H215O PET Study of Impairment of Nonverbal Recognition With Normal Aging
Abstract
Little research has been conducted regarding age-related changes in nonverbal memory. Using positron emission tomography (PET), the authors studied 17 elderly volunteers and 20 young volunteers, during nonverbal recognition task performance, to examine differences in brain blood flow. The subjects were asked to recognize a study list size (SLS) of shapes that was adjusted so that each subject performed at approximately 75% accuracy. Positron emission tomography results showed that, relative to younger individuals, elderly subjects engaged different regions, including the insula, during recognition. Elderly subjects did not show the relationship between parahippocampal flow and SLS, which was observed in younger subjects. These differences suggest that age-related functional brain changes partly explain performance deficits.
Although there is large interindividual variation, decline of certain cognitive functions is expected to occur with normal aging. These include memory, abstract reasoning, and visuospatial skills.1–3 The elderly, in particular, have been shown to have impaired recognition4 and recall.5
Prior positron emission tomography (PET) studies have shown that memory performance in young subjects, compared with elderly subjects, was associated with differential engagement of various regions, including frontal and temporal cortices, 6–9occipitotemporal regions,6,7,10 and frontal regions,6,7,11 depending on the tasks used.
Nonverbal memory change with normal aging has not been as extensively studied as verbal memory, and most studies have not corrected for task difficulty differences across young and elderly subjects. The current study used a continuous, nonverbal, recognition task that attempted to match all subjects for level of difficulty. We hypothesized that, relative to younger subjects, elderly subjects would show more brain blood flow changes during the continuous recognition task performance, especially in frontal lobe regions. These changes would reflect attempts by the elderly subjects to maintain performance in the face of changes related to normal aging.
METHOD
Subjects
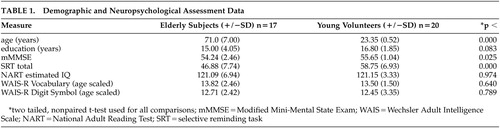
Potential subjects were screened to exclude those with neurological and psychiatric disorders and severe medical illnesses. All subjects were right-handed. Seventeen elderly volunteers (mean age 71.00 [±7] years, 8 men) and 20 young (mean age 23.35 [±2.35], 7 men) volunteers met criteria for entry into the study (Table 1). Neuropsychological evaluation was conducted with statistical comparison of test results to ensure that younger subjects and elderly subjects did not differ significantly on intelligence quotient (IQ). Groups were also matched on level of education. Neuropsychological tests used are shown in Table 1. The Institutional Review Board of Columbia reviewed and approved this protocol as meeting its ethical standards, and informed consent was obtained from subjects after procedures were fully explained.
Behavioral Tasks
Subjects were familiarized with the testing apparatus and trained in the tasks prior to scanning. A sample stimulus is shown in Figure 1. There were three conditions. The first condition (Low Demand) included study list size (SLS) (i.e., 1 study shape followed by 1 recognition probe which was either the target, a previously seen shape, or a foil). The second control (Nonmemory) was identical to Low Demand, except the same shape was presented at study each time. The final condition, Titrated demand, used an SLS that was predetermined in a training session. There were two 15-minute titration training sessions outside of the scanner, during which SLS was adjusted in a staircase manner such that recognition accuracy of 75% for each individual subject was attained (Figure 2). This SLS was then used in the Titrated demand condition. Stimuli were presented at a rate of 5 sec each for elderly subjects and 4 sec each for young subjects on a personal computer (PC). All subjects had 6 sec in which to respond when they were in the test phase of the study, after which the stimuli automatically disappeared from the screen. Stimuli automatically advanced if the subject responded prior to the time limit.
PET Scan Acquisition
Subject movement was controlled in the scanner by means of an individually molded thermoplastic mask. Each activation task was initiated 50 sec prior to the start of the scan and continued throughout the scan period. Subjects viewed the shape stimuli on an overhead monochrome monitor. Scans were separated by 10 minutes and were obtained in the following order: Nonmemory control, Low Demand, Titrate. Three other scans using a verbal task were also collected, but are not discussed here.
