Risk Factors in Psychosis Secondary to Traumatic Brain Injury
Abstract
Psychosis is a rare but devastating sequela of traumatic brain injury (TBI). This study examined risk factors for developing a psychosis secondary to TBI (PSTBI). Demographics of 25 inpatients with PSTBI were statistically analyzed for risk factors. Data from the PSTBI group were also compared with data from a control group of TBI patients without psychosis. Results indicate the PSTBI group was more likely to have had a previous congenital neurological disorder or to have sustained a head injury prior to adolescence. The PSTBI also had a higher proportion of males. Discussion focuses on potential models for developing PSTBI.
Psychosis is a devastating sequela of traumatic brain injury (TBI). Lifetime incidence rates of TBI survivors who later demonstrate psychotic symptoms vary across studies, but they are generally low. Davison and Bagley's review of papers published between 1917 and 1960 reveals incidences ranging from 0.07% to 9.8%.1 More recently published studies report similar incidence rates of 3.4% and 8.9%.2,3
The onset of psychosis after TBI is highly variable but is generally delayed. In their study of World War II veterans, Achte et al.4 reported that the occurrence of psychotic symptoms ranged from 2 days to 48 years after injury, with 42% experiencing their first psychotic episode 10 or more years after sustaining a missile wound to the head. Fujii and Ahmed5 reported a range from 3 months to 19 years with a mean onset of 5.9 years after closed head trauma. And Feinstein and Ron6 reported a mean latency of 11.7 years with a range of 0 to 52 years.
Evidence from World War II medical reports, case studies, and contemporary structural imaging data suggests that psychosis secondary to TBI is associated with damage to frontal and temporal areas. This association has been found in both open1,3,7 and closed6 head injury patients. Interestingly, these structures have also been implicated in schizophrenia.8–11
Despite a growing interest in this diagnosis, not much is known about risk factors for developing a psychosis after TBI. It is well established that delusions and hallucinations can result from temporal lobe epilepsy.12,13 Seizure disorder is a common sequela of TBI, and it has been speculated that kindling in temporal limbic areas may be one mechanism in developing a psychosis.13,14 Other investigators reported a preponderance of males in their sample of state hospital inpatients who developed a psychosis secondary to TBI.5 Psychosis has also been found to occur shortly after a TBI in persons who are predisposed to schizophrenia.15
One area that has not been examined as a risk factor for developing a psychosis secondary to TBI is the effect of prior brain damage or neurological condition. Fujii16 argues that the presentation of symptoms after TBI is an interaction of the injury and characteristics of the premorbid brain. Individuals differ in their brain organization, which could be affected by biological factors or experience. More profound effects on brain organization may result from conditions that actually damage the brain such as neurological disorders, traumatic brain injury, or drug abuse. Is it possible that changes resulting from previous brain damage can increase the risk for developing a psychosis with additional brain trauma?
The current study examines risk factors for developing a psychosis secondary to TBI. It is hypothesized that individuals who develop a psychosis secondary to TBI are more likely to have had a preexisting neurological condition. Prior existence of these conditions would be especially crucial for development of a psychosis after a mild head injury.
METHODS
Potential subjects for the TBI psychosis group were state hospital inpatients referred for a neuropsychological evaluation within a period of 7 years. Such referrals were generally based on performance on an intake neurocognitive screening battery consisting of the Trail Making Test parts A and B and the Bender Gestalt Test. Referrals were also made if a known neurological condition existed such as HIV-positive status.
Criteria for patients with psychosis secondary to TBI were based on the DSM-IV17 criteria for Psychotic Disorder due to a General Medical Condition—namely, the presence of 1) hallucinations or delusions, 2) historical or laboratory evidence indicating the psychosis is the direct physiological consequence of the medical condition, 3) psychotic symptoms not better accounted for by another mental disorder, and 4) psychotic symptoms not occurring exclusively within the course of delirium.
Because of cited difficulties in determining whether a psychotic condition is a direct consequence of the TBI,18 additional criteria described by Cummings were adopted to further operationalize DSM-IV criterion #2 for the disorder.19 Thus, to ensure the association between psychosis and TBI, PSTBI subjects had to also meet the following criteria: 1) no reported family history of psychotic illness, 2) no prior history of psychotic illness, 3) a history of TBI, 4) onset of psychotic symptoms after TBI, and 5) the existence of cognitive deficits.
