Long-Term Outcome of Neurosurgery for the Treatment of Resistant Depression
Abstract
Between 1973 and 1995, a total of 76 patients were treated with bilateral stereotactic, orbitomedial lesions for resistant severe depression at the Neuropsychiatric Institute, Sydney, Australia. On follow up after a mean 14.4 years, 24 (31.6%) subjects were confirmed dead, with six having committed suicide. Of the 52 patients still alive (mean age 62.9 years), 23 were interviewed in detail, and lesions verified in 18 with magnetic resonance imaging (MRI). On a 6-point global outcome rating scale, rated by consensus between two independent psychiatrists, five (22.7%) were judged to be completely recovered and another 11 (50%) showed significant improvement. The improvement was noted within days or weeks of the surgery. Adverse effects were epilepsy (2 subjects), marked personality change (1), weight gain (2), and mild personality change (5). Any reported cognitive impairment was mild. No definite predictors of improvement were identified.
Recent advances in the treatment of major depression have still left a small but significant proportion of patients who remain resistant to all forms of treatment.1 The morbidity and mortality in this group is very high, with as many as one in six of those severely affected with major depression ultimately killing themselves.2 A number of physical treatments are now being developed for the management of such patients. These include investigational strategies such as transcranial magnetic stimulation (TMS), vagus nerve stimulation (VNS), and deep brain stimulation (DBS).3 The advent of such treatments emphasizes the acceptance of the neurobiological model of depressive disorders, which has been further enhanced by an increased understanding of the functional neuroanatomy of affective disorders.4 In this context, it is pertinent to revisit neurosurgery as a treatment modality for resistant depression.
Neurosurgery for psychiatric disorders, or psychosurgery as it also referred to, involves the creation of lesions in the frontolimbic circuits for the treatment of psychiatric illness. Its use as a treatment has steadily declined from its peak in the 1950s, but it continues to be performed in a small number of centers around the world. There is a seeming consensus of international psychiatric opinion that the use of psychosurgery be primarily for the treatment of intractable, disabling and long-standing obsessive-compulsive disorder (OCD) and major depression.5–10 Recent research interest has been in the neurosurgical treatment of OCD,3 and the outcome of neurosurgery for depression has received less attention, despite the fact that resistant depression is a more common clinical imperative and has been treated with neurosurgery more often in the past.
Many different but related neurosurgical procedures have been used to treat depression, with four procedures having received some systematic evaluation: subcaudate tractomy (to interrupt white matter tracts between the prefrontal cortex, in particular the orbitofrontal region, and subcortical structures), anterior cingulotomy, limbic leucotomy (a combination of the previous two) and anterior capsulotomy (to interrupt fronto-thalamic connections in the anterior limb of the internal capsule). While the relative merits of the procedures have not been examined, the reported results from the various procedures appear to be comparable.10 In various studies11,12 between 30% and 68% of subjects showed significant improvement. Most studies however had a short-term follow up of 1–2 years with only a few exceptions.13–15 Whether the improvement with neurosurgery is sustained over the longer-term has not been adequately examined.
We report a follow-up study of all patients who received neurosurgery for the treatment of resistant severe depression in our facility between 1973 and 1995 and were followed up in 1996.
METHOD
Subjects
The study sample comprised consecutive patients with severe depression who were treated with neurosurgery at the Neuropsychiatric Institute, Prince Henry Hospital, Sydney, Australia, between 1973 and 1995. The criteria for inclusion were as follows: 1) Subjects had a definite diagnosis of Major Depression or Bipolar Disorder, currently depressed, with the patient being continuously depressed for over 1 year in the current episode. All subjects met DSM–IV criteria for major depressive episode on retrospective review. 2) The illness was judged to be resistant to medical therapies by the treating team. Specifically, the patient was judged to have had adequate trials of antidepressant drugs from various groups (tricyclics, MAOIs, SSRIs and SNRIs), as well as second line treatments such as lithium and anticonvulsants, and adjuncts such as neuroleptics, thyroid hormone, etc. They had had adequate trials of electroconvulsive therapy (ECT) on at least two separate occasions. 3) The patient had had an adequate trial of cognitive behavior therapy and, if appropriate, other forms of psychotherapy with no benefit. 4) The disorder was considered to be severe and resulting in significant disability. 5) The patient was capable of giving an informed consent to the procedure. 6) After 1977, approval was granted by the statutory review board of the New South Wales Department of Health. 7) The presence of a primary personality disorder was considered a relative contraindication to surgery.
