Subtle Prefrontal Neuropathology in a Pilot Magnetic Resonance Spectroscopy Study in Patients With Borderline Personality Disorder
Abstract
The authors examined the brains of patients with borderline personality disorder (BPD) by using short echo time single voxel spectroscopy and found a significant 19% reduction of absolute N-acetylaspartate concentrations in the dorsolateral prefrontal cortex in BPD (P=0.01) compared with control subjects.
Borderline personality disorder (BPD) is a persistent and severe mental disorder—characterized by patterns of instability of interpersonal relationships, self-image, and affect and by marked impulsivity—that begins in early adulthood and is present throughout adult life.
The prefrontal cortex and the amygdala are known to play an important role in emotional information processing and impulsivity, both of which are disturbed in BPD. Recent studies produced further evidence of an association between subtle brain pathology in these areas, impulsivity, and emotional instability.1,2 These observations have led to the hypothesis that dysfunction in these brain regions may underlie some of the psychopathological symptoms seen in BPD, in particular impulsive aggression.3
Functional brain imaging studies in BPD employing [18F]fluorodeoxyglucose positron emission tomography (FDG PET) showed evidence of frontal hypometabolism that was pronounced in prefrontal cortical areas.4 Although one structural neuroimaging study using CT scans did not find any evidence of brain pathology,5 the only magnetic resonance imaging (MRI) study in BPD found a slight and marginally significant reduction in frontal lobe volumes.6 In the present study we used hydrogen-1 magnetic resonance spectroscopy ([1H]MRS), one of the most sensitive techniques for the assessment of subtle neuronal dysfunction, to investigate a possible neurochemical correlate of the disturbed frontal metabolism reported earlier. To our knowledge, this is the first MRS study in patients with BPD. This technique permits the simultaneous measurement of N-acetylaspartate (NAA), compounds containing choline (Cho), creatine plus phosphocreatine (Cre), and lactate (Lac) signal intensities. NAA is of particular interest because it has been shown that NAA depletion reflects a state of neuronal damage often preceding cell death.7 In vitro experiments with rats showed that intermittent application of a mitochondrial toxin to striatal neurons resulted in disturbed cell metabolism along with reduced NAA concentrations as measured with MRS, both of which were partially reversible after 4 weeks of withdrawal of the toxin.8 This together with similar findings in other studies indicates that cerebral NAA concentration should be regarded as an indicator of cerebral neuronal integrity.7,8 Although conventional long echo time MRS allows a semiquantitative estimation of metabolite concentrations by calculating ratios of the different substances, short echo time MRS permits absolute quantification of these substances.9 In this study we used short and long echo time MRS to investigate possible frontal and striatal brain pathology as a possible cause of the glucose hypometabolism described in the literature.
METHODS
Approval of this study had been obtained from the local ethics committee. Unmedicated female patients were identified at the Borderline Personality Disorder Center of the Department of Psychiatry and Psychotherapy, University of Freiburg. All patients had been referred to participate in a specialized inpatient treatment program for BPD following the treatment principles of dialectic behavioral therapy.10
All patients were assessed by using standardized criteria for borderline personality disorder. The Structured Clinical Interview for DSM-IV Personality Disorders (SCID II) and the revised version of the Diagnostic Interview for Borderline Personality Disorder (DIB-R) were used by trained raters to establish ICD-IV and DIB-R criteria for BPD. Only patients fulfilling both criteria and achieving a minimum score of 8 out of 10 points in the DIB-R were included.11 The structured clinical interview (SCID I) was used to establish Axis I disorders. Patients with a lifetime diagnosis of schizophrenia, bipolar disorder, or learning disorders; alcohol or drug abuse within the past 6 months; current severe anorexia; or major depression were excluded. Only patients who had successfully finished regular schooling in the German system were included.
Twelve unmedicated patients and 14 age- and sex-matched healthy volunteers were included in this study and scanned after giving informed consent. Most patients fulfilled all but one or two DSM-IV criteria for BPD. Three patients obtained 8, two patients 9, and the remaining seven patients 10 out of a 10 possible points on the DIB-R. Because of this low dimensional variability within our BPD group, subdivision of BPD patients proved to result in groups that were too small for statistical comparison.
