Neurodevelopmental Pathways to Aggression: A Model to Understand and Target Treatment in Youth
Aggressive behavior, ranging from self-directed to other-directed destructive behavior, originates in multiple sources and can be associated with numerous Diagnostic and Statistical Manual IV-TR (DSM-IV-TR) defined mental disorders. 1 The impulsivity, hyperactivity, and distractible inattention associated with attention deficit hyperactivity disorder (ADHD) can lead to greater than expected accidents and injuries. The affective instability in bipolar disorder, borderline personality disorder, and intermittent explosive disorder can lead to emotionally charged aggressive behavior. The irritability associated with major depressive disorder and dysthymia can lead to aggressive and self-destructive behavior, including “acting out” aggression and suicide. Excessive anxiety associated with posttraumatic stress disorder (PTSD), autism spectrum disorders, and other developmental disabilities can result in low frustration tolerance and “striking out” aggressive behavior. Substance abuse and thought disorder (psychosis, schizophrenia) can impair judgment leading to inadvertent aggression and exposure to danger. Youth with conduct disorder and oppositional defiant disorder may seek the arousal, novelty, and stimulation of aggressive behavior, often with a predatory quality. While these DSM-IV-TR diagnoses may help guide interventions for associated aggression, the diagnosis by itself is often not sufficient to lead directly to effective treatment due to the multidetermined etiology of the aggression and comorbidity. 2 Until we understand the origins or etiologies of these episodes of aggression, we are limited in our ability to develop effective treatments for them.
Symptom Domains
This article is organized around symptom domains that can be understood through a neurodevelopmental model from the presenting or surface, primary symptom to underlying, related brain structures and functions. Early reports identified at least seven subtypes of aggression that were linked to neuroanatomical origins within the animal literature: predatory, intermale, territorial, maternal, irritable, fear-induced, and instrumental. 3 Traditionally, these aggressive behavior subtypes have been reduced to binary domains when dealing with humans: for example, hostile versus instrumental, overt versus covert, reactive versus proactive, defensive versus offensive, affective versus predatory, or impulsive versus controlled (for a complete review see Vitiello and Stoff, 1997). 4 Though helpful as a conceptual model, these binary constructs offer little for treatment options since many of the subtypes of aggression often present in a similar affective, impulsive manner. Therefore, based on the human and animal aggression literature as well as clinical experience using pharmacological and psychological target symptoms, we are proposing five primary symptom domains of aggressive behavior: impulsivity (rapid, thoughtless aggressive acts), affective instability (affectively charged attacks with seemingly little provocation), anxiety/hyperarousal (overwhelming anxiety and frustration leading to aggressive outbursts), cognitive disorganization (poorly organized and confused aggressive acts), and predatory/planned aggression (goal-directed preying on others).
The neurodevelopmental model proposes that brain development is a continuous process that is influenced by genetics, brain structure and function, and environmental interaction. All social influences affect brain development and all psychological constructs are implemented by brain mechanisms. Neurodevelopmental models trace healthy and pathological neurodevelopmental etiologies through brain mechanisms to surface symptoms. 5 In children and adolescents we see neurodevelopmental maturity leading to control of impulse, affect, anxiety, and cognition with individual differences based on individual and environmental factors. Thus, controlling aggression is a neurodevelopmental process that is increasingly understood using neuroscience research and will be used as the underpinning of support throughout this article. Future longitudinal research will determine if these targeted interventions for aggression enhance neurodevelopment. Identification of these potential targets and their biomarkers outlined in this article is a working model.
The brain regions that are of particular interest in understanding aggression are listed in Table 1 . Each primary symptom section will be identified clinically and linked to the neuroanatomical regions that are relevant to the origin of the target, primary symptom. Neuromodulators and functional circuits involving these regions will also be integrated.
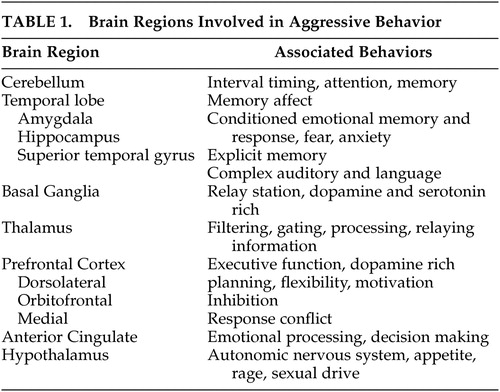 |
Therefore, this model-building paper will begin with the identification of key behavioral symptoms that lend themselves to treatment interventions, and then describe the neurostructural and neurofunctional underpinnings of these primary symptoms and relate these to treatment choice and response. Eventually, as the literature becomes available, the neuroendocrine system (testosterone, cortisol, cholesterol, etc.), which modulates many of the mentioned brain regions and has been implicated in aggressive behavior, and genetics, which confers both susceptibility (vulnerability) and resilience (resistance) to aggressive behavior, will be integrated into this model.
Impulsivity
Clinical Description
Impulsivity is the failure to resist an impulse, drive, or temptation, resulting in rapid, unplanned reactions to internal or external stimuli. It involves the inability to delay reward where the individual is unable to modify his or her behavior according to the context of the situation or to reflect on the consequences of the behavior, thus impairing judgment. With impulsivity there is an associated underestimated sense of harm and lack of regard for the negative consequences. 6 Impulsive aggression disorder has been defined as recurrent incidents of physical or verbal aggression that are out of proportion to the circumstances, occur at least twice a week for more than 1 month and lead to marked distress or impairment. 7
Subjects who demonstrate impulsive choice choose small or poor rewards that are immediately available over larger but delayed rewards. They are unable to calculate future risks or consequences and unable to delay acting on impulse, indicating impaired executive functioning. This type of aggression was shown to develop in almost 40% of children who sustained traumatic brain injury. 8 Impulsive aggression also appears to have a familial transmission. 9
Novelty or sensation seeking often is associated with impulsivity, but there are differences. Novelty seeking seems more likely to occur in youth who are seeking a means of stimulation rather than the “out-of-the-blue” impulsive behavior. 10 Novelty seeking can also occur in youth who are experiencing stress or are overstimulated, and are seeking distractions from their stress. This might occur with PTSD, depression, and/or substance abuse. Impulsive behavior is not sought, even indirectly, but it happens with no forethought. Although certain vulnerabilities (e.g., substance dependence or heightened anxiety) may occur in tandem with the impulsive act, it is not the preceding affect that seems to drive the impulsive act.
