet database query were performed to determine the types and frequency of alternative therapies used, with special attention given to the herbal medicines used in neuropsychiatric disorders. Clinical effects, mechanisms of action, interactions, and adverse reactions of the herbal treatments are detailed. Objective controlled trials will be needed to establish safety and efficacy of herbal supplements. Knowledge of the properties of these therapies can improve the care of neuropsychiatric patients.
This article provides a review of the current information on herbal alternative medicines (HAM) pertinent to physicians who treat patients with neurologic and psychiatric disorders. The findings reviewed were retrieved via a MEDLINE literature search of journals published since 1970 and an Internet database query.
BACKGROUND
Defining “Alternative”
An increasing number of people are using alternative therapies (AT; also referred to in the literature as “complementary and alternative medicine” [CAM]). CAM was defined in 1997 as “those [practices] that are currently not part of the dominant (conventional) medical system” for managing health and disease.1 Although this definition is only two years old, it is already obsolete because of the explosion of AT use throughout mainstream medicine.
Part of the confusion about the prevalence of AT use lies in the ambiguity of the definition of “alternative.” In the mid-1970s, AT in the United Kingdom was regarded as the “lunatic fringe” of medicine.2 This concept evolved into the term “fringe medicine.” In the early 1980s, British broadcast programs began referring to “alternative medicine.” Just three years ago, “alternative” stood for “curative procedures that diverge from those studied in universities and other official institutions [that are] carried out in everyday practice by the authorized (or conventional) physician.”3 To some patients, “alternative” calls attention to therapies not being offered by mainstream Western medicine—a residual definition of “anything not regular medicine.”4 The mid-1980s bore the British term “complementary medicine,” emphasizing collaboration between alternative and conventional therapies. In 1995 a term attributed to His Royal Highness the Prince of Wales appeared—”integrated medicine,”—emphasizing the integration of AT into standard health care patterns by education and funding research.2
Herbal alternative medicines, a subset of AT, will be discussed as the focus of this paper. Along with the pharmacology of the substances, five major issues regarding HAM will be briefly addressed at the outset: the pharmacological history of HAM, the population using HAM, the politics of HAM, the purity of the substances, and the issue of AT efficacy compared with placebo. It should be emphasized that the purpose of this review is to examine the current state of scientific knowledge and the sociopolitical issues surrounding HAM; it is intended neither to endorse nor to condemn their use.
Pharmacologic History
Allopathic medicine may at times consider traditional herbal drugs as the polar opposite of modern synthetic drugs, even though there is no pharmacological basis for such a view.5 Plants, however, have been used for centuries in the treatment of medical illness. Roughly one-quarter to one-half of current pharmaceuticals originally were procured from plants.6 Examples include foxglove leaf (digitalis), belladonna tops (atropine), poppy herb (morphine), white willow tree bark (salicin), and cinchona bark (quinine). Modern drugs developed from plant products include warfarin from the coumarin anticoagulants found in sweet clover silage, ergotamine from the ergot alkaloids of a fungus that infects rye grass, and the antineoplastic vincristine from the vinca alkaloid fractions of the rosy periwinkle.7
Proponents of herbal medicines describe a plant's therapeutic value as coming from the synergistic effects of the various components of the plants, in contrast to the individual chemicals of conventional medicines isolated by pharmacologists. Chung et al.8 studied the in vitro receptor-binding affinities of natural products used to treat psychotic illness in Korean traditional medicine. Extracts prepared from these five plants revealed potent binding affinities to monoamine receptors, especially alpha-2 adrenergic and dopaminergic receptors with variable selectivities. Cott9 has summarized receptor-binding activity of various HAM commercial extracts from a collection of herbal medicine texts (as noted in part in Table 1). This empiric evidence suggests a potential rational basis for some HAM therapies. The pharmacology of HAM will be examined more in depth later in the review.
The Population Using HAM
Prevalence of AT Use:
Although 20 years ago Americans would have considered “alternative medicine” an obscure term, surveys and reviews indicate that the number of patients using HAM and AT is rising steadily. A 1991 survey of adults in the United States determined that 34% of the 1,539 respondents had used at least one unconventional therapy in the past year.10 Only 28% of these patients informed their physicians that they attempted alternative forms of therapy. By 1998, of the 1,035 respondents surveyed, the number using some form of alternative health care during the past year had increased to 40%.11
Generally, HAM use in the United States is on the rise. Herbal medicine was found to be used by 3% of the U.S. population for the conditions described in the above-mentioned study by Eisenberg et al.10 The consumption of medicinal botanicals, including teas, powders, liquid extracts, capsules, tablets, and bulk plants or parts, is rising at a rate of approximately 15% per year in the United States.12 Currently, many HAM are readily used in Europe and are suggested to have beneficial effects in thousands of patients. Some 600 to 700 plant-based remedies are available in Germany, and an estimated 70% of German physicians prescribe phytomedicines. (From the Greek phyton, meaning plant, phytomedicines are defined as “advanced medicinal preparations made of herbs.”13)
European Physicians' Acceptance of HAM:
In 1976, the Federal Republic of Germany defined herbal remedies in the same manner as conventional medicines. Because of the wide use of herbal remedies in Europe, in 1978 the German government established an expert committee (“Commission E”) to evaluate the safety and efficacy of phytotherapy and herbal substances. The committee included physicians, pharmacists, pharmacologists, toxicologists, biostatisticians, pharmaceutical industry representatives, and nonmedical practitioners. The commission reviewed data from clinical trials, field studies, case reports, and scientific literature to establish with “reasonable certainty” the safety and efficacy of the herb in question. The German Commission E's findings were published as 462 monographs (GCEm), evaluating 360 herbs and 391 preparations of herb parts by the end of 1995. Approximately two-thirds of the monographs are positive assessments; negative assessments typically resulted from an unsatisfactory risk–benefit ratio. The GCEm provide a peer review of HAM for the European physicians to use as a resource for prescribing HAM. Along with the GCEm, one of the driving forces in phytomedicinal acceptance in the European medical community is the inclusion of phytomedicine in medical and pharmacy school curricula.13
American Physicians' Rejection of AT:
Questionnaires distributed to 295 family physicians in the Chesapeake region revealed that only 22.6% of the 176 respondents considered herbal medicine to be legitimate medical practice, with only 6.9% of the physicians having used HAM in actual practice.14 Chez and Jonas1 suggest that physician resistance to using AT stems from both a valid scientific concern and a lack of knowledge about the characteristics of these treatments. Up until recently, U.S. physicians received little, if any, training with HAM in medical school or in residency.
Status of Practice Guidelines:
Attempts to develop AT guidelines have been met by obstacles. Issues raised by the prospect of guidelines include concern that improper treatment practices may be adopted by prescribers without strong supporting scientific evidence, concern about possible implementation by health plan administrators in the absence of proven effectiveness, and worry that practice guidelines may increase medicolegal liability.15 Regarding HAM, unregulated medications may be unpredictably detrimental to the health of patients, and stringent treatment indications should be established to protect the patient.
Why People Seek Out AT:
Patients who seek alternative care have been thought to be subject to “neuroticism, ignorance and gullibility,”16 and have been characterized as “poorly educated, terminally ill patients who have exhausted conventional treatment.”17 (For contrasting findings, see below.) The high cost of health care drives some patients to seek self-medication to reduce costs. Turning to AT may be prompted by frustration with iatrogenic effects from chronic treatment or interventions. The belief that the scientific molecular model of medicine is not sufficient to address all of patients' physical and mental health needs may be another reason that patients use AT.18
Pharmacoepidemiologic profiles have described characteristics of AT consumers. European consumers using AT tend to feel that alternative practitioners are more patient-oriented than allopathic physicians; to believe that one can build up resistance to disease; to desire control over their health or disease; to have had a poor response to orthodox treatment in chronic disease; and to have less faith in conventional medicine.5 In 1998 Astin11 randomly surveyed 1,035 individuals living in the United States. Using multiple logistical regression, he found that predictors of alternative health care use were higher education, poorer health status, a holistic orientation to health, having had a transformational experience that changed the person's worldview, and symptoms of anxiety, back problems, chronic pain, or urinary tract problems. Cultural predictors of AT use included commitment to environmentalism or to feminism and interest in spirituality and personal growth psychology (referred to as “cultural creatives”). Contrary to prior descriptors of people who use AT in conjunction with conventional therapy, Astin's study did not find dissatisfaction with conventional medicine (found in only 9% of respondents) to be a predictor of AT use.
The Politics of HAM
In 1990, 60 million Americans used AT, and annual visits to AT providers (425 million) exceeded visits to all U.S. primary care physicians (338 million).10 It is a matter of concern that more than 70 percent of patients who acknowledged using AT never mentioned it to their physicians. It has been pointed out that allopathic physicians still incur a liability risk in prescribing medication to patients who take herbal remedies without their knowledge. Yet physicians in the United States are unable to regulate the administration of HAM through prescriptions, because many of these substances are classified as nutraceuticals and are marketed under the Dietary Supplement and Health Education Act of 1994 (DSHEA).19 The DSHEA preempts Federal Drug Administration (FDA) regulation of supplemental herbs and hormones.
Currently, extensive data supporting claimed benefits and effects are not readily available, in contrast to the situation for traditional pharmaceuticals. FDA-regulated drugs must undergo a rigorous, three-step process of evaluation in animals and humans to prove their relative efficacy and safety (on average over 15 years, costing $500 million per new drug). HAM, in contrast, are brought to market without regulation or oversight by a scientific or safety monitoring body. Pharmaceutical companies worldwide are taking greater interest in these herbal products, largely because of the burgeoning public interest.20 European consumers spent $7 billion on retail herbal remedies in 1996 (half being sold in Germany). U.S. consumers spent $441 million in 1997 on HAM in retail stores. An estimated $3.24 billion in U.S. sales were made through all channels of distribution (health food stores, mail order, and multilevel marketing organizations) in 1996.13 In comparison, U.S. patients spent $103 billion on prescription drugs in 1998.