For each scan, a bolus of 30 mCi H215O was injected intravenously. Scan acquisition was triggered by the detection of a threshold level of true counts from the camera. Employing a Siemens HR + PET camera, 2–30 sec scan frames were acquired in 2-Da mode and averaged. After measured attenuation correction (15-minute transmission scan) and reconstruction by filtered back-projection, image resolution was 4.6 mm full-width half-maximum (FWHM).
PET Data Processing and Analyses
The SPM99 program (Wellcome Department of Neurology) was used to implement the following steps: 1) a mean image was generated; 2) all images were then realigned to the mean image; 3) the mean image was used to determine a spatial transformation to the PET Montreal Neurological Institute (MNI) space template with SPM99; 4) this spatial transformation was then applied to the individual images; 5) normalized images were smoothed with an isotropic, Gaussian kernel (FWHM = 12 mm); 6) images were proportionally scaled by global image mean; 7) group data were modeled with a separate GLM (see below for model details) for each pair of conditions; 8) voxel-wise t-statistics corresponding to contrasts of interest were computed; and 9) MNI coordinates of local maxima of thresholded (α corrected=0.05) SPM {t} maps were converted to standard Talairach coordinates (using the MNI conversion program developed by Brett at http://www.mrc-cbu.cam.ac.uk/Imaging/mnispace.html). An automated procedure was used to assign an anatomic label to these coordinates by searching for the label associated with the nearest gray matter coordinate.16
In addition to subject effects, the design matrix represented condition (i.e., behavioral task), condition X group (i.e., the difference between groups in the effect of condition), condition X SLS (i.e., the dependence of the condition effect on SLS), and condition X group X SLS interactions (i.e., the difference between groups in the dependence of the condition effect on SLS). Specific comparisons studied were: 1) Mean during Titrate versus Low Demand—this comparison was intended to isolate the areas associated with the increased memory load in the Titrated condition. 2) Mean during Titrate versus Nonmemory control—this was done to separate out the memory component from differences related to simple task performance (viewing shapes, button pressing). 3) Mean during Low Demand versus Nonmemory control—this was done to examine the effects of low memory demand (SLS=1) versus the Nonmemory control. A direct comparison of elderly and young subjects was done for each of these contrasts. Condition X group X SLS interactions were examined for the Titrate versus Low Demand and for the Titrate versus simple comparisons.
RESULTS
Behavioral Data
Demographics and neuropsychological test results are summarized in Table 1. Elderly subjects’ scores were slightly below the young subjects’ on the modified Mini-Mental Status Exam, but their scores were well within normal range. As would be expected, total recall on the Selective Reminding Test was significantly lower in the elderly subjects.
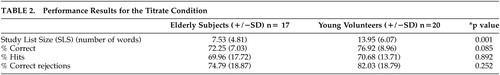
Young subjects attained a significantly longer SLS than elderly in the Titrate condition (13.95 compared with 7.53, respectively, p<0.001). However, as was intended, percent correct in the Titrate condition did not differ significantly between the two groups (76.92 compared with 72.25, respectively, p=0.085; Table 2).
PET Data Overview
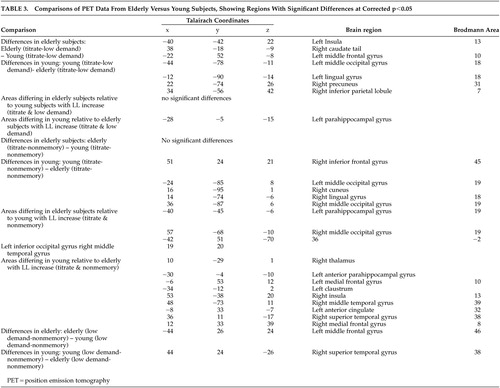
Results are summarized in Table 3, and shown in Figure 3 and Figure 4. Data from single group contrasts were used in an exploratory fashion to help with interpretation of group differences, but are not reported in detail here.
Titrate Versus Low Demand.