Patients were considered for the second operation if any initial improvement had waned within weeks or months, or a more partial response occurred to the first operation that was judged to have been inadequate.
Surgical Approach
Surgery was performed using a stereotactic procedure. The localization of the lesions was determined by pneumo-encephalography until 1989 when computerised tomography guided stereotaxis was introduced. This was replaced by magnetic resonance imaging (MRI) guidance in 1990. Surgery was performed under local anesthesia after heavily sedating the patient. A bilateral orbitomedial (OM) leucotomy was performed using the coordinates of Kelly et al.16 The first targets for the lesions were 10 mm superior and 15 mm anterior to the anterior clinoid processes, and 10 mm from the midline on either side. Second lesions were placed 8 mm lateral to, and 8 mm above, the first targets. Some patients had a second operation, which involved either an extension of the first lesions or the creation of additional anterior cingulate (AC) lesions. Additional OM lesions were placed laterally in the orbitomedial frontal lobe, 16 mm lateral, or 8 mm lateral to the first points. AC lesions were placed using a target point 5 mm above the ventricular wall over the anterior horns of the lateral ventricles. Two lesions were placed bilaterally: first, 10 mm from the midline; and second, 18 mm from the midline. Until 1989, lesions were made using a cryogenic probe that provides a tear-drop shaped lesion approximately 12 mm long and 8 mm in diameter.16 In 1989, a radiofrequency thermo-coagulation unit was introduced for greater accuracy of lesion.
Presurgical Assessment
Patients were assessed in detail as inpatients using a semistructured interview schedule prepared specifically for this purpose.17 The following rating scales were administered: Hamilton Rating Scale for Depression (HRSD)18 and Anxiety (HRSA),19 the Beck Depression Inventory (BDI),20 and the Strom-Olsen and Carlisle21 Ratings of Full Capacity and Capacity for Pleasure in the previous 6 months. Each patient was also administered some tests of the WAIS22 and Wechsler Memory Scale (WMS).23 In addition, each patient had a neurological examination and EEG, and from 1979 onward, a pre- and postoperative CT head scan.
Follow-Up Assessments
Early postoperative period.
Each patient was assessed 1 week after surgery, at 6 months, and at 1 and 2 years whenever possible using the scales described above. The neuropsychological tests were not repeated at these assessments.
The present study.
An attempt was made to follow up all the patients in 1995–96. Those who could be traced (by means of recorded addresses, enquiries from treating doctors, and electoral lists) were assessed in hospital or at home. Information was obtained from a closely related informant, and the current general practitioner and/or psychiatrist. For those who were deceased, death certificates were obtained to determine the cause of death. Subjects who underwent a detailed assessment were administered the Composite International Diagnostic Interview (CIDI)24 and completed the HRSD,18 the HRSA19 and the Strom-Olsen and Carlisle scale.21 A proportion (N=18) of subjects underwent a brain MRI scan on a GE (Signa)™ 1.5 T scanner.
Global Outcome Measure
Each patient was rated on a 6-point global outcome rating using a modification of the original Pippard Scale that has been previously adapted by our group.17 The ratings were:
1. Fully recovered: no residual depression, and without side-effects that impinged on functioning, but without consideration of other problems unrelated to depression.
2. Much improved: only mild residual symptoms, and/or mild relapse. A) Full occupational functioning and satisfactory relationships; B) Full occupational functioning or satisfactory relationships.
3. Mild improvement: evidence of symptomatic improvement, but to a minimum degree or without marked effect on social and occupational functioning.