The call for strict regulation of HAM is hindered by the industry's lack of incentive to investigate and develop plant-based drugs whose chemical constituents cannot be patented. Complementary medicine advocates argue that botanicals have been in common use without harm for centuries and should fall into a different category than typical pharmaceuticals. However, in the absence of documented safety for certain herbs, supplements, or chemical preparations, and given the lack of FDA scrutiny, there is no assurance of safety for patients or their doctors. These concerns have prompted some physicians to ask if herbal medicines should be transferred to the FDA's governance.
The Purity of HAM
Many patients are under the false assumption that “naturally derived” herbal medicines are safer and more “natural” and have fewer side effects. Documented case studies have shown that “natural” substances are not inherently safe or without adverse events, as discussed below. In the Canadian market, as in other countries, the quality of advice from health food stores varies greatly,20 and the purity of the products sold can vary as well.
Approximately 50 ginseng products sold in 11 countries were analyzed, and a number of them contained no ginsenosides when tested by oxidative cleavage procedures.21 Rigid quality control standards are not required for nutraceuticals, leading to substantial variability in the purity and the potency of these substances. Depending on the plant part, the age or ripeness when harvested, and the plant's growing environment and storage conditions, the therapeutic and toxic components of plants may vary considerably.22 Contamination with pesticide residues, microorganisms, aflatoxins, radioactive substances, and heavy metals has been documented, especially in Asian herbal preparations. Substitution of components, adulteration with pharmaceuticals, and incorrect preparation of crude plant material have yielded cases of toxicity from herbal medicines. Different components of each herb can exhibit different pharmacologic effects. Limited research on this subject offers little information about the biologic specificity of herbal concoctions. Drug–drug interactions between prescription drugs and HAM are poorly characterized, in part because of these great variations in the preparation of HAM.
HAM versus Placebo
Despite skepticism from the medical community regarding the scientific rigor and quality of the evidence that ATs are effective, their use continues to rise. In an effort to evaluate whether the effects of homeopathy were due to a placebo effect, Linde et al.23 conducted a meta-analysis of 89 placebo-controlled trials (randomized or double-blinded). Results revealed an odds ratio of 2.45 (95%, confidence interval 2.05–2.93) in favor of homeopathy but had insufficient data for efficacy in any single clinical condition. Criticism of the meta-analysis noted that disparate remedies for different conditions created an overly inclusive aggregate.24 Trials of HAM versus placebo have also demonstrated effects in support of HAM, as described below.
In October 1996, the Tzu Chi Institute was founded in British Columbia by a pediatric endocrinologist with the goal of researching and selecting the most useful alternative treatments and eventually integrating them into conventional treatment programs. Six hundred patients were identified within the first 6 months as potential research subjects for the institute's pilot studies with AT. In October 1993, the National Institutes of Health Office of Alternative Medicine (NIH OAM) awarded 30 therapists $30,000 exploratory grants to identify promising areas of future research with AT.25 The National Institute of Mental Health (NIMH) is now reviewing protocols and funding ongoing research to evaluate HAM effectiveness, including hypericum for depression and a multicenter trial of ginkgo in the elderly. To date, double-blind placebo-controlled randomized trials have been conducted with St. John's wort, ginkgo biloba, kava, ginseng, valerian, feverfew, garlic, and yohimbine, as described below.
Types of Alternative Therapies
The NIH OAM was established in 1992 for the investigation of the efficacy of AT. The therapies are divided into seven categories by the OAM:26 1) mind-body interventions, 2) bioelectromagnetic therapies, 3) alternative systems of medical practice, 4) manual healing methods, 5) pharmacologic and biologic treatments, 6) herbal medicine, and 7) diet and nutrition. We will review these categories briefly before focusing on specific herbal medications and their actions.
Mind-body interventions include biofeedback, relaxation therapies, meditation, body-oriented exercises (yoga, t'ai chi), hypnosis, and imagery. These techniques have been used for such conditions as asthma, hypertension, incontinence, insomnia, and chronic pain. Bioelectromagnetic therapies incorporate the interactions between living organisms and electromagnetic fields. Therapies include the use of electrical currents or magnetic fields to promote healing of fractures. Transcutaneous electrical nerve stimulation, or TENS, is included in AT although it is used in conventional chronic pain management. Transcranial electrostimulation is being investigated and has been used to treat depression and anxiety. Alternative systems of medical practice include Asian practices and homeopathy. Of the Asian techniques, acupuncture is the most studied and has been shown to increase endorphins, serotonin, and other neurotransmitters. There are sporadic reports of dysmenorrhea,27 pain,28 addiction, and alcoholism29 responding to acupuncture. Manual healing methods include osteopathic and chiropractic manipulation, physical therapy, massage, and therapeutic touch. These methods have been applied for treating low back pain, enhancing growth and development in premature babies, and improving general physiologic function. Pharmacologic and biologic treatments are used frequently by patients with life-threatening conditions. These treatments include chelation therapy for coronary artery disease, shark cartilage therapy for cancer, and intravenous ozone therapy for human immunodeficiency virus (HIV) infection. Herbal medicine is the mainstay of indigenous healing practices throughout the world, whereby plants and plant extracts are used as pharmacologic therapy (the focus of this paper). Lastly, diet and nutrition are widely used for both disease prevention and cure. Examples include vitamin supplements (vitamin E for blood clot and stroke prevention), folic acid for neural tube defect prevention, macrobiotic diet to treat cancer and chronic illness, and the ketogenic diet for epilepsy.
Among the principal medical conditions most frequently reported by patients seeking AT, five of the top ten fall within the province of neuropsychiatry. These conditions include back problems, insomnia, headache, anxiety, and depression (the other five being allergies, arthritis, sprains or strains, high blood pressure, and digestive problems).10 In a prospective study, Bullock et al.30 found presenting complaints similar to the above-stated five conditions, along with nicotine addiction, in patients who sought AT treatment.
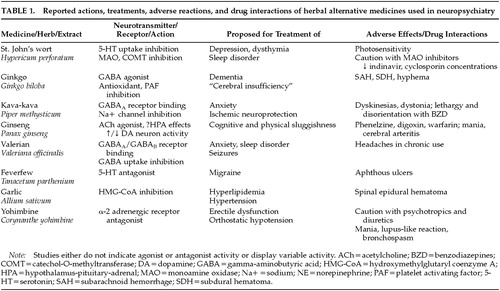
Listed below are the major herbal alternative medicines being used in neuropsychiatric disorders (Table 1). Current information is presented in the following general format: plant name, bioactive ingredient, neurotransmitter effect, pertinent studies, pharmacology, drug interactions, and side effects. Structures for selected bioactive ingredients are depicted in Figure 1.
REVIEW OF HERBAL ALTERNATIVE MEDICINES USED IN NEUROPSYCHIATRY
The following medicines will be reviewed: St. John's wort for depression, ginkgo for dementia, kava for anxiety, ginseng for cognitive and mood enhancement, valerian for insomnia, feverfew for migraine prophylaxis, garlic for atherosclerosis and stroke risk reduction, and yohimbine for erectile dysfunction. In light of the neuropsychiatrist's interaction with patients who have multiple sclerosis and HIV infection, the widely used immune stimulant echinacea is also reviewed. (Although frequently used today, nonherbal alternative treatments such as melatonin and S-adenosyl-methionine are not included in this review.)
St. John's Wort (Hypericum perforatum)
The aromatic perennial St. John's wort (SJW) has been prescribed in Germany as Johanniskraut over the past decade for treatment of depression, dysthymia, and sleep disorder. The leaves and yellow flowering tops yield about 0.1% hypericin, pseudohypericin, and related naphthodianthrones. Flavonoids, aromatic oxygen heterocyclic compounds found in certain plants, have also been identified in the plant.31
The plant's antidepressant mechanism of action initially was stated to be monoamine oxidase (MAO) inhibition, based on studies by Suzuki et al.;32 however, this finding was later attributed to a test product of only 80% purity. Crude plant extract xanthenones have demonstrated MAO inhibition in rat brain studies, but it is unclear if hypericin itself inhibits MAO-A or -B in the human nervous system. Plant fractions containing xanthones and flavonoids demonstrate marked MAO-A inhibition.33 Other unidentified components of the herb inhibit catechol-O-methyltransferase (COMT) and suppress interleukin release.34 Supporting the biogenic amine antidepressant effect of SJW, Perovic and Müller demonstrated hypericum extract inhibition of serotonin (5-HT) uptake in rat synaptosomes.35 It is not known whether the active components of hypericum are able to cross the blood–brain barrier.36
A 1996 European meta-analysis of hypericum in 23 randomized controlled trials in 1,757 outpatients concluded the herb extracts were more effective than placebo and were comparable to conventional antidepressants (ADs) in the treatment of mild to moderate depression.37 The side effect profile was better for hypericum than for conventional ADs. Side effects were experienced by 50 patients (19.8%) taking hypericum versus 84 (52.8%) given standard ADs. This study analyzed data from 15 placebo-controlled trials (largest N=120) and 8 controlled trials in which hypericum was compared with ADs (largest N=135). The limitations of the studies reviewed included short duration (4 to 8 weeks), lack of diagnostic precision with strict DSM/ICD criteria, the use of variable hypericin extract dosages, and lower standard antidepressant dosages (less than what most American psychiatrists consider therapeutic).38 Of the studies included in the meta-analysis, particularly noteworthy are the Hänsgen et al.39 and the Harrer et al.40 studies for evaluation of hypericum versus placebo and versus maprotiline, respectively, in a DSM-III-R/ICD-10 depressed population.