When compared with the younger group, elderly subjects showed greater mean differences between conditions in the left insula, right caudate tail, and left middle frontal gyrus (area 10). Young subjects showed greater mean differences than elderly subjects did in posterior regions (left middle occipital and lingual gyri and right precuneus and inferior parietal lobe). It should be noted that these comparisons do not control for the interaction between blood flow and SLS.
In this comparison, there was also a significant condition X group X SLS interaction only in the left parahippocampal gyrus. The interaction was such that the slope of the relationship between PET activation (i.e., Titrate-Low Demand) and SLS was greater in young subjects than in elderly subjects.
Titrated Demand Versus Nonmemory Control.
There were no areas on this comparison where elderly subjects showed a greater Titrate-Nonmemory difference than young subjects, though the young subjects demonstrated a larger mean Titrate-Nonmemory difference in frontal (right inferior frontal gyrus, area 45) and occipital (bilateral middle occipital gyri, right cuneus and lingual gyrus) regions.
There were several regions demonstrating condition X group X SLS interactions. Such regions where the slopes for elderly subjects were greater than those for young subjects included left posterior parahippocampal and inferior occipital gyri, and right middle occipital and middle temporal gyri. Areas associated with greater slopes for young subjects included the bilateral medial frontal gyri (area 10 on the left and area 8 on the right), left anterior parahippocampal gyrus and anterior cingulate, and the right thalamus, insula, middle temporal gyrus (area 39), superior temporal gyrus (area 38), and medial frontal gyrus (area 8).
Low Demand Versus Nonmemory.
Low Demand-Nonmemory differences were greater for the elderly in the left middle frontal gyrus (area 46). Young subjects showed greater differences in the superior temporal gyrus (area 38).
DISCUSSION
Young subjects attained a significantly longer study list size (SLS) than elderly subjects during nonverbal recognition. Between and within group differences in task difficulty were partially controlled for by titration. Even given this control for difficulty, residual differences in mean activation between the old and young subjects were seen for the Titrated demand task, compared with either one of the two controls. PET results showed, relative to young, elderly subjects engaged different regions, including the insula, during recognition, while young subjects engaged posterior brain regions. Regions where the two groups differed in the slope of Titrate minus control condition activation on SLS were also observed, with elderly subjects not showing the relationship seen in young between parahippocampal flow and SLS.
Our study differs from much of the work on aging and memory in that the Titrated demand memory condition attempted to match subjects on task difficulty. One critique of studies comparing different populations is that no attempt is made to match for differences between the groups, so that differences may be ascribed to amount of effort involved in task performance. In the current study, all subjects performed at approximately the same level of recognition accuracy. However, differences were still seen, with elderly subjects demonstrating greater differences in the bilateral insula and left middle frontal gyrus, among other regions. Young subjects showed greater mean activation in several regions, including bilateral posterior cortex. The overall recruitment of additional regions, relative to young subjects, by the elderly subjects during Titrate task performance was consistent with our hypothesis that age-related impairment would cause the elderly subjects to use alternate strategies in an attempt to maintain task performance. The decline in hippocampal changes, seen in elderly subjects relative to young, may reflect age-related impairment in this brain region.
Differences in posterior blood flow seen in young subjects on direct comparison with elderly subjects on the Titrate task relative to the contitions of the two controls may reflect use of a different strategy by young subjects (Figure 3). Occipital and temporal regions are part of a visual pathway involved in object perception17 and encoding.18 Prior work has found age-related decline in posterior blood flow during visual word identification.10 Another PET study found that young subjects showed greater differences than elderly subjects in occipito-temporal and parietal areas during performance of verbal memory tasks, despite a lack of age-related effect on performance.7 Thus, one of two differing strategies may be used, depending on age. Elderly subjects may no longer have access to posterior pathways, used by the young, due to age-associated neuronal changes. This may result in their utilization of alternate strategies, discussed below. Another explanation is that our differences in stimulus presentation times between the two groups, necessary to control for task difficulty, contributed to this effect in young subjects. Since young subjects spent more time viewing study stimuli at their faster presentation rate, they viewed more total stimuli during the Titrate condition.