Case Example
“Mr. A” is a 14-year-old boy with a long history of ADHD and erratic school performance, but no serious behavior problems or depression. There is a family history of alcoholism on both sides, but no other disorders in his upper-middle class family. Mr. A has not been physically aggressive toward others but is known to do things without thinking and is quick to respond to any challenge. While drinking a few beers with a group of mixed sex peers, he impulsively responds to a dare and jumps off a bridge into the water below, breaking one of his legs.
Brain Structure & Function
Behavioral inhibition is linked to four executive neuropsychological functions. These are working memory, self-regulation, internalization of speech, and reconstruction (behavioral analysis and synthesis). 11 These functions are processed in a network between the prefrontal cortex and the basal ganglia in the fronto-striatal region. The orbitofrontal cortex constrains impulsive outbursts, 12 and the ventromedial prefrontal cortex is involved with the delay of gratification 13 but is not directly involved in impulsive choice. In addition to the key role of the orbitofrontal cortex in restraining impulsive aggression, 7 , 14 the nucleus accumbens (reward and reinforcement) and the amygdala (identification of negative emotion) 15 are involved in impulsive behavior and the restraint of it. The amygdala is not involved in the regulation or mediation of impulsive and aggressive behavior but is involved in the acquisition of emotional memories used for decision-making based on experience. 16 Though it does not appear that the anterior cingulate is directly involved in impulsivity, it recruits other brain regions to contain impulsivity. 17 The usefulness of differentiating executive inhibition, thought to be frontal/fronto-striatal and dopaminergic, from motivational inhibition, thought to be noradrenergic/serotoninergic and limbic in nature, is proposed as an important way of subtyping aggression in youth. 18
Neuromodulators
Serotonin plays a central role in impulsivity 19 , 20 with the greatest effects in the orbitofrontal and cingulate cortex, 7 but serotonin neurotransmission is complex. 21 Impulsivity is also influenced by interconnections with the noradrenergic (irritable), dopaminergic (reward), glutaminergic and gamma-aminobutyric acid (GABA) systems. 22
Specific serotonin receptors and genes 23 are likely more involved with impulsive aggression than are others. For instance, knockout mice lacking the serotonin receptor 5-HT1B exhibited increased impulsive aggression. 24 Thus, the 5-HT1B receptors are suggested as a promising pharmacological target for reducing impulsive aggressive behavior in humans.
Treatment
Intervention is targeted at prolonging thinking before action, either with cognitive behavior techniques, removal from high-risk situations and overstimulation, or pharmacological intervention.
Pharmacological interventions that have been shown to decrease impulsive aggression include stimulants, SSRIs, and mood stabilizers ( Table 2 ), but often with different populations. 6 For instance, stimulants may be of greater benefit to individuals with distractible inattention leading to their impulsivity than to individuals without distractible inattention, even if they do not meet the criteria for ADHD.
 |
Psychosocial treatments for impulsivity usually begin with an educational and behavioral focus, progress to a cognitive focus, and conclude with a focus on relapse prevention. The major themes in intervention are practicing delay and improving response inhibition and reflection deficit.
Intervention is also directed toward strengthening the “cultural container,” the cultural protection offered by a strong culture surrounding the youngster and the youth’s identification with it. This is related to interventions that increase “reward dependence,” or the seeking of the positive aspects of approval. Other environmental interventions include changes in peer group and public education. After an initial evaluation of Mr. A, described above, effective treatment included substance abuse counseling, an SSRI, and therapy directed at impulse delay through anticipation, rehearsal, and modeling.
Affective Instability
Clinical Description
Affective instability is emotional dysregulation expressed as exaggerated reactions to negative or frustrating stimuli, 25 which may result in rage and aggression. In children and adolescents, affective instability usually occurs rapidly and is highly reactive. Emotional regulation is described as the ability to manage arousal or to modulate the intensity of emotional reactions. 26 According to Bhangoo and Leibenluft, 27 this ability develops biologically and its dysregulation is common to several disorders, including bipolar disorder and developmental disabilities like autism spectrum disorders.
Affective instability may be combined with impulsivity to result in risky and aggressive behavior, 4 but the two do not describe the same phenomenon; a person can be impulsive with or without an affective component. Purely impulsive aggression has no identifiable precedent, seemingly coming “out of the blue.” Affective aggression usually happens rapidly and may seem impulsive, but is differentiated by the preceding rush of affect, resulting in “hot tempered” aggression. Both affective instability and impulsivity are related to poor attention, attention shifting, and verbal self-control. 25
Affective instability, rages, euphoria, and rapid cycling or mixed moods are increasingly diagnosed as bipolar disorder in youth. 28 However, children with a number of psychiatric disorders manifest emotional dysregulation. 27 They may not meet the full criteria for bipolar disorder because they do not have grandiosity, decreased need for sleep, increased goal-directed activity, but do have hyperarousal, abnormal baseline mood, and extreme responses to frustration. Leibenluft et al. 25 suggest the phenotype in juvenile mania ranges from the narrow phenotype with all the classic bipolar symptoms to a broader phenotype that does not include all of the hallmark symptoms but shares symptoms of irritability and hyperarousal that are also commonly described in the narrower phenotype. Affective instability is clearly associated with this “broader” phenotype.
In a recent study of the personality changes due to brain injury, affective instability was noted to be the most common sequelae of traumatic brain injury in children, occurring in nearly 50% of the sample. 8 This affective dysregulation was most frequently characterized by very rapid shifts to pathological irritability.
Depression and irritability can lead to risk-taking and aggressive behavior that can be directed toward others or toward the self. 9 Factors that increase impulsivity, such as substance abuse or the availability of weapons, can increase the risk of harm, but treatment needs to be directed not only toward these factors but also to the underlying depression.
Case Example
“Ms. B” was a 12-year-old girl who had a history of severe temper tantrums and, over the past year, an aggression of increasing destructiveness. Ms. B would have episodes lasting 45 to 120 minutes in which she would seem beyond herself with rage and physical aggression. Episodes happened several times a week for several weeks, and then went away for a few weeks. They occurred more frequently in the late afternoon. She remembered the episodes when they were over but did not feel remorse. Ms. B had been diagnosed with ADHD and had benefited modestly from a regimen of amphetamine/dextroamphetamine, 10 mg, each weekday morning, especially at school. She was distractible, very impulsive, easily frustrated, mildly depressed at times, had no marked anxiety or tics, did well academically, and had poor social skills, although other kids “followed her.” A family history of mental disorder was denied. While Ms. B’s symptoms met the criteria for a diagnosis of attention deficit hyperactivity disorder–combined type, her additional symptoms of affective instability demonstrated by her moody, impulsive, and episodically aggressive behavior suggested bipolar disorder II, but she did not meet all the criteria for this disorder.