In 1997, the NIMH OAM and Office of Dietary Supplements provided funding for a multisite study to investigate hypericum in the treatment of depression. The three-arm study will compare hypericum, placebo, and sertraline in 300 patients with major depression.
SJW, also known as “goat weed” and “klamath weed,” is generally given orally at a dose of 300 mg, with 0.3% concentration (900 μg) of hypericin, three times daily. Onset of its mood-elevating effect usually occurs after several weeks.41 Pharmacologically, serum concentrations of hypericin peak in 5 hours and reach steady state in 4 days.42 The plasma half-life (t½) of pseudohypericin varies from 16.3 to 36 hours. The plasma t½ of hypericin is about 25 hours.
Interactions between SJW and monoamine oxidase inhibitors (MAOIs), selective serotonin reuptake inhibitors (SSRIs), dopaminergics, and sympathomimetics are unclear. Because of the potential for severe hyperpyretic or hypertensive crises, convulsions, or death, it is recommended that patients taking MAOIs not take SJW.13 Theoretically, SSRIs, dopaminergic agonists, and sympathomimetic agents may pose a risk when coadministered with hypericin.
The effect on cytochrome P450 (CYP450) enzymes was unknown until recently. Naphthodianthrones in SJW induce the CYP450 3A4 isoenzyme, potentially leading to the alteration of therapeutic levels of antivirals, immunosuppressants, anticoagulants, anti-arrhythmics, and oral contraceptives. The FDA released a public health advisory in February of this year on the risk of drug interactions with indinavir, a protease inhibitor, and other drugs metabolized by the CYP450 pathway. The recommendations in the advisory are based on a recent NIH study revealing a 57% area under the curve mean reduction of indinavir with SJW administration in 8 health non–HIV-infected volunteers.42a Because of the possibility of suboptimal antiretroviral drug concentrations leading to loss of virologic response, the advisory also recommended against concomitant administration of SJW with non-nucleoside reverse transcriptase inhibitors.
Two cases of acute cardiac cellular transplant rejection secondary to SJW-induced cyclosporin level reduction are described in a Lancet research letter.42b The 2 patients underwent heart transplantation 11 to 20 months prior to rejection for endstage ischemic cardiomyopathy. Both patients had event-free courses on the triple immunosuppressive regimen, cyclosporin, azathioprine, and low-dose corticosteroids. Within 3 weeks of beginning SJW (1 self-medicated and 1 prescribed by a psychiatrist), the patients presented for elective endomyocardial biopsy and were found to have subtherapeutic cyclosporin serum concentrations.
Prior to the Lancet publications, known adverse reactions with hypericin included photosensitivity in grazing animals and in one human report.36 The side effect profile includes gastrointestinal symptoms, allergic reactions, fatigue, dizziness, and xerostomia, and these occurred in 0.6% or less of 3,250 treated patients.43 Woelk et al.43 reviewed studies reporting the frequency of undesired effects with ADs to range from 2.5% (headache) to 17.6% (dry mouth). A 1.5% dropout rate in the hypericum-treated patients, versus reports of 3% to 12% dropout rates with ADs, was also noted.
Ginkgo (Ginkgo biloba)
Ginkgo comes as a concentrated extract from the G. biloba tree and is traditionally used as a treatment for improving mental alertness. Major components of the extract are flavonoids (ginkgo-flavone glycosides) and terpenoids (ginkgolides and bilobalide).44 These compounds are thought to act as free radical scavengers, providing cellular membrane protection and inhibiting platelet-activating factor (PAF), respectively.45 Bilobalide (but not ginkgolides) suppressed hypoxia-induced choline release from rat hippocampal slices when subjected to an oxygen-free buffer.46 Oral bilobalide also significantly increased gamma-aminobutyric acid (GABA) levels and glutamic acid decarboxylase activity in mouse brains as compared with controls.47 Finally, ginkgo extract administration increased the hippocampal muscarinic receptor population in aged rats, suggesting a possible prophylactic effect for ginkgo in the aging brain's normal decline in cholinergic function.48
Ginkgo has been prescribed in the United Kingdom over the past two decades for treatment of “cerebral insufficiency” (CI). CI is an inexact term used in Europe and is considered to indicate dementia secondary to impaired cerebral circulation. The symptom complex, roughly equivalent to vascular dementia, includes memory and concentration difficulties, absent-mindedness, confusion, anergia, tiredness, decreased physical performance, depressive moods, anxiety, dizziness, tinnitus, and headache.49
In Germany, the nootropic, or cognitive-enhancing, effects of ginkgo have been studied in patients with multi-infarct dementia or mild to moderate Alzheimer's dementia. A 24-week prospective randomized double-blind placebo-controlled multicenter trial evaluated ginkgo in 156 patients.50 The investigators used the Clinical Global Impression (CGI) scale “for psychopathological assessment,” the Syndrome Short Test (SST) for assessment of attention and memory, and the Nuremberg Geriatric Observation Scale for behavioral assessment of activities of daily life.51 The study demonstrated significant differences in responder rates on the CGI (32% vs. 17% placebo) and the SST (38% vs. 18% placebo), favoring the ginkgo-treated group. (Responders were defined as those scoring “much improved” or “very much improved” on the CGI and showing a decrease of at least 4 points on the SST.)
In the United States, a 1-year randomized double-blind placebo-controlled multicenter study with 120 mg of ginkgo extract (EGb) monitored cognitive impairment, daily living activity, and social behavior in 202 patients with Alzheimer's disease or multi-infarct dementia. Both cognitive and behavioral performance improved for 6 months to 1 year: 27% of ginkgo-treated subjects (vs. 14% on placebo) attained at least a 4-point improvement on the Alzheimer's Disease Assessment Scale (ADAS), and 37% were considered improved (vs. 23% placebo) on the Geriatric Evaluation by Relative's Rating Instrument.52 (These data can be compared contextually with the donepezil data from the 24-week trial, where 53.5% of subjects improved 4 ADAS points versus 26.8% on placebo.53) Although caregiver and cognitive tests demonstrated differences favoring the treated group in the ginkgo study, treatment effects were not detected by clinicians on the CGI.52
One major criticism of the study is the inclusion of the vascular dementia subgroup in the analysis of the Alzheimer's patient data. Another criticism is that the primary efficacy analysis was influenced by the substantial dropout rate after the halfway point in the study, necessitating secondary analysis based on intent-to-treat methodology. Of the 327 patients randomized to EGb or placebo, only 137 (78 and 59, respectively) completed the trial. Using the last observation carried forward to end could have produced an underestimate of the extent of natural deterioration of patients in the study. The NIH currently is sponsoring a three-and-a-half-year trial of ginkgo to study the prevention of Alzheimer's disease in people age 75 years or older without dementia.
Ginkgo also has been studied in a double-blind placebo-controlled trial of ginkgolide B in patients with acute multiple sclerosis (MS) exacerbations.54 This study showed no significant differences in ratings on functional scales with treatment. The MS study followed positive trial results examining PAF antagonism in rats with experimental allergic encephalomyelitis, the most widely used animal model of MS.55,56 Investigating PAF antagonist activity in the mixed ginkgolide compound, BN52063, Chung et al.57 demonstrated the compound's inhibition of platelet aggregation with human platelets.
The only side effect in the MS study was singultus (hiccups).54 The GCEm report other side effects as infrequent, including dyspepsia, headache, and allergic skin reactions.13 Although no severe side effects have been noted anecdotally, adverse reactions have been noted in various journal letters and case reports. Subarachnoid hemorrhage was reported in a 61-year-old man without other risk factors who was taking ginkgo 40 mg, three to four tablets per day.58 It was suggested that bleeding was induced by the extract's potent inhibition of PAF and association with increased bleeding time. Spontaneous bleeding into the anterior chamber of the eye was noted in a 70-year-old man after taking gingko 40 mg twice daily along with aspirin,59 again implicating ginkgolide-B PAF inhibition. A 33-year-old woman who developed bilateral subdural hematomas had a prolonged bleeding time while taking ginkgo 60 mg twice daily.60
Ginkgo is sold over the counter (OTC) in strengths of 40 mg to 60 mg in the United States. Most of the trials testing ginkgo in CI used 120 to 160 mg per day given in three divided doses. Increased dose efficacy has not been demonstrated in studies up to 240 mg. Peak absorption of flavone glycosides is attained in plasma within 2 to 3 hours and the t½ is 2 to 4 hours. Clinical effects may not be apparent for 4 to 6 weeks.61 There are no reports of interactions with psychiatric medications.
Kava-kava (Piper methysticum)
Kava is a member of the pepper family and contains psychoactive kava-pyrones. Polynesian islanders consume the extract as a beverage to experience a calming and relaxing effect without cognitive disturbances. Kava-pyrones possess a six-membered unsaturated lactone ring bound to a benzol ring by means of an ethylydene bridge.62 The α-pyrone constituents have been reported to possess anticonvulsive properties. Kavain, a synthetic kava pyrone, displayed inhibition of voltage-dependent sodium channels in rat cerebrocortical synaptosomes.63
Anticonvulsants have also shown reduction of brain injury from ischemia, and this property led to an investigation of whether kava was able to reduce cerebral infarct size in animals. Studies of the neuroprotective aspects of the kava extract and its constituents, methysticin and dihydromethysticin, have demonstrated protection against focal cerebral ischemia in rodents.64 The demonstration of selective binding to GABAA receptor complexes has been reported in the amygdala, hippocampus, and medulla regions of rat brain.65 Based on in vitro and in vivo binding studies in rats revealing that the resin and pyrones had only weak binding effects on benzodiazepine receptors, Davies et al.66 concluded that the pharmacological activities produced by kava pyrones are not due to a direct effect on GABA receptors.