The imaging technique we used is identical to that in other previously published studies.12 All experiments were performed on a 2-tesla whole body system (Medspec S200, Bruker, Ettlingen) using a standard quadrature head coil. T1- and T2-weighted coronal images were acquired to localize the voxels and to screen for brain pathology. Spectra were acquired in the left dorsolateral prefrontal cortex and the left striatum. We used PRESS (point-resolved spectroscopy) at echo times of 30 ms and 70 ms, a repetition time of 3 s, and a voxel size of 2 cm.3 128 scans were averaged. The previously published linear combination algorithm was used in combination with the internal water signal for estimation of the absolute concentration of the in vivo metabolites.9 Metabolite concentrations were corrected for the different cerebrospinal fluid content of the different voxels to adjust for local brain atrophy, using previously published methods.13 Results were compared between patient and control groups by use of two-tailed independent-sample t-tests. All statistical analyses were performed by using SPSS for Windows (Release 9.0.1).
RESULTS
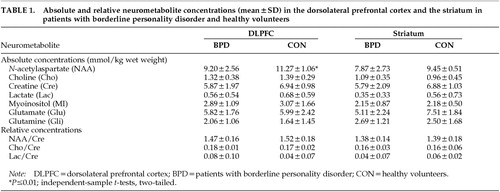
The patient and control groups were matched for age (mean±SD: BPD, 31.6±7.1 years; control subjects, 30.1±3.8 years) and sex (all female). All patients fulfilled DSM-IV and DIB-R criteria for BPD and were unmedicated at the time of the study. There was no evidence of any brain pathology on visual assessment of the T1- and T2-weighted images. Table 1 summarizes our MRS findings. Absolute quantitative short echo time MRS revealed a significant 19% reduction of dorsolateral prefrontal NAA concentration in patients with BPD (t=2.554, df=20, P=0.01). There were nonsignificant reductions of 15% in frontal and 16% in striatal creatine concentration (frontal, t=1.672, df=20, P=0.1; striatal, t=0.981, df=12, P=0.3). Frontal as well as striatal NAA/Cre and Cho/Cre ratios measured with long echo time MRS did not show any difference between the patient and control groups.
DISCUSSION
This is the first study employing MRS in patients with BPD. In summary, we found a significant reduction of the absolute NAA concentrations in the dorsolateral prefrontal cortex of patients with BPD. However, there were no differences in NAA/Cre or Cho/Cre ratios. This can be explained by our observation of a nonsignificant reduction in frontal and striatal creatine concentrations. This finding illustrates the limited usefulness of calculating the relative concentrations NAA/Cre or Cho/Cre in [1H]MRS because creatine is not a constant term but may vary in magnitude. Reductions of NAA and Cre concentrations at the same time may result in false negative findings. This observation is relevant because it demonstrates a potential risk of false negative findings if nonquantitative MRS is used alone.
Our main finding of reduced frontal NAA concentrations is in line with earlier reports of glucose hypometabolism in this area.14 With the finding of reduced cortical NAA signal in neurodegenerative disease, this neurometabolite traditionally had been regarded as a marker of local neuronal density.15 However, there is an increasing body of evidence indicating that NAA depletion might reflect a possibly reversible state of disturbed neuronal metabolism caused by various mechanisms like genetic defects7 or application of toxic substances.8 Alternatively, reduced NAA concentrations might reflect a reduced neuronal density or otherwise disturbed neuronal microstructure secondary to a neurodevelopmental pathology in brain subregions. This explanation is worth considering because evidence suggests that the symptoms of BPD are present at a very young age and do not progressively worsen over time. Further research will be needed to clarify the specific pathophysiology of cerebral NAA metabolism. At the moment, there is general agreement that NAA depletion reflects neuronal dysfunction.
Our finding of prefrontal NAA depletion relates well to reports of subtle frontal volume loss6 and frontal hypometabolism4 in BPD. Because the acquisition of high-quality MRS spectra is time-demanding, we were able to study only one frontal subregion. Thus we cannot comment on possible reductions of NAA in the orbitofrontal or cingulate cortex. The prefrontal cortex and limbic areas, in particular the amygdala, are known to play a crucial role in the regulation of affect and behavior.16 A dual brain pathology affecting prefrontal and limbic brain areas at the same time has been suggested as a correlate of emotional instability, impulsivity, and affective aggression,3 all of which are present in BPD. Similar findings have been described in attention-deficit/hyperactivity disorder,12,17 where these symptoms are also prevalent. Furthermore, prefrontal hypoperfusion18 as well as structural abnormalities2,19 have been reported in other patient groups displaying emotionally unstable, impulsive, and aggressive behavior. Thus decreased prefrontal NAA might only reflect the syndromal spectrum of impulsivity, emotional instability, and aggressive behavior, which is common to these disorders, rather than present a specific brain pathology for BPD.