Brain Structure & Function
Emotional regulation involves complex neural circuitry, including the orbital frontal cortex, amygdala, anterior cingulate, and cerebellum. In addition, emotional regulation also plays a role in functional involvement of the autonomic nervous system and neurotransmitters, particularly serotonin. 17 This neurobiology of emotion has been further divided into the ventral system, including the amygdala, insula, ventral striatum, ventral anterior cingulate, and the prefrontal cortex for the identification of the emotional significance of a stimulus and the automatic regulation of emotional responses. The dorsal system has also been included as well, demonstrating effortful regulation of affective states and subsequent behavior in structures including the hippocampus, dorsal anterior cingulate and prefrontal cortex. 29
Affective aggression is commonly found in temporal lobe epilepsy. 30 A recent well-executed study with bipolar adolescents and adults who were aggressive found significant amygdala volume reductions of 15.6% compared to healthy subjects. 31 Additional studies suggesting a link between temporal lobe structures and affective aggression include studies of anger in healthy men which link aggression with the bilateral temporal poles as well as with the left frontal cortex and right anterior cingulate. 32 It is possible that the prefrontal cortex and the anterior cingulate control the expression of angry aggression in healthy men. 33
Neuromodulators
The neuromodulators involved in affect regulation overlap with those involved in other symptom domains, which may account for the improvement in affective instability when using agents such as antidepressants also used for impulse control and atypical neuroleptics used for cognitive disorganization. For instance, serotonin is involved in both impulse control and in affect regulation. 21 , 32 In addition, the GABA system seems to be heavily involved in affective stabilization from anticonvulsants. 21
Treatment
Pharmacological treatments for affective instability are primarily atypical neuroleptics (e.g., risperidone) and mood stabilizers (e.g., divalproex) ( Table 2 ). 34 There are suggestions in the literature that those medications that improve affective instability have their primary action in those brain regions associated with affective instability, the temporal lobes, and the cerebellum.
Psychosocial treatments are similar to those used for impulsivity, but focus on strengthening the cognitive path from the perception of emotion to action. As described earlier, the path to emotional action is through the superior temporal lobe, so to modify this rapid rage response, the pathways through the cortex, including the prefrontal cortex, need to be strengthened through cognitive practice. This includes identification of emotion and pairing it with a pro-social response through practice with cognitive behavior and dialectic therapies. Sublimation of affective aggression through sports and martial arts may also be helpful for selected youth. For Ms. B in the example above, divalproex was added to stimulant treatment. This along with 10 sessions of cognitive behavior therapy resulted in relatively stable mood and behavior during an 18-month period.
Anxiety/Hyperarousal
Clinical Description
Fear is an adaptive response to a threatening stimulus that may lead to a protective response, whereas anxiety is an emotional response linked to a threatening stimulus, even in the absence of direct danger. Anxiety may be considered part of a normal response until it becomes excessive or difficult to tolerate, resulting in overstimulation. As coping tolerance is exceeded, the anxious hyperarousal may precipitate decompensation and disorganization, resulting in poorly directed aggression toward the self or others, sleep disturbances, irritability, difficulty concentrating, hypervigilance, and an exaggerated startle response. Oftentimes the aggressive act relieves the precipitating anxious hyperarousal sensations. These symptoms are commonly seen in children with PTSD, Cluster B disorders, depression-related anxiety, autism spectrum disorders, neurodevelopmental disorders, and other forms of mental retardation.
Case Example
“Ms. C,” a 13-year-old girl, came to the inpatient unit because of her unpredictable behavior. Her family had difficulty dealing with the random aggressive behavior Ms. C displayed toward her stepfather and younger siblings. Ms. C’s mother described her as a “difficult child” who was physically abused by her biological father. When Ms. C’s mother remarried, her behavior worsened, and eventually she spent 1 year in a group home where she was frequently abused physically, sexually, and verbally. During the hospitalization, Ms. C was guarded and often lashed out unexpectedly when she encountered an anxiety-evoking situation (i.e., in a group setting talking about her gender identity). She mentioned feelings of relief that often accompanied her aggressive behavior. Though she tried to minimize the impact of the abuse, when she actually talked about her abuse, she often became tearful. Ms. C was diagnosed with PTSD.
Brain Structure & Function
The limbic-hypothalamic-pituitary-adrenal (HPA) axis plays a vital role within the stress response. When stress is perceived at the level of the limbic system, the hypothalamus secretes corticotropin-releasing factor (CRF), which, in turn, stimulates the release of the adrenocorticotropin hormone (ACTH) from the pituitary. This, in turn, stimulates the release of glucocorticoid from the adrenal, which has a negative feedback effect on the axis of the limbic system (e.g., hippocampus and amygdala) and the hypothalamus, and the pituitary CRF stimulates other neurochemical responses in the brain, including the noradrenergic system within the locus coeruleus within the posterior pons. 35
The hippocampus typically has an inhibitory effect on the HPA. However, it is an area that is particularly sensitive to stress because of the high density of glucocorticoid receptors. Hypercortisolemia has been shown to decrease dendritic branching and neuronal loss in the CA3 region of the hippocampus, 36 and it has been associated with deficits in new learning. 37 Magnetic resonance imaging (MRI) volumetric studies comparing PTSD related to early childhood stress versus matched comparison subjects showed a 12% reduction in the left hippocampus volume, which is similar to results found in individuals with combat-related PTSD and women with early sexual abuse. 38 Hippocampal injury may be significant as it pertains to the amnesic, dissociative, anxiogenic, and disinhibitory symptoms of PTSD. 39
Likewise, the amygdala has been shown to be intimately involved in the regulation of the HPA system. 40 The amygdala in particular has been implicated in the development and retrieval of emotional memory, the learning of nonverbal motor patterns, the trigger of fight-or-flight response, threat detection, and the production of fear and anxiety. 41 Excessive amygdala and nucleus accumbens activity has been implicated in the development of aggressive hyperarousal within PTSD. 42 Similarly, amygdala abnormalities have been linked to heightened anxiety within autism spectrum disorders. 41 In each case, stimulation of the medial and cortical regions of the amygdala elicits additional corticosterone secretion. 43 Long-term exposure to cortisol leads to suppression of serotonin metabolism and down-regulation of the 5-HT1A and 5-HT2 receptors within the limbic system. 44
Neuromodulators
The serotonin system plays a key regulatory role within the CNS as it modulates internal affective states (anxiety, fear, depression, and aggression), control of sleep, reward circuits that mediate motivation, and hedonic states. 45 As a result of its broad-spectrum activity, serotonin modulation has become a first-line therapy. 46 Additionally, studies have shown increased brain-derived neurotrophic factors in the CA3 and CA1 regions of the hippocampus, which help reverse the effects of stress as well as increase dendritic branching and neurogenesis within the hippocampus when serotonin selective-reuptake inhibitors (SSRIs) are taken. 46
Noradrenergic cell bodies are primarily located in the locus coeruleus and project to the hippocampus, amygdala and other limbic structures within the brain. Exposure to stress increases noradrenergic modulation within these regions, 47 which generates hyperarousal and reexperiencing of symptoms 47 as well as stimulates the release of CRF from the hypothalamus. By targeting autonomic hyperarousal, improvements may be seen in anxiety level, concentration, mood, and behavioral impulsivity.