Extract WS 1490, with 70% kava-pyrone content, was tested in a double-blind placebo-controlled study in patients with various DSM-III-R anxiety disorders of nonpsychotic origin.67 (A limitation of the study was a diagnostically heterogeneous sample of patients with anxiety disorders and comorbid depression.) Patients reported significant improvement in anxiety symptoms as measured on the Hamilton Anxiety Scale from week 8 through week 24 in the kava group relative to placebo. The dose administered was 70 mg of 70% kava-lactones (synonymous with pyrones) by mouth three times a day.52 Suggested dosages are 150–200 mg (30% kava-lactones) po 1 to 3 times a day for anxiety and 500 mg po at bedtime for insomnia. More reliable doses are becoming available in the United States as the variety of OTC preparations increases.
Side effects reported in kava studies include stomach upset, vertigo, and a yellow, scaly rash at high doses. Elevations in liver enzymes, decreased albumin and protein, and increased cholesterol also have been noted.68 Adverse effects reported include orolingual dyskinesias and torticollis, when kava was taken alone,69 and lethargy and disorientation when the herb has been used in combination with a benzodiazepine.70
Ginseng (Panax ginseng, P. quinquefolius, and Eleutherococcus senticosus)
This Asian folk medicine has been used for thousands of years as a tonic for the restoration of strength and as a panacea for general healing. The dried root of Asian (Panax) ginseng contains at least 13 different ginseng saponins, called ginsenosides. The Siberian (Eleutherococcus) species is distinct from the Chinese in that it contains eleutherosides, or aglycon glycosides, which are completely different in chemical structure from the ginsenosides. Ginseng has been touted as an “adaptogen”—defined as a substance that increases resistance to biological, chemical, and physical stress—and as a product that improves vitality, including physical and mental work capacity. The root has been associated anecdotally with mood enhancement and improved quality of life. There is a lack of controlled data to suggest performance enhancement in fatigued humans.71
Preparations of the root can be administered orally, intranasally, or parenterally. Ginsenosides, the active ingredients, contain triterpenoid saponin glycosides. Central cholinergic and variable dopaminergic72 system effects, as well as stimulation of the hypothalamic-pituitary-adrenal axis, have been proposed.73 Ginseng appears to facilitate acetylcholine release and is associated with increased uptake of choline into hippocampal nerve endings.74 Ginseng reportedly has no effect on acetylcholinesterase or muscarinic receptor binding activity.75
The ginsenoside Rg1 exerts a “life-prolonging effect” on chick and rat cerebral cortex neurons in cell cultures as compared to nerve growth factor.76 Animal studies of ginseng describe stimulation of protein synthesis, inhibition of platelet aggregation, increased immune system activity, prevention of stress-induced ulcer, and anticonvulsive effects,77 but human data are lacking.
Few controlled clinical studies of ginseng have been reported. The ergogenic properties of the root concentrate were tested in a randomized double-blind placebo-controlled study, showing no effect on work performance or associated physiologic or psychologic measures.78 Cognitive functions were assessed in a study of 112 healthy volunteers, revealing no significant differences between ginseng and placebo group assessments of concentration, memory, or subjective experience.79
The usual recommended daily dose of ginseng dry root is 0.5 to 2.0 g. Capsules containing 250 mg of the root are commercially available. Concern has arisen regarding the content purity of commercial preparations of ginseng, as noted above.
Ginseng may have adverse interactions with psychoactive drugs and other drugs. The induction of manic-like symptoms was seen in a 42-year-old depressed woman taking ginseng concurrently with phenelzine.80 A 63-year-old man with membranous glomerulonephritis developed edema and hypertension after adding Korean ginseng (a germanium-containing ginseng product) to his regimen of furosemide and cyclosporine.81 Other case reports include decreased International Normalized Ratio (INR) in a patient on warfarin82 and elevated digoxin serum concentrations without signs of toxic effects in a 74-year-old man.83 With respect to the above case reports, the use of ginseng with cardiovascular medications should be discouraged. It is unknown whether ginseng interacts with the P450 enzyme system.
Adverse effects include the development of a manic state after initiation of one ginseng tablet per day for 10 days in a previously depressed 35-year-old woman.84 CNS stimulant activity (“irritable, uncooperative…, and overactive with disturbed sleep”) was reported in a patient with schizophrenia without worsening psychotic symptomatology.73 Ginseng was implicated as having induced Stevens-Johnson syndrome in a 27-year-old man.85 Cerebral arteritis was noted in a case report in a 28-year-old woman after ingestion of a large quantity of ethanol-extracted ginseng.86 “Ginseng abuse syndrome” has been described, characterized by nervousness, hypertension, sleeplessness, morning diarrhea, and skin eruptions in “chronic” root users taking 3 grams per day for at least 1 to 3 weeks.87
Valerian (Valeriana officinalis)
Valerian comes from the herbaceous perennial plant Valerian officinalis, which grows throughout North America, Europe, and Asia. This root is used as a sedative and hypnotic. Other proposed indications include anxiety and epilepsy; these applications have yet to be validated in humans. The rhizome of valerian contains two pharmacologically active ingredients: valepotriates and sesquiterpenes (valerenic acid and acetoxyvalerenic acid). Sedating effects of the active agents have been demonstrated in mice.88 The general pharmacological properties of these agents are unknown. Valerian crude extract, however, is noted to have GABAB receptor binding properties.9 Valerian extract also demonstrates GABA uptake inhibition in rat synaptosomes.89 Although the valepotriates are cytotoxic in cell culture, this has not been observed clinically.90
A placebo-controlled study tested the ability of valerian to decrease sleep latency and night awakenings and to improve sleep quality in 128 subjects without documented sleep disorder diagnoses.91 Although objective measures of sleep were unaffected by valerian, it produced a significant decrease in self-rated sleep latency scores and a significant improvement in self-reported sleep quality in habitually poor sleepers. In a separate double-blind placebo-controlled parallel-group-design study with 14 elderly female poor sleepers, Schulz et al.92 demonstrated an increase in slow wave sleep, without REM alteration, in a valerian-treated group of 8 subjects versus a placebo group of 6.
Valerian doses range from 500 mg to 12 g,90 given either in three divided doses or once nightly. No drug interactions or acute side effects from valerian have been reported in normal, limited dosing.
One case report, from Willey et al.,93 describes a valerian overdose in a woman who took 20 times (approximately 20 g) the recommended dose. Adverse effects included fatigue, abdominal pain, chest tightness, tremor of hands and feet, and lightheadedness. These symptoms resolved within 24 hours of ingestion. Adverse reactions reported by some chronic users included headaches, excitability, uneasiness, and cardiac disturbances.
Feverfew (Tanacetum parthenium)
Today, feverfew is generally used for migraine headache prophylaxis. The leaves of this bushy perennial contain parthenolide, a sesquiterpene lactone that inhibits platelet 5-HT release.94 An extract of feverfew has also been reported to inhibit prostaglandin (PG) biosynthesis via a mechanism that differs from salicylates, in that the extract did not inhibit cyclo-oxygenation by PG synthase.95 This action may explain the historical use of this plant as an antipyretic.
A pilot study to profile adverse effects and to establish the efficacy of feverfew in a sample of patients who had formerly used feverfew leaves daily showed a significant increase in the frequency and severity of headaches in former users taking placebo.96 This study was followed by an 8-month prospective randomized double-blind placebo-controlled crossover study of 72 migraineurs. The frequency of migraines was significantly decreased in subjects taking feverfew. Among the 60 patients who completed the study, 35 subjects (59%) in the crossover arm reported fewer headaches with feverfew during the 4-month treatment period. Subjects who had never taken feverfew in the past reported a 23% reduction in the number of attacks and in the severity of associated symptoms.97
In the United Kingdom the usual prescribed dosage is 50 to 100 mg daily, and the product is sold as dried leaves, capsules, concentrated drops, tinctures, and extracts. Chronic users report eating one to four small fresh leaves daily for migraine prophylaxis, usually with food to mask the bitter flavor of the plant. Manufacturers recommend daily doses of herb capsules varying from 600 to 1,800 mg in divided doses; however, 0.25 mg (250 μg) of parthenolide daily has been shown to be adequate for migraine prevention.
Chewing the leaves has been associated with ulcers of the mouth in 11% of subjects, with a reversible tongue irritation and lip swelling in these patients.96
Although a sublingual spray is available, the exact amount of the substance delivered in the spray is not known. The potency of this product is variable across manufacturers.
Garlic (Allium sativum)
Garlic is thought to have anti-atherosclerotic properties. Studies in humans and in animals have demonstrated lowered lipid levels, blood pressure, plasma viscosity, and inhibition of platelet aggregation.98 Garlic exerts its hypolipemic effect through the active ingredient, allicin, a sulfur-containing amino acid compound. The mechanism of action is unknown; however, hydroxymethylglutaryl coenzyme A reductase inhibition99 and remodeling of plasma lipoproteins and cell membranes100 have been proposed.