4. No change.
5. Worse: this included either symptomatically or functionally worse outcomes, and suicides.
Ratings were made by consensus by two independent psychiatrists who were presented with summaries of all data pertaining to the subject. The following measures were obtained to further document the impact of surgery objectively: number of ECT treatments, number of hospital admissions, and number of days spent in hospital, before and after surgery, coded usually in 5 year periods unless the follow-up period was shorter (N=3) in which case the presurgical period was matched with the follow-up period. A paired t test was used to determine any significant change.
RESULTS
Overall Sample
The overall sample (N=76) comprised 47 (62%) women and 29 (38%) men. The mean age of the subjects at the time of surgery was 51.0 (SD=13.5) years (range=24–82 years). A chart review of the subjects suggested that they all met, in retrospect, DSM–IV criteria for Major Depressive Episode at the time of surgery. Eleven (14.5%) had had at least one episode of mania or hypomania in the past, and therefore met criteria for Bipolar Disorder, although depression was the primary reason for the surgery. In 1 subject who was deceased at the time of follow up, the final diagnosis was Huntington’s disease, suggesting that the diagnosis of a primary affective disorder may have been in error in this patient. The mean duration of illness was 24.2 (SD=7.3) years. The mean duration of the index episode during which surgery was performed was 2.1 (SD=0.8) years. The subjects had been treated with a mean 5.6 antidepressant drugs in the index episode. In the 5 years preceding the surgery, they had been admitted to hospital on a mean 10.4 occasions and had received, on average, more than 50 ECTs.
Twenty-four (31.6%) subjects were confirmed dead. Of the remaining 52, 40 were traced to their current addresses. Ten subjects refused follow up and seven could not be assessed owing to geographical distance. Twenty-three subjects were personally interviewed by the authors and the outcome assessed in detail. The typical lesions on MRI are presented in Figure 1.
Deceased at Time of Follow up (N=24)
Of these subjects (14 men, 10 women), death certificates were available for 20. The mean age of this group at the time of surgery was 56.6 (16.2) years. Six (7 .9%) subjects were reported to have died from suicide (drug overdose,4 and gunshot2). The others died of causes apparently unrelated to the primary illness. One subject died within 2 months of surgery, and it is likely that postoperative respiratory complications contributed to his death. The majority died from cardio-respiratory problems; two had metastatic carcinoma; and one suffered from Huntington’s disease.
Detailed Follow-up Subjects (N=23)
This group comprised 18 (72%) women and five men (18%). The mean (SD) age at time of surgery was 48.5 years (range=32 to 72 years). The mean duration of follow up was 14.4 (5.3) years, and the mean age at the time of follow up was 62.9 (10.1) years. The high proportion of women in this group was partly accounted for by the higher mortality in men.
Outcome
A consensus was reached in 22 cases. In 1 subject, there was evidence of progressive cognitive decline that had begun within a year of surgery, making an informed decision on outcome impossible. Five (22.7%) subjects were judged to have completely recovered (rating I), and another 11 (50%) showed significant improvement (rating II). The outcome data are presented in Figure 2. No subject was judged to have worsened because of the surgery. The 16 “improved” subjects comprised 30% of the total living sample.
The Effect of Second Operation
Seven (30.4%) subjects had two operations, with the extension of previous OM lesions in 4 and additional AC lesions in 3. In all cases, the second surgery was performed because the patient had shown a significant but temporary improvement with the first operation. Four of these had a grade 2 improvement, and three showed minimal or no benefit.
Objective Indices of Improvement
The four objective indices are presented in Table 1. On paired t tests, there was significant improvement with surgery on all four indices.
Predictors of Improvement
In a logistic regression analysis, age, sex, duration of illness, severity of illness, and occurrence of perioperative delirium did not emerge as significant predictors of improvement in this sample.
Side Effects
Two subjects (8.7%) developed epilepsy, with both responding to anticonvulsant treatment. Mild personality change was reported in five (21.7%) cases. This was in the form of impulsivity (two cases), and poor motivation, mild disinhibition, and alcohol abuse (one case each). Personality change was severe in only one patient, who demonstrated overeating, incontinence and aggressive behavior, which gradually improved over 6 months. Two subjects had a significant weight gain after surgery. Eight (35%) subjects had a period of delirium, following the surgery, which usually cleared in a few days.