Treatment
The symptoms of anxious hyperarousal and spontaneous aggression represent abnormalities within the limbic system and serotonergic and noradrenergic activity, which have resulted in poor coping skills. Interventions aimed at decreasing overall stress and improvement of coping skills through case management, psychoeducation, and psychotherapy (which often combine principles of cognitive and behavior therapies) often combined with serotonergjic and noradrenergic modulation are most likely to be beneficial ( Table 2 ). Ms. C was initially started on an SSRI. Eventually, clonidine was added to her regimen. She received cognitive behavior therapy, and her family participated in weekly family counseling sessions. After 6 weeks of inpatient hospitalization, she returned back to her home and continued to work with her own therapist. Her behavior improved at home, but her relationship with her step-father continues to be strained.
Cognitive Disorganization
Clinical Description
Delusions, psychosis, or substance abuse can result in cognitive disorganization and aggressive behavior. While the behavior may appear impulsive to the casual observer, closer inquiry may reveal more long-standing, distorted perceptions or impaired reasoning as precedents to the aggressive behavior. Paranoia is particularly prominent in recurrently violent adolescents. 48 Delusions and psychosis are associated with schizophrenia, mood disorders, personality disorders, forms of mental retardation (e.g., fetal alcohol syndrome, autism), and brain injury (e.g., epilepsy or traumatic injuries). Often, substance abuse disorders are comorbid with many of the aforementioned categories of severe mental disorders and often exacerbate psychopathology. Recurring psychosis as experienced in schizophrenia has been demonstrated in neurocognitive deficits and worsening behavior. 49
In examining individuals with first-episode psychosis (cognitive disorganization), Foley et al. 50 showed 52 of 157 subjects presented with aggressive behavior (e.g., a hostile or destructive mental attitude, including verbal aggression, physical aggression, and/or violence). There was no statistical difference seen between subjects diagnosed with bipolar disorder or schizophrenia as the etiology of the cognitive disorganization. Nevertheless, those with comorbid substance abuse disorders were more likely to engage in aggressive behaviors. Similarly, results from the UK700 trial 51 suggested that personality disorder patients with comorbid psychosis were more prone to violent behaviors. Though there are many etiologies that result in cognitive disorganization, it is this cognitive disorganization component that often drives the aggressive behaviors and should be the target of intervention.
Case Example
“Mr. D,” an 18-year-old Mexican American boy, was drafted into the Mexican Army when he was 17 years old. He was discharged soon afterward because of his excessive aggression with peers and commanding officers. Mr. D believed that his commanding officers were singling him out and punishing him inappropriately. He believed that he had been elected to defend his nation. Mr. D deserted his unit, and later attacked his superior officer, which led to his first hospitalization. When released, Mr. D began experimenting with methamphetamine, marijuana, and cocaine, which worsened his delusions. He became increasingly aggressive with his family members and neighbors. Concerned, Mr. D’s family hospitalized him on numerous occasions for respite, protection, and treatment. He was diagnosed with schizophrenia–paranoid type and amphetamine, cocaine, and cannabis dependence.
Brain Structure & Function
The brain structure and function associated with cognitive disorganization can be inferred from studies of psychosis, schizophrenia, and the impaired thinking associated with substance abuse. Imaging studies demonstrate volume reductions of gray matter in soft tissue structures, particularly the medial temporal lobe (hippocampus and amygdala) 52 and the associated neocortex (prefrontal and superior temporal). 53 Animal lesion models (lesions of the ventral hippocampus and medial prefrontal cortex) have produced behavioral models consistent with schizophrenia patients. 54
The hippocampus and the prefrontal cortex have been implicated in the encoding, storage, and retrieval of memory, 55 which may be involved in the inappropriate generation of paranoid delusions. Heckers et al. 55 used positron emission tomography (PET) to evaluate relative function between the hippocampus and prefrontal cortex during verbal episodic memory retrieval in subjects with schizophrenia. They reported hyperfunctional hippocampal activity at rest and during cognitive activation, suggesting an improper encoding and storage of the memory. They also report robust activity of the prefrontal cortex (which is also seen in the comparison population) and hypofunctional hippocampal activity during memory recall, suggesting that an improper recruitment of neurons results in a poor retrieval of memory. Additionally, this impairment of recruitment during memory retrieval may represent a functional correlate of an abnormal corticohippocampal interaction seen in schizophrenia. 56
Likewise, the amygdala has been implicated in the development of emotional memory, threat detection, and fear production, which correspond to aggressive symptoms seen in paranoid delusions. Amygdala volume loss 52 seen in schizophrenia may account for the etiology of these symptoms. 57
Neuromodulators
The role of dopamine as a neuromodulator of psychosis became apparent when it was observed that psychostimulant agents, which caused the release of dopamine, induced psychosis, which in turn resolved with dopamine-blocking agents. Neuroimaging studies confirm that patients with schizophrenia have an elevated synthesis of dopamine 58 and an elevated release of dopamine in response to an impulse. 59 Other neurotransmitters (e.g., glutamate and GABA) and monoamines have been implicated in the pathology of schizophrenia. 60
The mesolimbic dopamine system, which makes projections to the limbic system and prefrontal cortex, has been implicated in the “attribution of salience,” a process whereby events and thoughts come to grab attention, drive action, and influence goal-directed behavior because of their association with reward or punishment. 61 Kapur 62 postulates that dopamine mediates the process but does not create it. Nevertheless, during the psychotic process, there is a dysregulated dopamine transmission, which is believed to usurp “the normal process of contextually driven salience and lead to aberrant assignment of salience to external objects and internal representations.” This leads to the improper linkage between “inappropriate salience and motivational significance to external and internal stimuli.” Antipsychotic medications do not change thoughts or ideas, but provide a neurochemical milieu where aberrant saliences are less likely to develop and existing saliences are more likely to subside. 62
Treatment
The symptoms of cognitive disorganization and continued paranoia represent abnormalities of the corticohippocampal pathway and dopaminergic activity. Interventions that improve executive function and memory are particularly helpful. Antipsychotic-D2 receptor blocking agents minimize hallucinations and delusional beliefs. 63 Psychosocial treatments that help with executive and social function prevent the development or accumulation of stressors and provide coping strategies that seem to benefit judgment, minimize external and internal stressors, and improve social relatedness, 49 , 63 which leads to decreased aggressive behavior. Additionally, efforts should be made to encourage substance abuse rehabilitation. Mr. D was initially started on an atypical antipsychotic. After an initial failed attempt, we attempted multiple combinations of atypicals, typicals, and decanoates. During this time, Mr. D continued to abuse street drugs and was resistant to drug counseling therapy. As such, his aggressive behavior continued to escalate, and eventually, he was incarcerated and lost to follow-up.