In a 12-week double-blind placebo-controlled study of 900 mg of garlic powder tablets, significantly lower total cholesterol and low-density lipoproteins (LDL) were reported in the treatment group.101 Side effects were limited to eructation (belching), and there were no significant odor problems. A German meta-analysis found that garlic reduced hypercholesterolemia by 5% to 20%.102 An American meta-analysis of five randomized placebo-controlled trials, incorporating patients with total cholesterol exceeding 200 mg/dl, revealed a pooled result of a 23 mg/dl drop in 164 patients treated with 600 to 1,000 mg garlic per day.103
The common dose ranges prescribed are 0.6 g to 0.9 g garlic powder daily. The GCEm reported the average dose to be 4 g fresh garlic daily.13 Dried garlic contains no allicin. Rather, it contains the precursor, alliin, and the alliinase enzyme necessary to convert alliin to allicin. Studies noted above recommend a dried garlic powder preparation standardized to 1.3% alliin for effective cholesterol and triglyceride reduction. Manufacturers' variability again brought concern regarding potency of the preparations after a German study revealed only 5 of 18 garlic supplements contained acceptable amounts of allicin.
There are no known drug interactions with garlic; however, it should be used cautiously in patients receiving anticoagulants because of a potential bleeding risk.
Uncommon side effects of garlic include gastrointestinal disturbance, asthma, contact dermatitis, and foul odor. Enteric coated dried garlic minimizes garlic taste and odor. Adverse effects include a case report of spinal hematoma in an 87-year-old man, attributed to the antiplatelet effect of excessive garlic ingestion.104
Yohimbine (Corynanthe yohimbine)
This medication is discussed because of its transformation from initial consideration as an herbal alternative medicine to mainstream acceptance as pharmacological therapy.
Yohimbine comes from the trunk of Pausinystalia yohimbe, a Central African tree of the Rubiaceae family. The substance has been reputed to be an aphrodisiac since the early part of this century.105 The bark contains indole alkaloids and acts as a treatment for male impotence and for orthostatic hypotension.
A review of seven double-blind placebo-controlled randomized trials was conducted and revealed that yohimbine is superior to placebo in the treatment of organic and psychogenic erectile dysfunction, with a range of positive response from 34% to 73%. Dosages ranged from a total of 16.2 to 30 mg per day in three divided doses. Although yohimbine is probably less effective than vasoactive intracavernous injection therapy, it is considerably less invasive.106 Reid et al.107 found a 46% partial or full response to yohimbine in a 10-week double-blind placebo-controlled partial crossover trial with 48 subjects having psychogenic impotence, and recommended that the drug be considered among first-line treatment options in the psychogenically impotent patient. Finally, clinical benefit has been reported from using lower doses of yohimbine in sexual dysfunction induced by clomipramine and fluoxetine.108 No information is yet available on comparing yohimbine with the newer agent sildenafil (Viagra).
Pharmacologically, yohimbine acts as a presynaptic α-2 adrenoreceptor antagonist. Alpha-adrenolytic drugs produce a rise in sympathetic drive by increasing noradrenaline turnover and central nervous system noradrenergic nuclei cellular firing rate.109,110 Other pharmacologic properties of yohimbine include dopamine receptor antagonism, MAO and cholinesterase inhibition, and 5-hydroxytryptamine receptor antagonist and agonist activity.111 The drug is not eliminated in the urine and is thought to be metabolized with rapid plasma clearance, demonstrating a biological t½ of 36 minutes.112,113 Morales et al.114 observed a 2- to 3-week latency between onset of daily yohimbine administration and erectile function improvement.
The GCEm recommend that the bark should not be taken by patients with renal disease, noting increases in blood pressure. Yohimbine is not recommended for use in patients with cardiac history or a history of gastrointestinal ulcer.13 Mild antidiuretic activity may be present from stimulation of antidiuretic hormone release. Side effects include mild anxiety and panic attacks.115 Treatment with yohimbine is advised with caution in patients taking psychotropic medications because of its potential effects on cholinergic and adrenergic activity.116
Adverse effects include the precipitation of transient, manic-like symptoms in depressed patients with a bipolar diathesis.117 Increased cholinergic and decreased adrenergic activity associated with yohimbine have also been implicated in increased mucous secretion and bronchoconstriction-induced bronchospasm.118 An idiosyncratic lupus-like syndrome has also been reported in a 42-year-old man.119 Direct autonomic effects from injected yohimbine include increased systolic blood pressure; increased anxiety; tachycardia; increased perspiration, salivation, lacrimation, and pupillary dilation; nausea; urgency; and erection.120
Echinacea (Echinacea purpurea and E. pallida)
Derived from the common purple cone flower, echinacea is one of the most popular supplements sold in the United States. Native Americans originally used the plant to treat respiratory infections. Extracts and preparations are derived from the leaves and flowers of the purpurea species and from the root of the pallida species.121 Given its widespread use for putative immune-stimulating effects, echinacea is referenced because of possible contraindications in immune-related neuropsychiatric illnesses such as multiple sclerosis (MS), HIV infection, and acquired immune deficiency syndrome (AIDS).
Echinacea has not been studied largely in the neuropsychiatric population. One study reported that of 129 patients with documented MS, 63% used 87 different AT and approximately 40% (n=32) used herbs for treatment of their illness. With echinacea being one of the five best selling HAMs in the United States, it is likely that some form of the preparation is being taken by these MS patients whose aim is “to participate actively in the healing process.”122
Studies have shown that the active ingredients in echinacea are isobutyl amides, caffeic acid derivatives, and heteroxylan, a high-molecular-weight polysaccharide. The actions noted from human in vitro studies include increasing the number of leukocytes, activating granulocytes, increasing phagocytosis by heteroxylan, inhibiting hyaluronidase, and stimulating tumor necrosis factor release by arabinogalactan.123
The GCEm recommend that patients with autoimmune diseases, such as MS, should not take the medicine because of its immunostimulant property. Administration of echinacea in AIDS has also been discouraged by the GCEm because of concern for impairment of T-cell functioning. There are no studies in vivo of this herb in immunodeficiency diseases such as HIV infection, although enhanced natural killer cell activity has been reported in vitro with echinacea in AIDS and chronic fatigue syndrome.124
CONCLUSION
Research Trends
Although many products sold over the counter are labeled as “natural,” this does not ensure the product's safety or efficacy. It would be of great benefit to patients if empiric, scientific research were conducted to investigate the biologic activity, safety, and efficacy of the substances reviewed herein, which are being used increasingly in the United States and abroad.
Various departments of pharmacognosy, which study the chemistry of modern natural products, rigorously investigate pharmacologically active compounds. At the Uppsala University in Sweden, pharmacognosists inventory and identify plants used by traditional healers, evaluate pharmacological activity of the plant extract, analyze results by bioassay-guided fractionation, isolate and characterize the active compounds, and conduct studies of structure-activity relationships.125 Using this research strategy, a Somali-Swedish project studied the gum-covered bark of Acacia tortilis, used traditionally as an asthma remedy. Bioassay-guided isolation resulted in finding quracol A and B, which inhibit cyclooxygenase and lipooxygenase and relax smooth muscle.
Positive results and discoveries enhance therapeutic potential. Negative results will lead to greater protection of patients by educating them on the possible dangers of folk or traditional practices. As noted by De Smet, “the question, whether the use of traditional drugs can entail a health risk, is a rhetorical one.”126 The medical and toxicological literature is replete with adverse reactions. There is urgent need for a systematic approach weighing the potential benefits and risks of traditional preparations compared with synthetic drugs. The issue of long-term effects with HAM has not been resolved. More studies directly comparing HAM with conventional medicines are needed to address these questions.
Need for Communication
Communicating with patients about HAM is essential. If we do not know what our patients are taking, we do not know what interactions may be occurring. Clinical research from family practice (FP) patient surveys and focus groups reveals that patients are aware of the ambivalence and even hostility that biomedicine demonstrates toward AT.127 Many patients do not raise the issue with their physicians because they do not expect their doctor to know about the therapy (safety, dosage, interactions) or it does not occur to the patient that the topic is germane in the presence of a “medical” doctor.128 It has been suggested that physicians ask an unimposing, “What else are you doing to take care of your health?” in every interview to gain awareness of the botanicals patients are taking. A more direct question could be, “Are you taking any over-the-counter or nonprescription medications, vitamins, or health supplements?”
There is a great need to educate the medical community about OTC drug uses and misuses in order to convey information to our patients. A large number of patients are self-diagnosing symptoms and self-prescribing treatment. Although the FDA may classify prescription and OTC drugs differently, to our bodies, as one physician noted, “a molecule is a molecule.” Physicians should educate patients that marketing-designed, direct-to-consumer television advertisements are no substitute for empiric objective data. The patient who uses HAM should also be made aware of the risk that quality/purity, efficacy, and potential interactions of these medicines may be unknown.
Communication between practitioners and researchers is also essential. A directory of CAM databases with bibliographic references currently exists.129 Although discussions about the need to evaluate quality standards and health claims are under way, there are no formal federal regulations in the United States for nutraceuticals.130 Having a central address for funded research projects, research findings, and adverse reactions would greatly benefit clinicians in the absence of FDA regulation and supervision of these compounds.
Future Directions
HAM are increasingly finding their way into the medical mainstream. This trend is demonstrated by the proliferation of medical school courses, programs for patients in hospitals and health maintenance organizations, and coverage for some AT in health plans.131 A 1995 survey of 124 U.S. medical schools and 390 FP residencies reported that 33 medical schools and 75 FP programs (approximately one-third of the respondents) offered instruction in complementary and alternative medicine.132 A 1998 survey of the U.S. medical schools revealed that 74 (64%) of the 117 respondents offer elective courses in CAM or include these topics in required courses.133 Based on the definition of alternative medicine given in the early 1990s that AT are “medical interventions not taught widely at U.S. medical schools or generally available at U.S. hospitals,” for a growing portion of our patients, AT are not “alternative” any more.