DISCUSSION
Our study has demonstrated that 16 (72.7%) out of 23 patients had a significant improvement from a severe and intractable depressive illness following neurosurgery, with five (22.7%) showing a full recovery. If all subjects at the time of follow up are included in the analysis, significant benefit was seen in at least 16/52 (30%). We suggest that the real proportion of good outcome in our sample was somewhere between 30% and 73%.
The improvement in depression, whenever it did happen, was apparent within days or weeks of the surgery, although full recovery could take many months. Usually, there was an obvious change in the patient’s mood after the postoperative delirium had cleared. In three patients mood improved after a delay; in one, it occurred over 6 months, and in two over 1 year. The occurrence of mood change soon after the surgery argues that it is an effect of the surgery, rather than an incidental change due to the natural course of the illness. That the improvement was sustained in many patients over the years of follow up makes it unlikely to be a placebo effect.
Since this is an open study, it would be useful to examine the outcome data in light of information about the long-term outcome of depression. Most of the long-term outcome studies were carried out before modern treatments were available.25,26 Many of the modern studies suffer from major deficiencies, but two studies,27,28 are of note for being prospective and rigorous. Both document a chronic course for a majority of severely depressed patients. Kiloh et al.27 followed up 145 patients with a primary depressive illness over 15 years, and found that 7% had suicided, 12% had remained incapacitated by illness, and only 20% had remained continuously well. The findings from the London study28 were not dissimilar. Another study29 has suggested that remission does occur in some patients after an unremitting major depression of more than 5 years duration. The recovery in this series, however, was slow and gradual, and those who recovered were the ones likely to have the shortest illnesses prior to entry into the study. The overall impression from the literature is that severe depression has a chronic course with a poor prognosis in the majority of cases, underscoring the beneficial effects of neurosurgery.
Comparison to Other Published Studies
The salient studies that have used stereotactic surgery to treat depression are listed in Table 2. The largest series of orbitomedial lesions, comparable to our method, is from the London group.6,12,25 Goktepe et al.12 reported grade 1 or 2 improvement in 55% of 96 patients treated until 1973. Hodgkiss et al.13 reported that 34% of patients had good improvement out of 183 patients so treated. The latter study, however, had a follow-up period of only 1 year. The Boston group has reported its experience with anterior cingulotomy for depression.11,14 The more recent study11 was prospective and used stringent assessments, concluding that about 30% of patients did well. The duration of follow up was again about 1 year. The Swedish group, that uses anterior capsulotomy, has largely reported results of surgery for OCD and anxiety disorders in recent years. An earlier report30 suggested that 47% of patients improved. The results of these other studies are therefore comparable to our study. The difference in our study is the long duration of follow up, which suggests that the improvement of neurosurgery is sustained over many years.
Suicide
Six (7.9%) patients in our sample were noted to have died from suicide. This rate does not differ greatly from that reported in the literature by others: 5% suicide deaths in the 16-month follow up of the limbic leucotomy;31 9% in a series of 198 patients treated with cingulotomy and followed up for 8.6 years.15 The lowest rate was reported at 1% by Bridges et al.6 Given the severity and chronicity of the illnesses being treated, these rates are not particularly high. In patients with uncontrolled depressive disorder, suicide rates of 15% have been described.2
The Safety Issue
The surgery was safe and relatively free of complications. One patient, who died within 2 months of surgery, had previously suffered from a chronic respiratory disease, and it was uncertain whether the surgery had played a role in his death. A mild delirium was not uncommon after the surgery, and this was usually not associated with evidence for edema surrounding the lesions. The occurrence of delirium was not necessary for therapeutic efficacy, contrary to what has sometimes been suggested.32 The rate of epilepsy in our sample was slightly higher than what has generally been reported in the literature (0.4% to 2%).33 Significant personality change occurred in one patient who had large lesions. While 5 patients were reported to have a mild personality change, it was difficult to decide whether this was a consequence of the surgery or resulted from the fact that the patients’ depression had improved. Cognitive deficits were not prominent in our patients. Of concern, however, was one patient who developed a progressive cognitive decline within 1 year of surgery. A retrospective review did not suggest an error in diagnosis, but it is possible that an underlying demented process had been missed, because of the depression.