Predatory Aggression
Clinical Description
Predatory aggression is probably the most difficult subtype of aggression to treat because it is premeditated and consciously executed. This type of aggression is also described as instrumental, cold-blooded, or planned. Clinical symptoms of this subtype of aggression are often apparent in patients with conduct disorder and oppositional defiant disorder (it should be noted that other subtypes of aggression, such as impulsive/affective aggression, can also be present in these disorders and in combination with predatory aggression). One study estimates that 3.5% of 13-year-olds demonstrate predatory aggression. 64 Children with this subtype of aggression are more likely to have antisocial personality disorder than adults.
Case Example
“Mr. E,” a 12-year-old boy with a history of conduct disorder was brought into the clinic because of escalating behavioral problems. Three days prior, Mr. E was suspended from school due to fighting. During the interview, he revealed that he knew another boy always carried “emergency money” (reportedly about $5.00) in his wallet. Mr. E related that between classes in a hallway “where there was never any teachers,” he pushed the other boy down and told this boy to give him the money. The other boy did not comply with his requests, and Mr. E subsequently kicked him repeatedly. Mr. E states that he wanted the money for a new pair of shoes “because my mom will only buy the stupid cheap shoes.”
Brain Structure & Function
Although this type of aggression is premeditated and consciously planned, people with predatory aggression have an underlying neurobiology which predisposes their aggressive behaviors. This neural pathway involves the midbrain, lateral hypothalamus, thalamus, hippocampus, amygdala, and usually normal performance from the prefrontal cortex. A neuroimaging study investigating the neurobiology of planned aggression noted that predatory-aggressive subjects exhibited hyperactive subcortical signaling, which was hypothesized to make them more prone to act aggressively. 65 The prefrontal function in these subjects was comparable to healthy subjects. We hypothesized that because these subjects demonstrated intact prefrontal function, their aggressive acts were planned and predatory in nature (rather than impulsive, which would be expected in patients with impaired prefrontal function). This idea is supported by an imaging study of violent antisocial personality disordered patients who showed a decrease in prefrontal gray matter, suggesting a link to the decision making and executive function deficits in “hot-blooded,” affectively unstable, aggression. 66
Animal models have demonstrated that overstimulation of the lateral hypothalamus leads to predatory type aggressive behavior. 67 Furthermore, other animal studies have shown that this predatory aggression, which is elicited by stimulation of the lateral thalamus, can be suppressed with a D2 antagonist. 68 Further research suggests that children exposed to abusive environments are prone to develop neurobiological changes which lead to antisocial behaviors. 39 Therefore, chronic childhood stress due to an abusive environment leads to neuroregulatory changes in the limbic system, which result in aggressive behavior.
Neuromodulators
Dopamine plays a key role in the modulation of predatory aggression as well as in the brain’s reward system, which reinforces this behavior. Dopamine neurons within the ventral tegmental area and substantia nigra process rewarding stimuli. They send axon attachments to brain structures (e.g., striatum, nucleus accumbens, and frontal cortex) involved in motivation and goal-directed behavior. Dopamine activation occurs as novel stimuli are encountered and produce a primary reward. Eventually, the primary reward does not produce a phasic response (dopamine activation), and instead, the phasic response (dopamine activation) occurs during the anticipation of the event. 69
Treatment
Clinicians should consider the role of the child’s environment in creating predatory aggression. The possibility of child abuse should be considered. Children exposed to maltreatment and stressful environments can develop neurobiological changes which lead to antisocial behaviors. 40 For the same reasons, other modifiable stressors should be considered and modified when possible.
Other approaches (e.g., multi-systemic therapy) optimize the role of the frontal cortex for conscious control of underlying aggressive tendencies. This treatment involves the identification of factors, which makes the subject more likely to act aggressively, along with trained anticipation using alternative strategies. Consequently, both the psychosocial environment and supports for adaptive behaviors are addressed in the comprehensive treatment. 70
By targeting the underlying aggressive pathology caused by overactive subcortical activation (which makes a person prone to behave aggressively, albeit in a planned or controlled fashion), a clinician could diminish predatory aggression with pharmacological agents. Antipsychotic medications demonstrate benefits in the treatment of aggression. Valproate, which can correct GABA deficiencies, is effective in the treatment of aggression, although this seems more likely to benefit affective instability and impulsivity. Mr. E, in the example above, demonstrated increased predatory aggression while he was attending a mixed diagnosis adolescent group, and modest improvement in his predatory aggression when he was placed in a group home with several other aggressive teenagers where a behavioral program was strictly enforced. The longer-term outcome is unknown.