So-called “natural” products may provide a new source of beneficial neuropsychotropic drugs. Monotherapy or augmentation with HAM could produce treatment strategies with the possibility of fewer or minimal side effects for patients. Biologic investigations to characterize individual active substances (reflecting historical pharmacologic approaches) and whole plants (realizing the possibility of synergistic effects of components) are needed, as are rigorous studies to evaluate both the risks and the benefits of such treatments. These procedures—coupled with animal models, in vivo behavioral experiments, and rigorous clinical patient trials—may separate the wheat from the chaff and may provide new treatment applications for these alternative medicines.
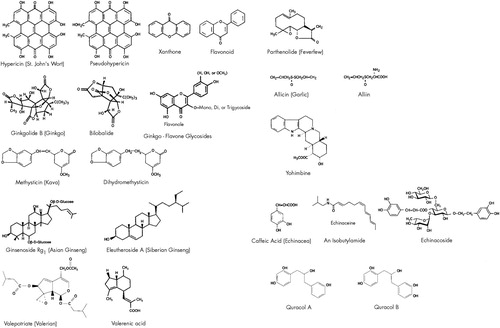
FIGURE 1. Structures of selected active compounds in herbal medicinesStructures redrawn and adapted from references 31 and 134–138. The authors thank D. Scott Davis, Ph.D., and Larry Walker, Ph.D., for their assistance in assembling these structures and John T. Knight for drawing the figure.
 |
1 Chez RA, Jonas WB: The challenge of complementary and alternative medicine. Am J Obstet Gynecol 1997; 177:1156–1161Google Scholar
2 Davey RW: A perspective on complementary medicine. Med Leg J 1997; 65:65–85Crossref, Medline, Google Scholar
3 Koutouvidis N, Papamichael E, Fotiadou A: Aristophanes' wealth: ancient alternative medicine and its modern survival. J R Soc Med 1996; 89:651–653Crossref, Medline, Google Scholar
4 Wardwell WI: Alternative medicine in the United States. Social Science Medicine 1994; 38:1061–1068Google Scholar
5 De Smet PAGM: An introduction to herbal pharmacoepidemiology. J Ethnopharmacol 1993; 38:197–208Crossref, Medline, Google Scholar
6 Fugh-Berman A: Clinical trials of herbs. Primary Care 1997; 24:889–903Crossref, Medline, Google Scholar
7 Clark AM: Natural products as a resource for new drugs. Pharm Res 1996; 13:1133–1141Google Scholar
8 Chung IW, Kim YS, Ahn JS, et al: Pharmacologic profile of natural products used to treat psychotic illnesses. Psychopharmacol Bull 1995; 31:139–145Medline, Google Scholar
9 Cott J: Medicinal plants and dietary supplements: sources for innovative treatments or adjuncts? Psychopharmacol Bull 1995; 31:131–137Google Scholar
10 Eisenberg DM, Kessler RC, Foster C, et al: Unconventional medicine in the United States: prevalence, cost, and patterns of use. N Engl J Med 1993; 328:246–252Crossref, Medline, Google Scholar
11 Astin JA: Why patients use alternative medicine: results of a national survey. JAMA 1998; 279:1548–1553Google Scholar
12 Borchers AT, Hackman RM, Keen CL, et al: Complementary medicine: a review of immunomodulatory effects of Chinese herbal medicines. Am J Clin Nutr 1997; 66:1303–1312Google Scholar
13 Blumenthal M (ed): The Complete German Commission E Monographs: Therapeutic Guide to Herbal Medicine. Austin, TX, American Botanical Council, 1998Google Scholar
14 Berman BM, Singh BK, Lixing L, et al: Physicians' attitudes toward complementary or alternative medicine: a regional survey. J Am Board Fam Pract 1995; 8:361–366Medline, Google Scholar
15 Woolf SH, Bell HS, Berman B, et al: Clinical practice guidelines in complementary and alternative medicine. Arch Fam Med 1997; 6:149–154Crossref, Medline, Google Scholar
16 Thompson WG: Alternatives to medicine. CMAJ 1990; 142:105–106Medline, Google Scholar
17 Cassileth BR, Lusk EJ, Strouse TB, et al: Contemporary unorthodox treatments in cancer medicine: a study of patients, treatments, and practitioners. Ann Intern Med 1984; 101:105–112Crossref, Medline, Google Scholar
18 Spiro HM: Doctors, Patients, and Placebos. New Haven, CT, Yale University Press, 1986Google Scholar
19 103rd Congress: Dietary Supplement Health and Education Act of 1994. (Public Law No. 103-417), 1994Google Scholar
20 Cottrell K: Herbal products begin to attract the attention of brand-name drug companies. CMAJ 1996; 155:216–219Medline, Google Scholar
21 Cui J, Garle M, Eneroth P, et al: What do commercial ginseng preparations contain? (letter). Lancet 1994; 344:134Crossref, Medline, Google Scholar
22 Drew AK, Myers SP: Safety issues in herbal medicine: implications for the health professional. Med J Aust 1997; 166:538–541Crossref, Medline, Google Scholar
23 Linde K, Clausius N, Ramirez G, et al: Are the clinical effects of homeopathy placebo effects? A meta-analysis of placebo-controlled trials. Lancet 1997; 350:834–843Crossref, Medline, Google Scholar
24 Langman MJS: Homeopathy trials: reason for good ones, but are they warranted? Lancet 1997; 350:825Google Scholar
25 Marshall E: The politics of alternative medicine. Science 1994; 265:2000–2002Google Scholar
26 Gordon JS: Alternative medicine and the family physician. Am Fam Physician 1996; 54:2205–2212Google Scholar
27 Helms J: Acupuncture for the management of primary dysmenorrhea. Obstet Gynecol 1987; 69:51–56Medline, Google Scholar
28 Patel M, Gutzwiller F, Paccaud F, et al: A meta-analysis of acupuncture for chronic pain. Int J Epidemiol 1989; 18:900–906Crossref, Medline, Google Scholar
29 Bullock M, Culliton PD, Olander RT: Controlled trial of acupuncture for severe recidivist alcoholism. Lancet 1989; i:1435–1439Google Scholar
30 Bullock ML, Pheley AM, Kiresuk TJ, et al: Characteristics and complaints of patients seeking therapy at a hospital-based alternative medicine clinic. The Journal of Alternative and Complementary Medicine 1997; 3:31–37Crossref, Medline, Google Scholar
31 Wagner H, Bladt S: Pharmaceutical quality of hypericum extracts. J Geriatr Psychiatry Neurol 1994; 7(suppl 1):S65–68Google Scholar
32 Suzuki O, Katsumata Y, Oya M, et al: Inhibition of monoamine oxidase by hypericin. Planta Med 1984; 50:272–274Crossref, Medline, Google Scholar
33 Bladt S, Wagner H: Inhibition of MAO by fractions and constituents of hypericum extract. J Geriatr Psychiatry Neurol 1994; 7(suppl 1):S57–S59Google Scholar
34 Thiede HM, Walper A: Inhibition of MAO and COMT by hypericum extracts and hypericin. J Geriatr Psychiatry Neurol 1994; 7(suppl 1):S54–S56Google Scholar
35 Perovic S, Müller WEG: Pharmacological profile of hypericum extract: effect on serotonin uptake by postsynaptic receptors. Arzneimittel-Forschung/Drug Research 1995; 45:1145–1148Google Scholar
36 Abramowicz M, Ed: St. John's wort. The Med Lett Drugs Ther 1997; 39(1014):107–108Google Scholar
37 Linde K, Ramirez G, Mulrow CD, et al: St. John's wort for depression: an overview and meta-analysis of randomised clinical trials. BMJ 1996; 313:253–258Crossref, Medline, Google Scholar
38 De Smet PAGM, Nolen WA: St John's wort as an antidepressant. BMJ 1996; 313:241–242Crossref, Medline, Google Scholar
39 Hänsgen KD, Vesper J, Ploch M: Multicenter double-blind study examining the antidepressant effectiveness of the hypericum extract LI 160. J Geriatr Psychiatry Neurol 1994; 7(suppl 1):S15–S18Google Scholar
40 Harrer G, Hübner WD, Podzuweit H: Effectiveness and tolerance of the hypericum extract LI 160 compared to maprotiline: a multicenter double-blind study. J Geriatr Psychiatry Neurol 1994; 7(suppl 1):S24–S28Google Scholar
41 Sherman C: “Natural” antidepressant is put to the test. Internal Medicine News, Dec 1997, p 18Google Scholar
42 Staffeldt B, Kerb R, Brockmöller J, et al: Pharmacokinetics of hypericin and pseudohypericin after oral intake of the Hypericum perforatum extract LI 160 in healthy volunteers. J Geriatr Psychiatry Neurol 1994; 7(suppl 1):S47–S53Google Scholar
42a Piscitelli SC, Burstein AH, Chaitt D, et al: Indinavir concentrations and St John's wort. Lancet 2000; 355:547–548Crossref, Medline, Google Scholar
42b Ruschitzka F, Meier PJ, Turina M, et al: Acute heart transplant rejection due to St John's wort. Lancet 2000; 355:548–549Crossref, Medline, Google Scholar
43 Woelk H, Burkard G, Grünwald J: Benefits and risks of the hypericum extract LI 160: drug monitoring study with 3250 patients. J Geriatr Psychiatry Neurol 1994; 7(suppl 1):S34–S38Google Scholar
44 Kleijnen J, Knipschild P: Ginkgo biloba. Lancet 1992; 340:1136–1139Google Scholar