Severity of TBI was based on criteria set by the Mild Traumatic Brain Injury Committee of the Head Injury Interdisciplinary Special Interest Group, American Congress of Rehabilitation Medicine.20 Criteria are based on duration of loss of consciousness (LOC). A head injury is classified as mild if LOC is 30 minutes or less and moderate to severe if LOC is longer than 30 minutes.
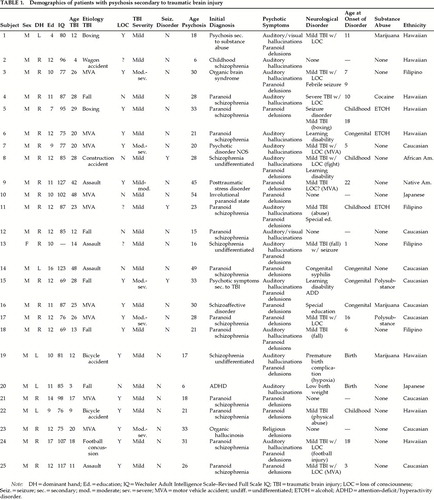
A total of 284 patients were referred for neuropsychological testing during this period. Of the total, 25 patients met inclusion criteria for psychosis secondary to TBI. The following are initial admitting diagnoses upon onset of symptoms; thus, in some cases, the diagnosis listed was received prior to admission into the state hospital. Seventeen subjects were diagnosed with schizophrenic illness: 12 paranoid schizophrenia, 3 chronic undifferentiated type, 1 childhood schizophrenia, and 1 schizoaffective disorder. Four subjects were diagnosed with an organic disorder: 1 organic hallucinosis, 1 organic brain syndrome, 1 psychotic disorder due to substance abuse, and 1 psychotic disorder due to traumatic brain injury. The other 4 subjects received diagnoses of posttraumatic stress disorder, attention-deficit/hyperactivity disorder (ADHD), psychotic disorder not otherwise specified, and involutional paranoid state.
The sample comprised 12 Pacific Islanders, 9 Caucasians, 2 Asians, 1 African American, and 1 Native American. Prior history of substance abuse was reported in 14 subjects. Of these subjects, 8 reported use of substances that have the potential to induce a psychosis with long-term use (e.g., methamphetamine, cocaine, LSD). None of these subjects were reported to be using these substances at the time of psychosis onset.
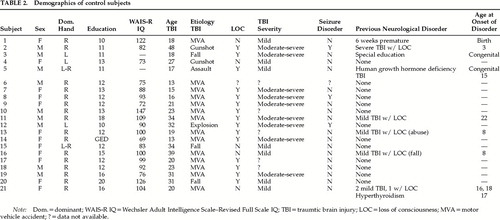
The control group consisted of outpatient TBI patients who were referred for neuropsychological evaluation to address issues of TBI. Referrals were made to the same department during the same time period. There were 21 subjects in the control group. This sample comprised 8 Caucasians, 5 Asians, 4 Pacific Islanders, 2 African Americans, and 2 Hispanics. Of these subjects, 3 reported past abuse of substances that have the potential to induce a psychosis.
The design of the study was a retrospective chart review. Data from the TBI subjects with psychosis were analyzed both within group and with the control group where appropriate. Analyses included chi-squares, two-tailed t-tests, and descriptive statistics. Significance level was set at 0.05.
RESULTS
Data for TBI subjects with psychosis are presented in Table 1. The control group is characterized in Table 2.
The gender of the TBI sample was predominantly male (24:1). This gender ratio was significantly different from the expected 2:1 gender base rates for TBI (χ2=4.21, P<0.05). Gender proportions (9:12) were also significantly different from those of the control group (χ2=16.8, P<0.001). The gender ratio for the psychosis group, however, is not significant if statistics are calculated on the gender base rates of the institution (χ2=0.91, P>0.05).
The ratio of right versus left/mixed hand dominance for the psychosis group (20 right; 5 non-right) was proportionally similar to the ratios in both the general population (χ2=0.50, P>0.05) and the control group (χ2=0.07, P>0.05). There were no differences between the highest level of education attained between the psychosis and control groups (mean±SD: 11.4±2.1 vs. 12.6±3.7 years; t=0.202, P>0.05). The groups were also similar in WAIS-R Full Scale IQ scores: psychosis, 89.0±16.2; control, 94.3±21.6 (t=0.371, P>0.05). No differences were found in proportion of past use of substances that can induce a psychosis with long-term use (χ2=2.57, P>0.05).