Condition X group X SLS interactions for Titrate, compared with both control conditions were greater for the young than the elderly subjects in the left anterior parahippocampus. Primate work has shown that parahippocampal regions are engaged during recognition memory performance.19,20 Human studies have shown the parahippocampal regions are crucial for encoding.21 Early PET studies comparing young and elderly volunteers found reduced flow in the parahippocampal gyri at rest in elderly subjects, relative to young subjects, suggesting that function may decline with normal aging.22In a functional magnetic resonance imaging (fMRI) study of young subjects, Witter and others23 found, that anterior parahippocampal gyri differences occurred with object recognition. They suggested that the parahippocampal gyri coordinates bidirectional, topographically arranged transfer of information between higher cortical association areas and the hippocampus.20,24 This suggests that the differences in modulation of frontal cortex seen in elderly subjects versus young volunteers in this study may be due, in part, to age-related parahippocampal changes.
Insular differences were seen in elderly subjects relative to young subjects in the Titrate versus Low Demand comparison (Figure 4). The insula is implicated in language and semantic memory retrieval (see Cabeza et al.). Young subjects’ suppression of the insula during difficult task performance may have contributed to their ability to attain significantly longer list lengths, since suppression of pathways through the insula may lead to improved episodic memory performance.7 The differences in insular blood flow with increasing SLS on the correlational analyses of the Titrate versus Nonmemory control indicate that elderly subjects with higher SLS used the insula less. These findings are supported by the fact that elderly subjects showed insular differences on the Titrate versus Low Demand comparison and also demonstrated correlations with SLS in insula for the Titrate condition, compared to the Nonmemory condition. Thus, the elderly subjects showed insular differences with the more difficult task and modulated the insula differently than young subjects. An alternative interpretation is that the insular differences were related to covert naming by some subjects. The left insula has been implicated in object naming in other imaging work.26,27 It may be that those subjects with poorer performance used a naming strategy, involving the insula.
On direct comparison, elderly subjects, relative to young, demonstrated greater mean differences in the left middle frontal gyrus on comparisons between Titrate and Low Demand and the two control conditions (areas 10 and 46, respectively, Figure 4). Young subjects showed right-sided frontal differences, relative to elderly subjects, only on Titrate-Nonmemory (area 45) but not on the comparison of the two controls. This may be due to the fact that the Low Demand control poses more difficulty to elderly subjects than to young subjects, causing them to recruit areas used, in young, only for the more difficult tasks. Numerous other PET studies have reported differential difficulty-related frontal blood flow increases by young subjects, relative to elderly subjects. Jonides and others8 found left lateral prefrontal cortex differences, when an n-back memory task was made more difficult. The prefrontal cortex change with increased task challenge was not seen in elderly subjects, who also showed more interference during task performance. Similarly, Grady and others28 in a study of face recognition found changes in prefrontal cortex blood flow (areas 9 and 46), as difficulty of face identification was increased by image degradation. Prior work by the same group29 found that elderly subjects engaged regions associated with more difficult task performance in young subjects (such as prefrontal cortex) during performance of less difficult tasks. One alternative explanation that should be considered is that fatigue and motor learning effects on blood flow may have played a role since our tasks were not counterbalanced across conditions.30
Area 46 and the entire mid dorsolateral prefrontal cortex have been implicated in spatial memory, since lesions in this area cause selective spatial mnemonic impairment on spatial delayed response and alteration tasks.31,32 Recent primate studies by Petrides33 demonstrated a specific role for mid dorsolateral prefrontal cortex in monitoring information during tasks that require executive function. A PET study with young and elderly volunteers using delayed visual discrimination also found differences between young and old in brain regions engaged during task performance.34 When networks connected to hippocampal differences were examined, they found that elderly subjects recruited a network including BA 9 and 46, and also middle cingulate and caudate, while young subjects engaged BA 10, fusiform, and posterior cingulate. Since groups were matched on performance, difficulty effects could not account for this finding. The authors suggest that large-scale network operations may change with normal aging, especially as hippocampal function declines. These frontal cortex findings in the current study may be indicative of the lack of hippocampal differences seen in elderly subjects, compared with young subjects, as task performance (SLS attained) increased.