Further research in larger patient samples is needed to establish the sensitivity and specificity of this finding. Researchers conducting longitudinal studies should try to investigate the dynamics of different cerebral metabolites in the course of BPD and in response to therapy, thus clarifying if this finding presents a vulnerability factor for BPD or alternatively is an epiphenomenon of the disorder.
 |
1 Tebartz van Elst L, Woermann F, Lemieux L, et al: Affective aggression in patients with temporal lobe epilepsy: a quantitative magnetic resonance study of the amygdala. Brain 2000; 123:234-243Crossref, Medline, Google Scholar
2 Woermann F, Tebartz van Elst L, Koepp MJ, et al: Reduction of frontal neocortical grey matter associated with affective aggression in patients with temporal lobe epilepsy: an objective voxel-by-voxel analysis of automatically segmented MRI. J Neurol Neurosurg Psychiatry 2000; 68:162-169Crossref, Medline, Google Scholar
3 Tebartz van Elst L, Ebert D, Trimble MR: Dual brain pathology in patients with affective aggression. Arch Gen Psychiatry (in press)Google Scholar
4 Soloff PH: A fenfluramine-activated FDG-PET study of borderline personality disorder. Biol Psychiatry 2000; 47:540-547Crossref, Medline, Google Scholar
5 Lucas PB, Gardner DL, Cowdry RW, et al: Cerebral structure in borderline personality disorder. Psychiatry Res 1989; 27:111-115Crossref, Medline, Google Scholar
6 Lyoo IK: A brain MRI study in subjects with borderline personality disorder. J Affect Disord 1998; 50:235-243Crossref, Medline, Google Scholar
7 Jenkins BG: Nonlinear decrease over time in N-acetyl aspartate levels in the absence of neuronal loss and increases in glutamine and glucose in transgenic Huntington's disease mice. J Neurochem 2000; 74:2108-2119Crossref, Medline, Google Scholar
8 Dautry C: Early N-acetylaspartate depletion is a marker of neuronal dysfunction in rats and primates chronically treated with the mitochondrial toxin 3-nitropropionic acid. J Cereb Blood Flow Metab 2000; 20:789-799Crossref, Medline, Google Scholar
9 Provencher SW: Estimation of metabolite concentration from localised in vivo proton NMR spectra. Magn Reson Imaging 1993; 30:672-679Google Scholar
10 Linehan MM, Armstrong HE, Suarez A, et al: Cognitive-behavioral treatment of chronically parasuicidal borderline patients. Arch Gen Psychiatry 1991; 48:1060-1064Crossref, Medline, Google Scholar
11 Zanarini MC, Gunderson JG, Frankenburg FR, et al: The revised diagnostic interview for borderlines: discriminating BPD from other axis II disorders. J Pers Disord 1989; 3:10-18Crossref, Google Scholar
12 Hesslinger B, Thiel T, Tebartz van Elst L, et al: Attention-deficit disorder in adults with and without hyperactivity: where is the difference? A study using short echo 1H-magnetic-resonance spectroscopy. Neurosci Lett 2001; 304:117-119Crossref, Medline, Google Scholar
13 Ernst T: Absolute quantitation of water and metabolites in the human brain, II: metabolite concentrations. J Magn Reson 1993; 192:9-19Google Scholar
14 De la Fuente JM: Brain glucose metabolism in borderline personality disorder. J Psychiatr Res 1997; 31:531-541Crossref, Medline, Google Scholar
15 Ebert D, Speck O, Konig A, et al: 1H-magnetic resonance spectroscopy in obsessive-compulsive disorder: evidence for neuronal loss in the cingulate gyrus and the right striatum. Psychiatr Res 1997; 74:173-176Crossref, Medline, Google Scholar
16 Davidson RJ, Putnam KM, Larson CL: Dysfunction in the neural circuitry of emotion regulation: a possible prelude to violence. Science 2000; 289:591-594Crossref, Medline, Google Scholar
17 Castellanos FX, Giedd JN, Berquin PC, et al: Quantitative brain magnetic resonance imaging in girls with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry 2001; 58:289-295Crossref, Medline, Google Scholar
18 Pietrini P, Guazzelli M, Basso G, et al: Neural correlates of imaginal aggressive behavior assessed by positron emission tomography in healthy subjects. Am J Psychiatry 2000; 157:1772-1781Crossref, Medline, Google Scholar
19 Raine A, Lencz T, Bihrle S, et al: Reduced prefrontal gray matter volume and reduced autonomic activity in antisocial personality disorder. Arch Gen Psychiatry 2000; 57:119-127Crossref, Medline, Google Scholar