Limitations of the Study
This study had a number of limitations. As mentioned above, it was an open study and therefore did not have the benefit of a prospectively assessed comparison group. In arguing that the beneficial effect seen in some patients is a direct consequence of the surgery, we relied on two observations: the temporal association of the surgery with the change, and the natural course of severe depression previously mentioned. The patients who improved did so within days or weeks of the surgery, with only two patients showing a delayed response. Given the duration and resistant status of the patients’ illnesses, a placebo effect seems very unlikely.
The significant dropout rate of the study is of some concern, but it is felt that valid conclusions can be drawn from the results. With 52 patients currently alive, we estimate a lower positive outcome rate—assuming that all subjects lost to follow up had not improved—of at least 30%, which is similar to the rate reported by Price and colleagues.11 The surgery was performed over a span of two decades, and the practice of psychiatric therapy has changed much in this period. In addition, many newer drugs became available in the 1990s. It is therefore likely that the nature of “resistant” depression changed in this period. However, our study does not show that the outcome of neurosurgery was less favorable in the second half of the series when compared to the first half. Bridges et al.6 reported a less favorable outcome of depression in the period 1979–91 (34% good outcome) in comparison with surgery performed prior to 1973 (55% good outcome) even though the surgical procedure had been the same.
Theoretical Implications
The benefit from neurosurgery has often been used as an argument to build an anatomical model of affective disorders. The results of this study provide further evidence that dysfunctional activity in frontal-subcortical circuits underpins depressive disorder. The persistence of benefit from surgery supports the salience of the circuits, such that the benefit is not diluted by activity in “intact” brain regions. The lesions used in this study are known to destroy fibers connecting the prefrontal cortex and the limbic system as well as the subcortical nuclei, which include the striatum and thalamus.33
Disconnection of the orbitomedial cortex may be a key factor, although anterior cingulate cortex is likely to be similarly affected by these lesions. There are of course many aspects of the treatment that are not easily reconciled. It is difficult to understand, if indeed there is a common neurobiological basis to these disorders, why only a proportion of patients respond to the treatment. Moreover, the benefit from the treatment is not immediate, but evolves over days and weeks, and in some cases over months. While this may reflect the plasticity of the systems involved, it argues against a simple mechanistic neuroanatomical model for depression. The abnormal mood state of an individual appears to be based on a complex and interactive brain state, which can be perturbed by surgical lesions, and this perturbation may or may not result in a new state of relatively normal mood.
CONCLUSION
Our study demonstrated that a proportion of patients with resistant depression respond to orbitomedial frontal surgical lesions with significant improvement or complete remission, and this improvement is sustained in many cases. It can be argued on this basis that neurosurgical treatment should continue to have a place in the panoply of psychiatric treatments available. There have however been no significant new developments in psychiatric neurosurgery in the last 3 decades, and no robust prognostic indicators are available. Future research should address these deficiencies if neurosurgery is to remain an available and mainstream psychiatric treatment.
ACKNOWLEDGMENTS
The authors thank Dr. John Sydney Smith for providing several case notes, Teresa Lee and Stasia Rogers for assistance in data collection, Drs. Cathy Mason and Julian Trollor for outcome ratings, and Angie Russell for manuscript preparation.
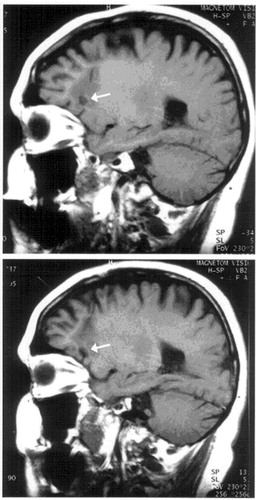
FIGURE 1. MRI Sagittal Sections Demonstrating Bilateral Orbitomedial Lesions in One Subject
Some atrophy in frontal white matter exists.