The mean sample age for sustaining a TBI that predated the psychotic condition was 21.4±11.9 years (range 3–48 years). The majority of head injuries were caused by motor vehicle accidents (9). The next most frequent cause of injury was falls (5), followed by assault (4). The other causes included 2 bicycle accidents, 2 boxing accidents, a construction accident, a wagon accident, and a football accident. The majority of subjects experienced a loss of consciousness (16/22; 3 unknown). Most head injuries were considered mild (16/22; 3 could not be determined).
There were no differences in age at occurrence of head injury between the psychosis and control groups (t=0.364, P>0.05) or in proportion of closed to open head injury (χ2=1.89, P>0.05). There was a trend for difference in the proportion of mild to moderate-severe injuries (χ2=3.24, P<0.10), with the control group having a greater proportion of moderate to severe injuries.
Secondary seizures occurred in only 3 of the 25 psychosis subjects, which is similar to the general TBI population's 5% incidence rate (χ2=0.25, P>0.05). There were no differences in proportion of seizure occurrence between psychosis and control subjects (3/21 control subjects; χ2=0.03, P>0.05). In addition, no differences were found in the proportion of subjects who had a history of abusing substances that can induce a psychosis (χ2=1.75, P>0.10).
The mean age for onset of psychosis was 25.6±12.1 years (range 6–54 years). The mean delay for onset of psychotic symptoms was 4.6±4.4 years (range 0–15 years). Eighteen subjects experienced paranoid delusions, 15 had auditory hallucinations, and 2 had visual hallucinations. For the control group, the mean length of time since the injury was 9.2±8.4 years (range 1–23 years). Fifteen control subjects had symptom-free postinjury durations that were longer than the mean for symptom onset in the psychosis group.
The majority of subjects in the psychosis group reported a prior history of head injury or symptoms of a neurological disorder (20/25; 80%). Fourteen of the subjects had sustained a prior head injury. Three experienced seizures, 3 were diagnosed with a learning disability or were placed in special education, 2 experienced birth complications (1 was premature with hypoxia; the other was premature with a low birth weight), 1 was diagnosed with ADHD, and 1 was diagnosed with congenital syphilis. The proportion of subjects in the psychosis group that experienced a prior head injury or prior neurological disorder was greater than chance (χ2=4.50, P<0.05) and significantly differed from the control group (χ2=7.99, P<0.01).
In addition, the majority of subjects in the psychosis group (17/20) reported either a congenital condition or a head injury sustained prior to the onset of adolescence. This proportion is again greater than chance (χ2=4.9, P<0.05), but it is not significantly different from the control group (χ2=0.42, P>0.05).
DISCUSSION
Our findings support the general hypothesis that a preexisting head injury (probably causing brain injury) or a neurological condition are risk factors for developing a psychosis secondary to TBI. Eighty percent of our psychotic TBI sample reported previous neurological conditions, compared with less than 40% of the control group. In a significant majority of the psychosis group, the condition occurred prior to or during childhood. This finding is consistent with Fujii's argument that the presentation of symptoms after TBI is an interaction of the injury and characteristics of the premorbid brain.16
Whereas other studies have reported that secondary psychosis generally occurs after moderate to severe head injury,4,5 the majority of our cases involved mild head injury. The fact that most of our subjects had experienced a previous head injury or neurological condition supports our corollary prediction that the existence of a prior neurological condition may be an important factor for developing a psychosis after mild brain injury.
A trend did exist, however, for proportionately more of the TBIs to be at the moderate or severe level in the control group than in the psychosis group. This finding is counterintuitive and contradicts findings from other studies.4,5 We believe that this trend may be due to biases in data collection. Subjects in the control group were those referred for neuropsychological evaluation and treatment. It is therefore likely that the more severe cases would be overrepresented among those referred.
Another finding was that males significantly outnumbered females in developing a psychosis when the gender ratio in the psychosis group was compared with those for the control group and for people with head injuries in the general population. However, this ratio was not significant when compared with the base ratio of males to females in the institution from which the data were collected. This finding may thus be an artifact of the sample.
Although our findings with regard to gender are equivocal, there are many reasons why males might be at higher risk than females for developing a secondary psychosis. Males are found to have a higher incidence rate of neurodevelopmental disorders such as learning disabilities,17,21 and males also are more likely to sustain TBI in childhood.22 Another potential mechanism may be sexual dimorphism in brain organization. Imaging studies have demonstrated that the brains of males are more lateralized than the brains of females.23,24 This difference in lateralization is believed to be an important factor in females having a better prognosis for recovery from aphasia after a left hemisphere cerebral vascular accident.25,26 More studies are definitely needed to examine whether males are similarly more vulnerable to developing a psychosis after TBI.