Choosing Treatment Based on Primary Symptom Domains
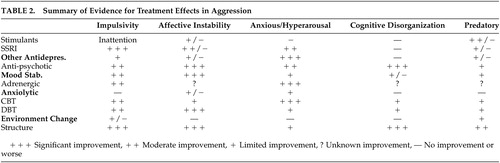
As initially alluded to, children and adolescents with aggressive tendencies are often challenging to treat. Many of the children and adolescents are particularly likely to terminate treatment prematurely. Kazdin 71 identified the following familial risk factors that may lead to premature termination: socioeconomic disadvantage, facets of the family constellation (younger mothers, single-parent families), high parent stress, adverse child-rearing practices, and parental history of antisocial behaviors. With this present, the selection of a treatment or treatments begins with a definition of the behaviors and events occurring immediately before the aggressive behavior. This leads to the identification of the underlying, primary target symptom or combination of symptoms and is usually the most productive focus for treatment selection. This also should lead beneath the surface symptom to the neurobiological origins or etiology of the risky behavior. Table 2 summarizes the effects of treatment on the proposed symptom domains discussed in more detail in the text below. Since clinical trials are reported for DSM-IV-TR categories, it is not possible to do a literature search for evidence of the effectiveness of each of these approaches based on the symptom domains proposed here. As a compromise, Table 2 is based on a careful review of the literature and clinical experience. The literature reviewed to develop this table is summarized in the text, but this does not represent a meta-analysis or a rigorous, comprehensive review. The ratings of improvement are based on the literature and clinical experience.
In the text below, medications used to treat aggressive behavior are reviewed first, including those that act primarily in the dopaminergic, serotonergic, adrenergic, and GABA systems followed by psychosocial treatments.
Stimulants
Stimulant treatment may improve aggressive behavior, 72 especially when related to impulsivity. However, stimulants are known to increase cognitive disorganization and anxious hyperarousal 73 and thus may worsen aggression in some individuals within these symptom domains.
A regimen of methylphenidate, up to 60 mg a day for 5 weeks, resulted in improved antisocial behavior (provocative, aggressive, mean behavior, as well as lying and cheating) in 41 conduct-disordered youths between 6 and 15 years old, compared with 42 conduct-disordered comparison subjects. Two-thirds of the children also met criteria for ADHD, but response was not related to severity. Effectiveness is probably due to decreased impulsivity and hyperactivity. 74 Stimulants and/or clonidine are shown to improve aggressive, oppositional, and conduct disorders associated with ADHD. 75
Stimulants improve executive function by acting on dopamine receptors in the prefrontal cortex 76 and basal ganglia, 77 but also exert effects on the noradrenergic receptors from the locus coeruleus to the prefrontal cortex and on the cerebellum. 78 Neuroimaging studies suggest stimulant medications activate the prefrontal cortex and the basal ganglia in subjects with ADHD. 79 Stimulants act in the striatum by binding to the dopamine transporter and this increased striatal dopamine activity may play a role in enhancing prefrontal executive function. 80
Antidepressants
Antidepressants may benefit aggression of an impulsive, affective instability or anxious hyperarousal origin. However, they are not of benefit to cognitive disorganization or predatory aggression and may lead to troubling side effects. Selective serotonin reuptake inhibitors (SSRIs) have been used in the treatment of aggressive behavior with benefits, although these aggressive or irritable patients may have a propensity to demonstrate “activating” or “disinhibiting” side effects (anxiety-like symptoms sometimes likened to hypomania 81 from SSRIs). While decreasing anger, irritability, and aggression, SSRIs may also precipitate rapid cycling in patients with bipolar disorder. 81 SSRIs may be most effective with moderately aggressive patients, at least with adults; 82 impulsive aggression that does not respond to an SSRI may respond to a mood stabilizer. 83
Long-term administration of selective serotonin reuptake inhibitors raises intrasynaptic serotonin in the cortex of animals. 84 SSRI administration has also been shown to increase the number of serotonin receptors in rat pups 85 and to increase neurogenesis in the adult rat hippocampus. 46 These effects seem to be more profound in the developing brain. Six weeks of paroxetine treatment significantly increased the 5-HT agonism on 5-HT2A receptors in the cortex of young patients with depression but not with older patients. 86
Antidepressant medication decreases metabolism in the left amygdala and the left subgenual anterior cingulate cortex in depressed patients. Further, amygdala metabolism is shown to decrease in responders compared with nonresponders. 87
Antipsychotics
Antipsychotic medications may be helpful for impulsive, affective instability, cognitive disorganization, and anxious hyperarousal-based aggression, and may even be helpful to predatory aggression. However, side effects limit their routine use, and initiation must thoughtfully consider risk/benefit and carefully monitor side effects.
Compounds that target dopamine receptors, which have prominent roles in motivation and the initiation of behavior, 69 reduce aggressive behavior, but those that more particularly target the D2 receptor appear more specific in their inhibition of aggression. The broad use of atypical neuroleptics in the treatment of aggression may be due to the more specific action on D2 receptors and block 5-HT2 receptors. Aggressive behaviors often result from a failure to balance the generation and screening of action. Restoration of these processes often occurs by balancing dopamine, which has a significant role in activation and initiation of behaviors, and inhibiting serotonin. 88
Antipsychotic therapy has been shown to increase striatum volume, modify ultrastructural changes in synapse morphology, gene expression, and protein synthesis within the striatum, 89 as well as decrease the metabolism within the hippocampus and the cortico-striato-thalamic circuits. The reduction in metabolism associated with antipsychotic treatment predicts a reduction in symptoms of hallucinations and delusions. 90 Additionally, cellular activation studies of typical and atypical antipsychotics have shown increased expression of protein Fos, a marker for cellular activation in the nucleus accumbens, central amygdala, and central medial thalamus. 91
Mood Stabilizers
Mood stabilizers are recommended as a first-line treatment of aggression in patients with impulsive aggression and bipolar symptoms. 92 Research studies have shown valproate to be effective in treating aggression in patients with pervasive developmental disorders, 93 conduct disorder, 94 and oppositional defiant disorder, 95 although it is suggested that those with an affective component respond to the greatest extent. In a study comparing the treatment response of subgroups with impulsive aggression to divalproex, the Cluster B personality disorders (e.g., antisocial personality disorder) subgroup demonstrated the best results. 96 A comprehensive review of valproate research revealed an overall 77% response rate in the treatment of aggression. 97
Lithium treatment is shown to be more effective for affective aggression as compared to predatory aggression. 98 Greater response of affective symptoms, compared with impulsive symptoms, was seen in inpatients treated with divalproex.