In summary, this study demonstrates differences between elderly subjects and young subjects during performance of a nonverbal memory task. Relative to younger subjects, elderly subjects demonstrated blood flow changes in different regions, including the left insula, during performance of the titrated memory condition and failed to recruit prefrontal cortex. They also did not show the preponderance of occipital differences seen in young subjects on difficult tasks and failed to modulate parahippocampal blood flow, relative to the youngs, with increasing SLS. These differences may help delineate the locus of decline in processing abilities as an effect of normal aging.
ACKNOWLEDGMENTS
This study was conducted at the Cognitive Neuroscience Division of the Sergievsky Center, Columbia University, New York, NY.
This study was supported by National Institute of Health federal grants AG14671 and RR00645.
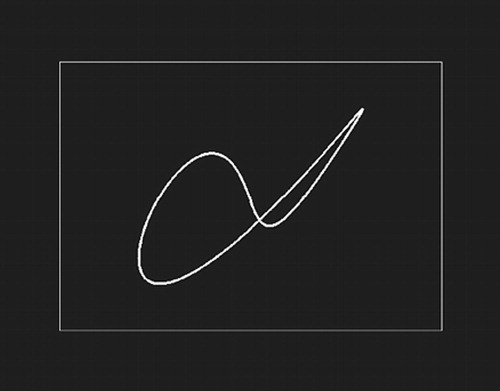
FIGURE 1. Example of Shape Stimuli Used in All Three Conditions. Box Surrounding Stimuli Indicates That Subject Is to Answer “Yes” or “No” As to Whether This Stimulus Has Been Seen Before.
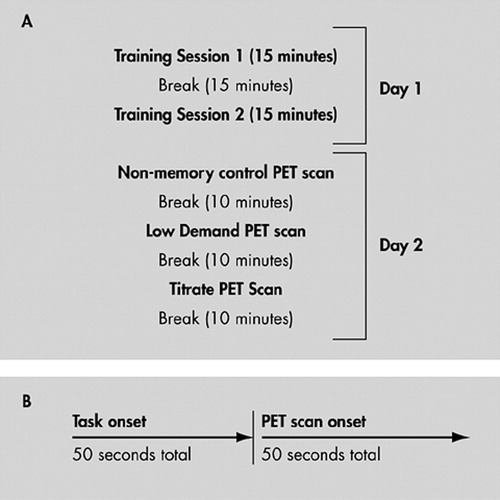
FIGURE 2. Overall Study Design (A) and Timeline for PET Scan
[(B) Timeline was the same for all three PET scans, regardless of task performed during scan.]
PET=positron emission tomography
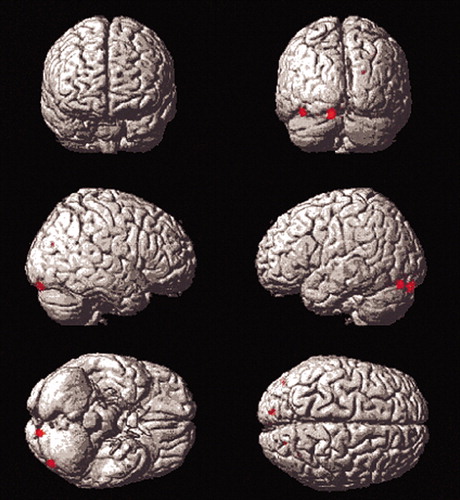
FIGURE 3. Results for the Titrate Versus Low Demand Comparison of Young Subjects to Older Subjects
Young subjects showed greater mean differences than older subjects in several posterior regions (left middle occipital and lingual gyri and right precuneus and inferior parietal lobe). It should be noted that these comparisons do not control for the interaction between blood flow and study list size.