Other factors including left-handedness, education, posttraumatic IQ, or abuse of substances that may induce a psychosis did not appear to be risk factors in our sample, since there were no differences between the two groups. The proportions of subjects with seizure disorder in both groups were very similar to overall base rates in the population. The low incidence of seizure disorder in our psychosis sample is surprising, given reports of much higher incidence rates in other studies.18,27
Although the data support our hypothesis that prior neurological condition is a risk factor for developing a psychosis secondary to TBI, there may be alternative explanations. Smeltzer et al.28 argue that those who are biologically predisposed to developing a psychosis may also be predisposed to sustaining a brain injury. For instance, persons with schizophrenia have been found to have a higher incidence of brain injury than the general population.1,29
In our sample, we controlled for family history of mental illness. However, there may be unknown genetic or acquired risk factors other than schizophrenia that predispose an individual to both TBI and psychosis secondary to TBI. For example, presence of the apolipoprotein E E4 allele has been found to be a risk factor for both Alzheimer's dementia and psychotic symptoms in Alzheimer's patients.30,31
Another possibility is that the initial TBI is not a risk factor for developing a psychosis after a second TBI. Instead, the initial TBI may place an individual at risk for sustaining a delayed-onset psychosis, such as schizophrenia, or for subsequent brain injuries independent of the psychosis.32 The development of psychosis may be a delayed sequela of the pathophysiological changes resulting from either the earlier or the later TBI.14 In the latter case, the initial injury may contribute only indirectly to the processes of developing a psychosis.
Given our results concerning a prior neurological condition, particularly with onset in childhood as a risk factor for psychosis secondary to TBI, what are some possible mechanisms for developing this condition? We propose two possible models of development based on conceptualizations of two other severe disorders of brain dysfunction: schizophrenia and dementia.
Schizophrenia is currently conceptualized as a neuro-developmental disorder that may be caused by genetic factors combined with prenatal insult to the central nervous system (CNS).33,34 Despite early brain abnormalities and possible soft neurological signs, the onset of positive symptoms is generally delayed, manifesting itself in adolescence or early adulthood. It is believed that some type of cerebral maturation (such as neuroendocrine changes, synaptic pruning, or neuronal myelinization), or processes of cell death (such as apoptosis or necrosis) are necessary for the effects of the lesion to become manifest.33,35
In a significant majority of our sample, the onset of a prior neurological condition occurred congenitally or in childhood, whereas the TBI associated with the onset of psychosis occurred in young adulthood. The presence of an early CNS insult may result in pathophysiological processes in the brain that can interact with neurologic insults to the brain, possibly from TBI, to cause a psychosis. The latter injury may precipitate or propagate disease processes from earlier TBI or other neurological conditions.
There is some evidence to support this model. Traumatic brain injury can result in cell death, damage to white matter, and neuroendocrine changes—processes that may mimic or interact with proposed maturational changes that trigger psychotic illness in schizophrenics.1,15,36
In addition, studies have reported a relatively high rate of early TBI in schizophrenic populations. For example, in one retrospective study schizophrenic subjects had a significantly greater incidence of head trauma before the age of 10 than bipolar, depressive, and control subjects.29 The authors' interpretation was that TBI may lower the threshold for psychosis in some people. In another study examining obstetric complications and early brain injuries in schizophrenic patients, 61.5% of the schizophrenic sample reported such events, compared with only 8.3% in the bipolar group.37
Dementia is the other neurological disorder that may provide insight into the mechanism for secondary psychosis. The Satz theory of cognitive reserve38 addresses the acquisition of dementia. According to Satz, individuals differ in the amount of brain capacity available for general functioning. This cognitive reserve declines throughout life, through either the normal aging process or accumulated insults from disease or external stressors such as toxins or trauma. Dementia occurs once a certain threshold of reduced cognitive capacity is reached. Thus, individuals with prior brain damage of any type are at higher risk for developing a dementia.