Some research suggests that long-term treatment with lithium, which can be neurotoxic at high serum levels, is paradoxically neuroprotective at therapeutic serum levels and may enhance hippocampal neurogenesis. 99 Additionally, increases in gray matter were found in bipolar patients after 4 weeks of lithium treatment. 100 However, the short-term effects of lithium treatment include cognitive side effects. Therefore, anticonvulsant medications have been used as an alternative to lithium, and these drugs appear to be less detrimental to cognitive functioning. When compared to bipolar patients treated with lithium, patients treated with divalproex sodium were noted to perform better on memory tests. 101 Valproate is also thought to promote neurite outgrowth. 99
Some reports suggest positive behavioral effects of carbamazepine; however, research studies have demonstrated unclear results 102 and ineffectiveness 103 in the treatment of aggression. On the other hand, recent research suggests that oxcarbazepine, an analogue of carbazepine, may be effective in treatment-resistant aggression in adults. 104
Adrenergic Agents
Deficits in the noradrenergic system are implicated in the production of hyperarousal. Alpha adrenergic agents can be helpful in targeting aggression related to anxious hyperarousal. Alpha 2-adrenoceptors, which mediate noradrenergic modulation, have been classified into three receptor subtypes (2A, 2B, and 2C), each with a unique distribution. The alpha 2A is found throughout the brain, especially the nucleus coeruleus. The alpha 2B is localized to the thalamus, and the alpha 2C also has wide distribution with an increased distribution in the basal ganglion. 105 Agents like guanfacine are specific to the alpha 2A receptor, whereas clonidine targets all of the alpha-receptors. 106 Recent literature suggests that clonidine is more effective in modulating the noradrenergic system in the prefrontal cortex, premotor cortex, parietal region, and insula and decreasing activating behavioral symptoms versus guanfacine. 107 Postural hypotension, one of the major side effects of clonidine, has been attributed to alpha 2B and 2C activity. 106
Anxiolytics
Benzodiazepines have been integral in the treatment of anxiety disorders and inducing sedation. They enhance the activity of the GABA receptor throughout the CNS, especially those found within the central amygdala complex. 91 Caution surrounds the extensive use because of addiction potential, sedation, and serious consequences when mixed with other agents (e.g., alcohol). Consequently, benzodiazepines should be used cautiously in the treatment of aggression.
Psychosocial and Other Nonpharmacological Interventions
Over 550 psychotherapies have been identified for treating children and adolescents. Many of these have been based on similar principles, and many have never been studied empirically. 71 Additionally, it is important to identify participants who are more likely to follow through the rigors of therapy.
Therapies that enhance behavioral control, such as cognitive behavior therapy, dialectical behavioral therapy, and problem-solving skills training, are shown to benefit aggression associated with impulsivity, affective instability, and anxiety by engaging children in a step-by-step approach to solve interpersonal problems. 71 Similarly, cognitive enhancement therapy based on similar principles has shown improved cognitive function behavior in stable schizophrenics. 49 Because of normal cognitive development, meta-analysis indicated children need to be 10 or 11 years old before they begin to benefit from these therapies. 108
Therapies that help parents alter their child’s behavior, such as parent management training, are shown to benefit aggression associated with predatory aggression in subjects from 2 to 17 years of age and with children with anxiety/hyperarousal aggression. 71 Effective therapy involves a parent who meets regularly with the therapist to learn effective procedures to curb aggressive tendencies.
Therapies that help reduce stress, such as autogenic relaxation training, are found effective in reducing behavioral and emotional problems associated with anxiety/hyperarousal and cognitive disorganization aggression. 109 Likewise, stress reduction in parents is shown to reduce aggression in children referred for aggressive and antisocial behavior. 110
Therapies aimed at creating a health-promoting environment range from school-based interventions, such as small, structured classrooms, to community-based interventions, such as after-school programs, to the highly organized, comprehensive multi-systemic therapy, which benefits all types of aggression, but particularly predatory aggression. 70
As of today, there have not been any studies identifying brain regions and psychological interventions with aggressive adolescents. Promising work by Goldapple et al. 111 has shown, with PET imaging, increased glucose metabolism in the anterior cingulate and hippocampus and decreased activity in the frontal cortex among depressed subjects receiving cognitive behavior therapy compared with depressed subjects receiving paroxetine. Conversely, healthy comparison subjects receiving paroxetine showed an opposite activation pattern. 111 Improved anterior cingulate function (emotional processing and decision making) and lessened frontal cortex function (proposed cognitive behavior therapy-related, learned ability to reduce sensory stimuli) 111 may likely occur within other symptom domains and similarly would provide similar cognitive support.
Discussion
Aggressive behavior in youth can be broken down into five primary symptom domains with underlying neurodevelopmental underpinnings relevant for targeted treatment programs. By focusing on these symptom domains and brain circuits, the clinician can plan a more strategic intervention which is increasingly able to target the origin or origins of aggressive behavior.
Impulsive aggression benefits from treatments that affect the prefrontal cortex, the basal ganglia, and the serotonin neurotransmitter system. Affective instability-related aggression benefits from treatments that affect the temporal lobe and the cerebellum, although the prefrontal cortex and the basal ganglia are clearly involved. Anxious/hyperarousal-based aggression benefits from treatments that modulate the prefrontal cortex and the amygdala through the noradrenergic, and to some extent the serotonergic neurotransmitter systems. Cognitive disorganization-related aggression, the broadest of the symptom domains, benefits from treatments that affect the prefrontal cortex, thalamus, basal ganglia, and the temporal lobes through the serotonin and dopamine neurotransmitter systems. Predatory aggression responds best to treatments that affect temporal lobe structures and the basal ganglia, and enhance prefrontal function, particularly through the dopamine neurotransmitter systems.
This model has limitations. Clearly, there is overlap in particular brain regions for each of these domains, and the symptoms also have some overlap, although perhaps less if sequencing is taken into consideration. Greater understanding of subregions and complex circuitry between regions will likely help identify the more specific networks involved in the “surface” or target symptom. This process, in turn, might help define the biomarkers used to define the target symptom.