45 Packer L, Haramaki N, Kawabata T: Ginkgo biloba extract (EGb 761), in Effects of Ginkgo Biloba Extract (EGb 761) on Aging and Age-Related Disorders, edited by Christen Y, Courtois Y, Droy-Lefaix MT. Paris, Editions Scientifiques Elsevier Paris, 1995, pp 23–47Google Scholar
46 Klein J, Chatterjee SS, Loffelholz K: Phospholipid breakdown and choline release under hypoxic conditions: inhibition by bilobalide, a constituent of Ginkgo biloba. Brain Res 1997; 755:347–350Crossref, Medline, Google Scholar
47 Sasaki K, Hatta S, Haga M, et al: Effects of bilobalide on gamma-aminobutyric acid levels and glutamic acid decarboxylase in mouse brain. Eur J Pharmacol 1999; 367(2–3):165–173Google Scholar
48 Taylor JE: Neuromediator binding to receptors in the rat brain: the effect of chronic administration of Ginkgo biloba extract. Presse Medicine 1986; 15:1491–1493Google Scholar
49 Kleijnen J, Knipschild P: Ginkgo biloba for cerebral insufficiency. Br J Pharmacol 1992; 34:352–358Crossref, Google Scholar
50 Kanowski S, Herrmann WM, Stephan K, et al: Proof of efficacy of the Ginkgo biloba special extract EGb 761 in outpatients suffering from mild to moderate primary degenerative dementia of the Alzheimer type or multi-infarct dementia. Pharmacopsychiatry 1996; 29:47–56Crossref, Medline, Google Scholar
51 Oswald WD, Fleischmann UM: Nürnberger-Alters-Inventar (NAI: Nuremberg Age Inventory). Universität Erlangen-Nürnberg, Psychologisches Institut II, 1986Google Scholar
52 Le Bars PL, Katz MM, Berman N, et al: A placebo-controlled, double-blind, randomized trial of an extract of Ginkgo biloba for dementia. JAMA 1997; 278:1327–1332Google Scholar
53 Rogers SL, Farlow MR, Doody RS, et al: A 24-week, double-blind, placebo-controlled trial of donepezil in patients with Alzheimer's disease. Neurology 1998; 50:136–145Crossref, Medline, Google Scholar
54 Brochet B, Guinot P, Orgogozo JM, et al: Double blind placebo controlled multicentre study of ginkgolide B in treatment of acute exacerbations of multiple sclerosis. J Neurol Neurosurg Psychiatry 1995; 58:360–362Crossref, Medline, Google Scholar
55 Howat DW, Chand N, Moore AR, et al: The effects of platelet-activating factor and its specific antagonist BN52021 on the development of experimental allergic encephalomyelitis in rats. International Journal of Immunopathology and Pharmacology 1988; 1:11–15Google Scholar
56 Howat DW, Chand N, Braquet P, et al: An investigation into the possible involvement of platelet activating factor in experimental allergic encephalomyelitis in rats. Agents and Actions 1989; 27(3/4):473–476Google Scholar
57 Chung KF, Dent G, McCusker M, et al: Effect of a ginkgolide mixture (BN 52063) in antagonising skin and platelet responses to platelet activating factor in man. Lancet 1987; i:248–251Google Scholar
58 Vale S: Subarachnoid haemorrhage associated with Ginkgo biloba (letter). Lancet 1998; 352:36Crossref, Medline, Google Scholar
59 Rosenblatt M, Mindel J: Spontaneous hyphema associated with ingestion of Ginkgo biloba extract. N Engl J Med 1997; 336:1108Crossref, Medline, Google Scholar
60 Rowin J, Lewis SL: Spontaneous bilateral subdural hematomas associated with chronic Ginkgo biloba ingestion. Neurology 1996; 46:1775–1776Google Scholar
61 Kleijnen J, Knipschild P: Ginkgo biloba. Lancet 1992; 340:1136–1139Google Scholar
62 Keller F, Klohs MW: A review of the chemistry and pharmacology of the constituents of Piper methysticum. Lloydia 1963; 26:1–15Google Scholar
63 Gleitz J, Beile A, Peters T: (+/–)–Kavain inhibits veratridine-activated voltage-dependent Na(+)–channels in synaptosomes prepared from rat cerebral cortex. Neuropharmacology 1995; 34:1133–1138Google Scholar
64 Backhauss C, Krieglstein J: Extract of kava (Piper methysticum) and its methysticin constituents protect brain tissue against ischemic damage in rodents. Eur J Pharmacol 1992; 215:265–269Crossref, Medline, Google Scholar
65 Jussofie A, Schmiz, Hiemke C: Kavapyrone enriched extract from Piper methysticum as modulator of the GABA binding site in different regions of rat brain. Psychopharmacology 1994; 116:469–474Crossref, Medline, Google Scholar
66 Davies LP, Drew CA, Duffield P, et al: Kava pyrones and resin: studies on GABAA, GABAB and benzodiazepine binding sites in rodent brain. Pharmacol Toxicol 1992; 71:120–126Crossref, Medline, Google Scholar
67 Volz HP, Kieser M: Kava-kava extract WS 1490 versus placebo in anxiety disorders: a randomized placebo-controlled 25-week outpatient trial. Pharmacopsychiatry 1997; 30:1–5Crossref, Medline, Google Scholar
68 Mathews JD, Riley MD, Fejo L, et al: Effects of the heavy usage of kava on physical health: summary of a pilot survey in an aboriginal community. Med J Aust 1988; 148:548–555Medline, Google Scholar
69 Schelosky L, Raffauf C, Jendroska K, et al: Kava and dopamine antagonism. J Neurol Neurosurg Psychiatry 1995; 58:639–640Crossref, Medline, Google Scholar
70 Almeida JC, Grimsley EW: Coma from the health food store: interaction between kava and alprazolam. Ann Intern Med 1996; 125:940–941Crossref, Medline, Google Scholar
71 Bahrke MS, Morgan WP: Evaluation of the ergogenic properties of ginseng. Sports Med 1994; 18:229–248Crossref, Medline, Google Scholar
72 Watanabe H, Ohta H, Imamura L, et al: Effect of Panax ginseng on age-related changes in the spontaneous motor activity and dopaminergic nervous system in the rat. Jpn J Pharmacol 1991; 55:51–56Crossref, Medline, Google Scholar
73 Wilkie A, Cordess C: Ginseng: a root just like a carrot? J R Soc Med 1994; 87:594–595Google Scholar
74 Benishin CG: Actions of ginsenoside Rb1 on choline uptake in central cholinergic nerve endings. Neurochem Int 1992; 21:1–5Crossref, Medline, Google Scholar
75 Benishin CG, Lee R, Wang LCH, et al: Effects of ginsenoside Rb1 on central cholinergic metabolism. Pharmacology 1991; 42:223–229Crossref, Medline, Google Scholar
76 Himi T, Saito H, Nishiyama N: Effect of Ginseng saponins on the survival of cerebral cortex neurons in cell cultures. Chemistry and Pharmacology Bulletin 1989; 37:481–484Crossref, Medline, Google Scholar
77 Barna P: The case of ginseng (letter). Lancet 1985; ii:548Google Scholar
78 Engels HJ, Wirth JC: No ergogenic effects of ginseng (Panax ginseng C.A. Meyer) during graded maximal aerobic exercise. J Am Diet Assoc 1997; 97:1110–1115Google Scholar
79 Sorensen H, Sonne J: A double-masked study of the effects of ginseng on cognitive functions. Current Therapeutic Research 1996; 57:959–968Crossref, Google Scholar
80 Jones BD, Runikis AM: Interaction of ginseng with phenelzine. J Clin Psychopharmacol 1987; 7:201–202Crossref, Medline, Google Scholar
81 Becker BN, Greene J, Evanson J, et al: Ginseng-induced diuretic resistance. JAMA 1996; 276:606–607Crossref, Medline, Google Scholar
82 Janetzky K, Morreale AP: Probable interaction between warfarin and ginseng. Am J Health Syst Pharm 1997; 54:692–693Crossref, Medline, Google Scholar
83 McRae S: Elevated serum digoxin levels in a patient taking digoxin and Siberian ginseng. CMAJ 1996; 155:293–295Medline, Google Scholar
84 González-Seijo JC, Ramos YM, Lastra I: Manic episode and ginseng: report of a possible cause. J Clin Psychopharmacol 1995; 15:447–448Crossref, Medline, Google Scholar
85 Dega H, Laporte JL, Francès C, et al: Ginseng as a cause for Stevens-Johnson syndrome? (letter). Lancet 1996; 347:1344Crossref, Medline, Google Scholar
86 Ryu SJ, Chien YY: Ginseng-associated cerebral arteritis. Neurology 1995; 45:829–230Crossref, Medline, Google Scholar
87 Siegel RK: Ginseng abuse syndrome: problems with the panacea. JAMA 1979; 241:1614–1615Google Scholar
88 Von Eickstedt KW, Rahman S: Psychopharmacologic effects of valepotriates. Arzneimittel-Forschung 1969; 19:316–319Medline, Google Scholar
89 Ortiz JG, Nieves-Natal J, Chaves P: Effects of Valeriana officinalis extracts on [3H] flunitrazepam binding, synaptosomal [3H] GABA uptake, and hippocampal [3H] GABA release. Neurochemistry Research 1999; 24:1373–1378Google Scholar
90 Heiligenstein E, Guenther G: Over-the-counter psychotropics: a review of melatonin, St John's wort, valerian, and kava-kava. J Am Coll Health 1998; 46:271–276Crossref, Medline, Google Scholar