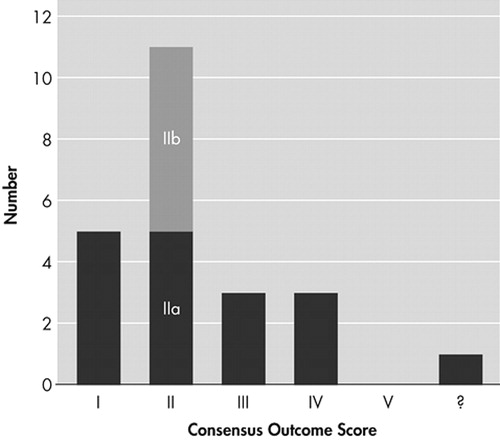
FIGURE 2. Global Outcome Scores for Neurosurgical Treatment of Depression (N=23) Using the Modified Pippard Scale
 |
 |
1 Judd LL: The clinical course of unipolar major depressive disorders. Arch Gen Psychiatry 1997; 54:989–991Crossref, Medline, Google Scholar
2 Morgan G: Long-term risks after attempted suicide. BMJ 1993; 306:1626–1627Crossref, Medline, Google Scholar
3 Malhi GS, Sachdev P: Novel physical treatments for the management of neuropsychiatric disorders. J Psychosom Res 2002; 53:709–719Crossref, Medline, Google Scholar
4 Mayberg HS: Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. Br Med Bull 2003; 65:193–207Crossref, Medline, Google Scholar
5 Sachdev P, Sachdev J: Sixty years of psychosurgery. Aust NZ J Psychiatry 1997; 31:457–464Crossref, Medline, Google Scholar
6 Bridges PK, Bartlett JR, Hale AS, et al: Psychosurgery: stereotactic subcaudate tractotomy. An indispensable treatment. Br J Psychiatry 1994; 165:599–611Crossref, Medline, Google Scholar
7 RANZCP. Statement number 29. The Royal Australian and New Zealand College of Psychiatrists. Position Statement on Psychosurgery. Melbourne: RANZCP, 1990Google Scholar
8 Cosnys P, Caemaert J, Haaijman W, et al: Functional stereotactic neurosurgery for psychiatric disorders: an experience in belgium and the netherlands. Advances & Technical Standards in Neurosurgery 1994; 21:239–279Crossref, Medline, Google Scholar
9 Cosgrove GR, Rauch SL. Psychosurgery. Neurosurg Clin Nth Am 1995; 6:167–176Crossref, Medline, Google Scholar
10 Malhi GS, Bartlett JR: Depression: a role for neurosurgery? Br J Neurosurgery 2000; 14:415–422Crossref, Medline, Google Scholar
11 Price T, Cosgrove R The outcome of cingulotomy for intractable major depression. 149th Annual Meeting of the American Psychiatric Association, N.Y., May 4–9 1996Google Scholar
12 Goktepe EO, Young LB, Bridges PK: A further review of the results of stereotactic subcaudate tractotomy. Br J Psychiatry 1975; 126:270–280Crossref, Medline, Google Scholar
13 Hodgkiss AD, Malizia AL, Bartlett JR, et al: Outcome after the psychosurgical operation of stereotactic subcaudate leucotomy 1979-1991. J Neuropsychiatry Clin Neurosci 1995; 7:230–234Link, Google Scholar
14 Corkin S. A prospective study of cingulotomy, in The Psychosurgery Debate, Edited by Valenstein ES. San Francisco, WH Freeman & Co, 1980, pp 164-204Google Scholar
15 Ballantine Books HT Jr., Bouckoms AJ, Thomas ET, et al: Treatment of psychiatric illness by stereotactic cingulotomy. Biol Psychiatry 1987; 22:807–819Crossref, Medline, Google Scholar
16 Kelly D, Richardson A, Mitchell-Heggs N: Stereotactic limbic leucotomy: neurophysiological aspects and operative technique. Br J Psychiatry 1973; 123:133–140Crossref, Medline, Google Scholar
17 Hay P, Sachdev P, Cumming S, et al: Treatment of obsessive-compulsive disorder by psychosurgery. Acta Psychiatr Scand 1993; 87:197–207Crossref, Medline, Google Scholar
18 Hamilton M: A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56–62Crossref, Medline, Google Scholar