Similar to dementia, psychosis is a neurological syndrome resulting in severe cognitive and behavioral impairments. In our sample, subjects experienced a minimum of two neurological conditions, including TBI, prior to the manifestation of psychosis. Is it possible that additive effects of CNS insults would predispose an individual to developing a psychosis? Although there is no direct evidence for this hypothesis, studies have demonstrated an increase in the incidence of neurobehavioral and emotional symptoms with repeated brain injury.32
Regardless of the model being used, it is believed that TBI contributes to the development of a psychosis by damaging frontal and temporal structures. These areas are highly vulnerable to lesions in TBI because of the surrounding bony structures. Converging evidence from studies examining both open and closed head injury indicates there is damage to temporal and frontal lobes in a preponderance of TBI patients who develop a psychosis.1,3,5,7,27,36 Interestingly, both temporal and frontal areas have been implicated in schizophrenia.8–11
Despite interesting findings, our study has several limitations. First, the sample size is small, which would affect the power and generalizability of our findings. Second, the study is based on retrospective chart review and may be subject to biases in data collection. Third, our initial pool of subjects came from referrals for neuropsychological testing. Thus, our findings may be limited to a specialized subsample of patients who demonstrate cognitive deficits on such testing. Similarly, comparisons between the psychosis and control groups may be based on artifacts of our specific samples.
Prospective studies with larger sample sizes are needed to replicate our findings. Potential directions for future studies would include 1) further exploration of male gender as a risk factor, 2) examination of patients in a nonacute psychiatric setting, and 3) further examination of mechanisms for developing a psychosis secondary to TBI.
 |
 |
1 Davison K, Bagley CR: Schizophrenia-like psychoses associated with organic disorders of the central nervous system: a review of the literature. Br J Psychiatry 1969; 114(suppl):113–162Google Scholar
2 Violin A, De Mol J: Psychological sequelae after head traumas in adults. Acta Neurochir (Wien) 1987; 85:96–102Crossref, Medline, Google Scholar
3 Achte K, Jarho L, Kyykka T, et al: Paranoid disorders following war brain damage. Psychopathology 1991; 24:309–315Crossref, Medline, Google Scholar
4 Achte KA, Hillbom E, Aalberg V: Psychoses following war injuries. Acta Psychiatr Scand 1969; 45:1–18Crossref, Medline, Google Scholar
5 Fujii DE, Ahmed I: Psychosis secondary to traumatic brain injury. Neuropsychiatry Neuropsychol Behav Neurol 1996; 9:133– 138Google Scholar
6 Feinstein A, Ron M: A longitudinal study of psychosis due to a general medical condition: establishing predictive and construct validity. J Neuropsychiatry Clin Neurosci 1998; 10:448–452Link, Google Scholar
7 Hillbom E: After-effects of brain injuries. Acta Psychiatr Neurol Scan Suppl 1960; 142:1–195Google Scholar
8 Ingvar DH: Evidence for frontal/pre-frontal cortical dysfunction in chronic schizophrenia: the phenomenon of hypofrontality reconsidered, in Biological Perspectives of Schizophrenia, edited by Helmchen H, Henn FA. New York, Wiley, 1987, pp 201–211Google Scholar
9 Weinberger DR, Berman KF: Speculation on the meaning of cerebral metabolic hypofrontality in schizophrenia. Schizophr Bull 1988; 14:157–168Crossref, Medline, Google Scholar
10 Rossi A, Stratta P, Casacchia M, et al: Reduced temporal lobe areas in schizophrenia: preliminary evidences from a controlled multiplanar magnetic resonance imaging study. Biol Psychiatry 1990; 27:61–68Crossref, Medline, Google Scholar
11 Yates WR, Swayze VW, Andreasen NC: Neuropsychological effect of global and cerebral atrophy is schizophrenia. Neuropsychiatry Neuropsychol Behav Neurol 1990; 3:98–106Google Scholar
12 Flor-Henry P: Psychosis and temporal lobe epilepsy: a controlled investigation. Epilepsia 1969; 10:363–395Crossref, Medline, Google Scholar
13 McKenna PJ, Kane JM, Parrish K: Psychotic syndromes in epilepsy. Am J Psychiatry 1985; 142:895–904Crossref, Medline, Google Scholar