91 Leathwood PD, Chauffard F, Heck E, et al: Aqueous extract of valerian root (Valeriana officinalis L.) improves sleep quality in man. Pharmacol Biochem Behav 1982; 17:65–71Crossref, Medline, Google Scholar
92 Schulz H, Stolz C, Müller J: The effect of valerian extract on sleep polygraphy in poor sleepers: a pilot study. Pharmacopsychiatry 1994; 27:147–151Crossref, Medline, Google Scholar
93 Willey LB, Mady SP, Cobaugh DJ, et al: Valerian overdose: a case report. Veterinary and Human Toxicology 1995; 37:364–365Medline, Google Scholar
94 Heptinstall S, White A, Williamson L, et al: Extracts of feverfew inhibit granule secretion in blood platelets and polymorphonuclear leukocytes. Lancet 1985; i:1071–1074Google Scholar
95 Collier HOJ, Butt NM, McDonald-Gibson WJ, et al: Extract of feverfew inhibits prostaglandin biosynthesis. Lancet 1980; ii:922–923Google Scholar
96 Johnson ES, Kadam NP, Hylands DM, et al: Efficacy of feverfew as prophylactic treatment of migraine. British Medical Journal 1985; 291:569–573Crossref, Medline, Google Scholar
97 Murphy JJ, Heptinstall S, Mitchell JRA: Randomised double-blind placebo-controlled trial of feverfew in migraine prevention. Lancet 1988; ii:189–192Google Scholar
98 Mansell P, Reckless JPD: Garlic: effects on serum lipids, blood pressure, coagulation, platelet aggregation, and vasodilatation. BMJ 1991; 303:379–380Crossref, Medline, Google Scholar
99 Brosche T, Platt D: Garlic (letter). BMJ 1991; 303:785Crossref, Medline, Google Scholar
100 Brosche T, Platt D, Dorner H: The effect of a garlic preparation on the composition of plasma lipoproteins and erythrocyte membranes in geriatric subjects. The British Journal of Clinical Practice 1990; 69 (suppl):12–19Google Scholar
101 Jain AK, Vargas R, Gotzkowsky S, et al: Can garlic reduce levels of serum lipids? A controlled clinical study. Am J Med 1993; 94:632–635Crossref, Medline, Google Scholar
102 Warshafsky S, Kamer RS, Sivak SL: Effect of garlic on total serum cholesterol: a meta-analysis. Ann Intern Med 1993; 119:599–605Crossref, Medline, Google Scholar
103 Brosche VT, Platt D: Knoblauch als pflanzlicher Lipidsenker [Garlic as a lipid-lowering drug]. Fortschritte der Medizin 1990; 108:703–706Medline, Google Scholar
104 Rose KD, Croissant PD, Parliament CF, et al: Spontaneous spinal epidural hematoma with associated platelet dysfunction from excessive garlic ingestion. Neurosurgery 1990; 26:880–882Crossref, Medline, Google Scholar
105 Riley AJ, Goodman RE, Kellett JM, et al: Double blind trial of yohimbine hydrochloride in the treatment of erection inadequacy. Sexual and Marital Therapy 1989; 4:17–26Crossref, Google Scholar
106 Ernst E, Pittler MH: Yohimbine for erectile dysfunction: a systematic review and meta-analysis of randomized clinical trials. J Urol 1998; 159:433–436Crossref, Medline, Google Scholar
107 Reid K, Morales A, Harris C, et al: Double-blind trial of yohimbine in treatment of psychogenic impotence. Lancet 1987; ii:421–423Google Scholar
108 Jacobsen FM: Fluoxetine-induced sexual dysfunction and an open trial of yohimbine. J Clin Psychiatry 1992; 53:119–122Medline, Google Scholar
109 Cedarbaum JM, Aghajanian GK: Noradrenergic neurons of the locus coeruleus: inhibition by epinephrine and activation by the α-antagonist piperoxane. Brain Res 1976; 112:413–419Crossref, Medline, Google Scholar
110 Bolme P, Corrodi H, Fuxe K, et al: Possible involvement of central adrenaline neurons in vasomotor and respiratory control: studies with clonidine and its interactions with piperoxane and yohimbine. Eur J Pharmacol 1974; 28:89–94Crossref, Medline, Google Scholar
111 Goldberg MR, Robertson D: Yohimbine: a pharmacologic probe for study of the α-2 adrenoreceptor. Pharmacologic Reviews 1983; 35:143–180Medline, Google Scholar
112 Owen JA, Nakatsu SL, Fenemore J, et al: The pharmacokinetics of yohimbine in man. Eur J Clin Pharmacol 1987; 32:577–582Crossref, Medline, Google Scholar
113 Owen JA, Nakatsu SL, Condra M, et al: Sub-nanogram analysis of yohimbine and related compounds by high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl 1985; 342:333–340Crossref, Google Scholar
114 Morales A, Condra M, Owen J, et al: Is yohimbine effective in the treatment of organic impotence? Results of a controlled trial. J Urol 1987; 137;1168–1172Google Scholar
115 Wong AHC, Smith M, Boon HS: Herbal remedies in psychiatric practice. Arch Gen Psychiatry 1998; 55:1033–1044Google Scholar
116 Arky R, Med Cnslt: Yocon: Brand of yohimbine hydrochloride, in Physicians' Desk Reference, 52nd edition. Montvale, NJ, Medical Economics Company, 1998, p 1185Google Scholar
117 Price LH, Charney DS, Heninger GR: Three cases of manic symptoms following yohimbine administration. Am J Psychiatry 1984; 141:1267–1268Google Scholar
118 Landis E, Shore E: Yohimbine-induced bronchospasm. Chest 1989; 96:1424Crossref, Medline, Google Scholar
119 Sandler B, Aronson P: Yohimbine-induced cutaneous drug eruption, progressive renal failure, and lupus-like syndrome. Urology 1993; 41:343–345Crossref, Medline, Google Scholar
120 Holmberg G, Gershon S: Autonomic and psychic effects of yohimbine hydrochloride. Psychopharmacologia 1961; 2:93–106Crossref, Medline, Google Scholar
121 Alschuler L, Benjamin SA, Duke JA: Herbal medicine: what works, what's safe. Patient Care 1997; 31:49–68Google Scholar
122 Winterholler M, Erbguth F, Neundorfer B: The use of alternative medicine by multiple sclerosis patients: patient characteristics and patterns of use. Fortschr Neurol Psychiatr 1997; 65:555–561Crossref, Medline, Google Scholar
123 Burger RA, Torres AR, Warren RP, et al: Echinacea-induced cytokine production by human macrophages. Int J Immunopharmacol 1997; 19:371–379Crossref, Medline, Google Scholar
124 See DM, Broumand N, Sahl L, et al: In vitro effects of echinacea and ginseng on natural killer and antibody-dependent cell cytotoxicity in healthy subjects and chronic fatigue syndrome or acquired immunodeficiency syndrome patients. Immunopharmacology 1997; 35:229–235Crossref, Medline, Google Scholar
125 Bohlin L: Research on pharmacologically active natural products at the Department of Pharmacognosy, Uppsala University. J Ethnopharmacol 1993; 38:225–231Crossref, Medline, Google Scholar
126 De Smet PAGM: Is there any danger in using traditional remedies? J Ethnopharmacol 1991; 32:43–50Google Scholar
127 Elder NC, Gillcrist A, Minz R: Use of alternative health care by family practice patients. Arch Fam Med 1997; 6:181–184Crossref, Medline, Google Scholar
128 Lazar JS, O'Connor BB: Talking with patients about their use of alternative therapies. Primary Care 1997; 24:699–712Crossref, Medline, Google Scholar
129 Wootton JC: Directory of databases for research into alternative and complimentary medicine. J Altern Complement Med 1997; 3:179–190Crossref, Medline, Google Scholar
130 Marwick C: Growing use of medicinal botanicals forces assessment by drug regulators. JAMA 1995; 273:607–609Crossref, Medline, Google Scholar
131 Angell M, Kassirer JP: Alternative medicine: the risks of untested and unregulated remedies. N Engl J Med 1998; 339:839–841Crossref, Medline, Google Scholar
132 Carlston M, Stuart MR, Jonas W: Alternative medicine instruction in medical schools and family practice residency programs. Fam Med 1997; 29:559–562Medline, Google Scholar
133 Wetzel MS, Eisenberg DM, Kaptchuk TJ: Courses involving complementary and alternative medicine at US medical schools. JAMA 1998; 280:784–787Crossref, Medline, Google Scholar
134 Bisset NG (ed, trans): Herbal Drugs and Phytopharmaceuticals, 2nd German edition, edited by Wichtl M. Stuttgart, Germany, and Boca Raton, FL, Medpharm/CRC Press, 1994Google Scholar
135 Budavari S (ed): The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals. 11th edition. Rahway, NJ, Merck, 1989Google Scholar
136 Dictionary of Natural Products (CD-ROM), release 7.2. London, Chapman and Hall, 1999Google Scholar
137 Cott J: Natural product formulations available in Europe for psychotropic indications: NCDEU update. Psychopharmacol Bull 1995; 31:745–751Medline, Google Scholar
138 DeFeudis FV: Ginkgo biloba extract (Egb 761), in Pharmacological Activities and Clinical Applications. New York, Elsevier, 1991Google Scholar