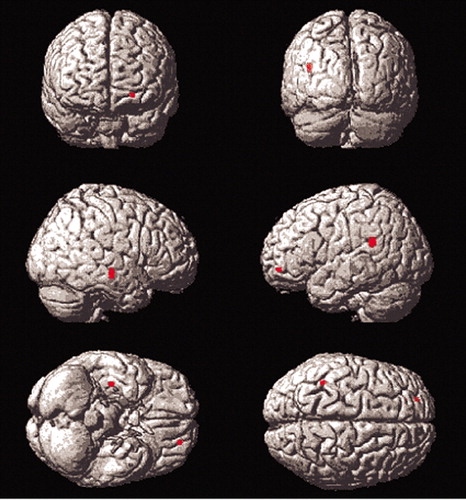
FIGURE 4. Results for the Titrate-Low Demand Comparison of Older Subjects to Young Subjects
Older subjects showed greater mean differences between conditions than younger subjects in the left insula, right caudate tail, and left middle frontal gyrus (area 10). These comparisons do not control for the interaction between blood flow and study list size.
 |
 |
 |
1 Erber JT, Galt D Jr, Botwinick J: Age differences in the effects of contextual framework and word-familiarity on episodic memory. Exp Aging Res 1985; 11:101–103Crossref, Medline, Google Scholar
2 Craik FIM, Jennings JM: Human memory, in The Handbook of Aging and Cognition, Edited by Craik FIM, Salthouse TA. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc., 1992, ix, pp 51–110Google Scholar
3 Verhaeghen P, Marcoen A, Goossens L: Facts and fiction about memory aging: A quantitative integration of research findings. J Gerontol 1993; 48:157–171Crossref, Google Scholar
4 McCormack PD: Aging and recognition memory: methodological and interpretive problems. Exper Aging Res 1984; 10:215–219Crossref, Medline, Google Scholar
5 Rockey, LS: Memory assessment of the older adult, in Handbook of Neuropsychology and Aging. Critical issues in neuropsychology. Edited by Nussbaum, PD. New York: Plenum Press 1997, 559Google Scholar
6 Grady CL, McIntosh AR, Horwitz B, et al: Age-related reductions in human recognition memory due to impaired encoding. Science 1995; 269:218–221Crossref, Medline, Google Scholar
7 Cabeza R, Grady CL, Nyberg L, et al: Age-related differences in neural activity during memory encoding and retrieval: a positron emission tomography study. J Neurosci 1997; 17:391–400Crossref, Medline, Google Scholar
8 Jonides J, Marshuetz C, Smith EE, et al: Age differences in behavior and PET activation reveal differences in interference resolution in verbal working memory. J Cogn Neuroscience 2000; 12:188–196Crossref, Medline, Google Scholar
9 Esposito G, Kirkby BS, Van Horn JD, et al: Context-dependent, neural system-specific neurophysiological concomitants of ageing: mapping PET correlates during cognitive activation. Brain 1999; 122:963–979Crossref, Medline, Google Scholar
10 Madden DJ, Turkington TG, Coleman RE, et al: Adult age differences in regional cerebral blood flow during visual world identification: evidence from H215O PET. Neuroimage 1996; 3:127–142Crossref, Medline, Google Scholar
11 Reuter-Lorenz PA, Jonides J, Smith EE, et al: Age differences in the frontal lateralization of verbal and spatial working memory revealed by PET. J Cogn Neuroscience 2000; 12:174–187Crossref, Medline, Google Scholar
12 Nelson HE. The National Adult Reading Test (NART): Test Manual. Windsor, Berks, UK: NFER-Nelson, 1982Google Scholar
13 Wechsler D. Wechsler Memory Scale-Revised manual. San Antonio, Texas: The Psychological Corporation, 1987Google Scholar
14 Buschke H, Fuld PA: Evaluation of storage, retention, and retrieval in disordered memory and learning. Neurol 1974; 11:1019–1025Crossref, Google Scholar
15 Stern Y, Sano M, Paulson J, Mayeux R: Modified mini-mental state examination: validity and reliability. Neurol 37(suppl):179,1987Google Scholar
16 Lancaster JL, Woldorff MG, Parsons LM, et al: Automated talairach atlas labels for functional brain mapping. Human Brain Mapp 2000; 10:120–131Crossref, Medline, Google Scholar