19 Hamilton M: The assessment of anxiety states by rating. Br J Med Psychol 1959; 32:50–55Crossref, Medline, Google Scholar
20 Yesavage JA, Brink TL, Rose TL, et al: Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res 1983; 17:37–49Crossref, Medline, Google Scholar
21 Strom-Olsen R, Carlisle S: Bi-frontal stereotactic tractotomy. A followup study of its effects on 210 patients. Br J Psychiatyr 1971; 118:141–154Crossref, Medline, Google Scholar
22 Wechsler D. Wechsler Adult Intelligence Scale. New York: The Psychological Corporation, 1955Google Scholar
23 Wechsler D Wechsler Memory Scale - Revised. San Antonio: The Psychological Corporation, 1987Google Scholar
24 Robins LN, Wing J, Wittchen HU, et al: The composite international diagnostic interview. An epidemiologic instrument suitable for use in conjunction with different diagnostic systems and in different cultures. Arch Gen Psychiatry 1988; 45:1069–1077Crossref, Medline, Google Scholar
25 Rennie TAC: Prognosis in manic-depressive psychoses. Am J Psychiatry 1942; 88:801–814Crossref, Google Scholar
26 Lundquist G: Prognosis and course in manic-depressive sychoses: a follow-p study of 319 admissions. Acta Psychiatr et Neurol Scand Suppl 1945; 35:1–96Google Scholar
27 Kiloh LG, Andrews G, Neilson M: The long-term outcome of depressive illness. Br J Psychiatry 1988a; 153:752-757Google Scholar
28 Lee AS, Murray RM: The long-term outcome of maudsley depressives. Br J Psychiatry 1988a; 153:741-751Google Scholar
29 Mueller TI, Keller MB, Leon AC, et al: Recovery after 5 years of unremitting major depressive disorder. Arch Gen Psychiatry 1996; 53:794–799Crossref, Medline, Google Scholar
30 Herner T: Treatment of mental disorders with frontal stereotactic thermo-lesions. A follow-up study of 116 cases. Acta Psychiatr et Neurol Scand Suppl 1961; 158:1–11Google Scholar
31 Mitchell-Heggs N, Kelly D, Richardson A: Stereotactic limbic leucotomy—a follow-up at 16 months. Br J Psychiatry 1976; 128:226–240Crossref, Medline, Google Scholar
32 Kiloh LG, Smith JS, Johnson GF Physical treatments in psychiatry. Melbourne, Blackwell Scientific Publications, 1988b, pp 277-333Google Scholar
33 Corsellis J, Jack AB, Laitinen LV, et al (eds.): Surgical approaches to psychiatry. Lancaster, Medical and Technical Publishing Co, 1973Google Scholar
34 Meyer G, McElhaney M, Martin W, et al. Stereotactic cingulotomy with results of acute stimulation and serial psychological testing, in Surgical Approaches in Psychiatry, Edited by Laitinen LV, Livingston KE. Lancaster, UK, Medical & Technical Publishing, 1973, pp 39-58Google Scholar
35 Poynton AM, Kartsounis LD, Bridges PK: A prospective clinical study of stereotactic subcaudate tractotomy. Psychol Med 1995; 25:763–770Crossref, Medline, Google Scholar
36 Vilkki J: Late psychological and clinical effects of subrostral cingulotomy and anterior mesoloviotomy in psychiatric illness, in Neurosurgical Treatment in Psychiatry, Pain and Epilepsy. Edited by Sweet WH, Obrador S, Martin-Rodriguez JG. Baltimore, University Park Press, 1977, pp 253-259Google Scholar