14 Gualteri CT, Cox DR: The delayed neurobehavioral sequelae of traumatic brain injury. Brain Inj 1991; 5:219–232Crossref, Medline, Google Scholar
15 Lishman WA: Organic Psychiatry: The Psychological Consequences of Cerebral Disorder, 2nd edition. Oxford, UK, Blackwell Scientific, 1987Google Scholar
16 Fujii DE: Kolb's learning styles and potential cognitive remediations of brain injured individuals: an exploratory factor analytic study involving undergraduates. Professional Psychology Research and Practice 1996; 27:266–271Crossref, Google Scholar
17 American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th edition. Washington, DC, American Psychiatric Association, 1994Google Scholar
18 Ahmed I, Fujii DE: Posttraumatic psychosis. Seminars in Clinical Neuropsychiatry 1998; 3:23–33Medline, Google Scholar
19 Cummings JL: Organic psychosis. Psychosomatics 1988; 29:16–26Crossref, Medline, Google Scholar
20 Mild Traumatic Brain Injury Committee of the Head Injury Interdisciplinary Special Interest Group of the American Congress of Rehabilitation Medicine: Definition of mild traumatic brain injury. J Head Trauma Rehabil 1993; 8:86–87Crossref, Google Scholar
21 Geschwind N, Galaburda AM: Cerebral lateralization, biological mechanisms, associations, and pathology. Arch Neurol 1985; 42:428–459Crossref, Medline, Google Scholar
22 Annegers JF, Gradow JD, Kurland LT, et al: The incidence, causes, and secular trends of head trauma in Olmstead County, Minnesota 1935–1974. Neurol 1980; 30:912–919Crossref, Medline, Google Scholar
23 Gur RC, Gur RE, Obrist WD, et al: Sex and handedness differences in cerebral blood flow during test and cognitive activity. Science 1982; 217:659–660Crossref, Medline, Google Scholar
24 Levy J, Heller W: Gender differences in human neuropsychological functioning, in Handbook of Behavioral Neurobiology, vol 11: Sexual Differentiation, edited by Gerall AA, Ward IL. New York, Plenum, 1992, pp 245–274Google Scholar
25 Pizzamiglio L, Mammucari A, Razzano C: Evidence for sex differences in brain organization in recovery in aphasia. Brain Lang 1985; 25:213–223Crossref, Medline, Google Scholar
26 Basso A, Capitani E, Moraschini S: Sex differences in recovery from aphasia. Cortex 1982; 18:469–475Crossref, Medline, Google Scholar
27 Feinstein A, Ron MA: Psychosis associated with demonstrable brain disease. Psychol Med 1990; 20:793–803Crossref, Medline, Google Scholar
28 Smeltzer DJ, Nasrallah HA, Miller SC: Psychotic disorders, in Neuropsychiatry of Traumatic Brain Injury, edited by Silver JM, Yudofsky SC, Hales RE. Washington, DC, American Psychiatric Press, 1994, pp 251–283Google Scholar
29 Wilcox JA, Nasrallah HA: Childhood head trauma and psychosis. Psychiatry Res 1987; 21:303–306Crossref, Medline, Google Scholar
30 Harwood DG, Barker WW, Ownby RL, et al: Apolipoprotein-E (APO-E) genotype and symptoms of psychosis in Alzheimer's disease. Am J Geriatr Psychiatry 1999; 7:119–123Medline, Google Scholar
31 Harrington CR, Roth M: Susceptibility genetics in the etiopathogenesis of Alzheimer's disease: role for potential confounding factors. Int Psychogeriatr 1997; 9:229–244Crossref, Medline, Google Scholar
32 Carlsson GS, Svardsudd K, Welin L: Long-term effects of head injuries sustained during life in three male populations. J Neurosurg 1987; 67:197–205Crossref, Medline, Google Scholar
33 Waddington JL, Lane A, Scully PJ, et al: Neurodevelopmental and neuroprogressive processes in schizophrenia. Psychiatr Clin North Am 1998; 21:123–149Crossref, Medline, Google Scholar
34 Nasrallah HA: The neuropsychiatry of schizophrenia, in Textbook of Neuropsychiatry, 2nd edition, edited by Yudofsky SC, Hales RE. Washington, DC, American Psychiatric Press, 1992, pp 621–638Google Scholar
35 Woods BT: Is schizophrenia a progressive neurodevelopmental disorder? Toward a unitary pathogenetic mechanism. Am J Psychiatr 1998; 155:1661–1670Google Scholar
36 Shapiro LB: Schizophrenic-like psychosis following head injuries. Illinois Medical Journal 1939; 76:250–254Google Scholar
37 Gureje O, Bamidele R, Raji O: Early brain trauma and schizophrenia in Nigerian patients. Am J Psychiatry 1994; 151:368–371Crossref, Medline, Google Scholar
38 Satz P: Brain reserve capacity on symptom onset after brain injury: a formulation and review of evidence for threshold theory. Neuropsychology 1993; 3:273–295Crossref, Google Scholar