17 Mishkin M, Ungerleider LG: Contribution of striate inputs to the visuospatial functions of parieto-preoccipital cortex in monkeys. Behav Brain Res 1982; 6:57–77Crossref, Medline, Google Scholar
18 Haxby JV, Ungerleider LG, Horwitz B, et al: Face encoding and recognition in the human brain. Proc Natl Acad Sci U S A 1996; 93:922–927Crossref, Medline, Google Scholar
19 Meunier M, Bachevalier J, Mishkin M, et al: Effects on visual recognition of combined and separate ablations of the entorhinal and perirhinal cortex in rhesus monkeys. J Neurosci 1993; 13:5418–5432Crossref, Medline, Google Scholar
20 Suzuki WA, Zola-Morgan S, Squire LR, et al: Lesions of the perirhinal and parahippocampal cortices in the monkey produce long-lasting memory impairment in the visual and tactual modalities. J Neurosci 1993; 13:2430–2451Crossref, Medline, Google Scholar
21 Gabrieli JDE, Brewer JB, Desmond JE, et al: Separate neural bases of two fundamental memory processes in the human medial temporal lobe. Science 1997; 276:264–266Crossref, Medline, Google Scholar
22 Martin AJ, Friston KJ, Colebatch JG, et al: Decreases in regional cerebral blood flow with normal aging. J Cereb Blood Flow Metab 1991; 11:684–689Crossref, Medline, Google Scholar
23 Witter MP, Naber PA, van Haeften T, et al: Cortico-hippocampal communication by way of parallel parahippocampal-subicular pathways. Hippocampus 2000; 10:398–410Crossref, Medline, Google Scholar
24 Insausti R, Herrero MT, Witter MP: Entorhinal cortex of the rat: cytoarchitectonic subdivisions and the origin and distribution of cortical efferents. Hippocampus, 1997 1997; 7:146–183Google Scholar
25 Cabeza R, Nyberg L: Imaging cognition: An empirical review of PET studies with normal subjects. J Cogn Neuroscience 2000; 9:1–26Crossref, Google Scholar
26 van Turennout M. Bielamowicz L. Martin A. Modulation of neural activity during object naming: effects of time and practice. Cerebral Cortex. 13(4):381–391Google Scholar
27 Price CJ, Moore CJ, Humphreys GW, et al:The neural regions sustaining object recognition and naming. Proc R Soc Lond B Biol Sci 1996; 263:1501–157Crossref, Google Scholar
28 Grady CL, Horwitz B, Pietrini P, et al: Effect of task difficulty on cerebral blood flow during perceptual matching of faces. Human Brain Mapp 1996; 4074:227–239Crossref, Google Scholar
29 Grady CL, Maisog JM, Horwitz B, et al: Age-related changes in cortical blood flow activation during visual processing of faces and location. J Neurosci 1994; 14:1450–1462Crossref, Medline, Google Scholar
30 Rajah M, Hussey D, Houle S, et al: Task-independent effect of time on RCBF. Neuroimage 1998; 7:314–325Crossref, Medline, Google Scholar
31 Butters N, Pandya D, Sanders K, et al: Behavioral deficits in monkeys after selective lesions within the middle third of sulcus principalis. J Comparative Physiology and Psychol 1971; 76:8–14Crossref, Medline, Google Scholar
32 Goldman PS, Rosvold HE: Localization of function within the dorsolateral prefrontal cortex of the rhesus monkey. Exp Neurol 1970; 27:291–204Crossref, Medline, Google Scholar
33 Petrides M: The role of the mid-dorsolateral prefrontal cortex in working memory. Exp Brain Res 2000; 133:44–54Crossref, Medline, Google Scholar
34 Della-Maggiore V, Sekuler AB, Grady CL, et al: Corticolimbic interactions associated with performance on a short-term memory task are modified by age. J Neurosci 2000; 20:8410–8416Crossref, Medline, Google Scholar