Though more research is necessary to further elucidate the neurobiology and to validate the specific treatment response of aggression, the clinical utility of dissecting the particular symptom domains of aggression by using neurobiological and neurodevelopmental models is a logical approach to a complex problem.
1. American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th ed, Text-Revision (DSM-IV-TR). Washington, DC, American Psychiatric Association, 2000Google Scholar
2. Miczek KA, Fish EW, De Bold JF, et al: Social and neural determinants of aggressive behavior: pharmacotherapeutic targets at serotonin, dopamine and gamma-aminobutyric acid systems. Psychopharmacology (Berl) 2002; 163 : 434–458 Google Scholar
3. Moyer K: The Psychobiology of Aggression. New York, Harper & Row, 1976Google Scholar
4. Vitiello B, Stoff DM: Subtypes of aggression and their relevance to child psychiatry. J Am Acad Child Adolesc Psychiatry 1997; 36 : 307–315 Google Scholar
5. Pennington BF: The Development of Psychopathology: Nature and Nurture. New York, Guilford, 2002Google Scholar
6. Moeller FG, Barratt ES, Dougherty DM, et al: Psychiatric aspects of impulsivity. Am J Psychiatry 2001; 158 : 1783–1793 Google Scholar
7. Siever LJ, Buchsbaum MS, New AS, et al: d,l-fenfluramine response in impulsive personality disorder assessed with [18F]fluorodeoxyglucose positron emission tomography. Neuropsychopharmacology 1999; 20 : 413–423 Google Scholar
8. Max JE, Robertson BA, Lansing AE: The phenomenology of personality change due to traumatic brain injury in children and adolescents. J Neuropsychiatry Clin Neurosci 2001; 13:161–170Google Scholar
9. Brent DA, Oquendo M, Birmaher B, et al: Peripubertal suicide attempts in offspring of suicide attempters with siblings concordant for suicidal behavior. Am J Psychiatry 2003; 160:1486–1493Google Scholar
10. Spear LP: The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev 2000; 24 : 417–463 Google Scholar
11. Barkley RA: Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol Bull 1997; 121 : 65–94 Google Scholar
12. Volavka J: The neurobiology of violence: an update. J Neuropsychiatry Clin Neurosci 1999; 11 : 307–314 Google Scholar
13. Bechara A: Neurobiology of decision-making: risk and reward. Semin Clin Neuropsychiatry 2001; 6 : 205–216 Google Scholar
14. Pietrini P, Guazzelli M, Basso G, et al: Neural correlates of imaginal aggressive behavior assessed by positron emission tomography in healthy subjects. Am J Psychiatry 2000; 157 : 1772–1781 Google Scholar
15. Hollander E, Evers M: New developments in impulsivity. Lancet 2001; 358 : 949–650 Google Scholar
16. Bechara A, Damasio H, Damasio AR: Role of the amygdala in decision-making. Ann N Y Acad Sci 2003; 985 : 356–369 Google Scholar
17. Davidson RJ, Putnam KM, Larson CL: Dysfunction in the neural circuitry of emotion regulation—a possible prelude to violence. Science 2000; 289 : 591–594 Google Scholar
18. Hinshaw SP: Impulsivity, emotion regulation, and developmental psychopathology: specificity versus generality of linkages. Ann N Y Acad Sci 2003; 1008 : 149–159 Google Scholar
19. Higley JD, Linnoila M: Low central nervous system serotonergic activity is traitlike and correlates with impulsive behavior: a nonhuman primate model investigating genetic and environmental influences on neurotransmission. Ann N Y Acad Sci 1997; 836 : 39–56 Google Scholar
20. Kruesi MJ, Rapoport JL, Hamburger S, et al: Cerebrospinal fluid monoamine metabolites, aggression, and impulsivity in disruptive behavior disorders of children and adolescents. Arch Gen Psychiatry 1990; 47 : 419–426 Google Scholar
21. Krakowski M: Violence and serotonin: influence of impulse control, affect regulation, and social functioning. J Neuropsychiatry Clin Neurosci 2003; 15 : 294–305 Google Scholar
22. Hollander E, DeCaria CM, Finkell JN, et al: A randomized double-blind fluvoxamine/placebo crossover trial in pathologic gambling. Biol Psychiatry 2000; 47 : 813–817 Google Scholar
23. Evans J, Reeves B, Platt H, et al: Impulsiveness, serotonin genes and repetition of deliberate self-harm (DSH). Psychol Med 2000; 30 : 1327–1334 Google Scholar
24. Brunner D, Hen R: Insights into the neurobiology of impulsive behavior from serotonin receptor knockout mice. Ann N Y Acad Sci 1997; 836 : 81–105 Google Scholar
25. Leibenluft E, Charney DS, Towbin KE, et al: Defining clinical phenotypes of juvenile mania. Am J Psychiatry 2003; 160 : 430–437 Google Scholar
26. Fox NA: Temperament and regulation of emotion in the first years of life. Pediatrics 1998; 102 : 1230–1235 Google Scholar
27. Bhangoo RK, Leibenluft E: Affective neuroscience and the study of normal and abnormal emotion regulation. Child Adolesc Psychiatr Clin N Am 2002; 11 : 519–532 Google Scholar
28. Wozniak J: Pediatric bipolar disorder: the new perspective on severe mood dysfunction in children. J Child Adolesc Psychopharmacol 2003; 13 : 449–451 Google Scholar
29. Phillips ML, Drevets WC, Rauch SL, et al: Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biol Psychiatry 2003; 54 : 515–528 Google Scholar
30. Nakaji P, Meltzer HS, Singel SA, et al: Improvement of aggressive and antisocial behavior after resection of temporal lobe tumors. Pediatrics 2003; 112 : e430 Google Scholar
31. Blumberg HP, Kaufman J, Martin A, et al: Amygdala and hippocampal volumes in adolescents and adults with bipolar disorder. Arch Gen Psychiatry 2003; 60 : 1201–1208 Google Scholar
32. Dougherty DD, Shin LM, Alpert NM, et al: Anger in healthy men: a PET study using script-driven imagery. Biol Psychiatry 1999; 46 : 466–472 Google Scholar
33. Critchley HD, Simmons A, Daly EM, et al: Prefrontal and medial temporal correlates of repetitive violence to self and others. Biol Psychiatry 2000; 47 : 928–934 Google Scholar
34. Wagner KD, Ambrosini PJ: Childhood depression: pharmacological therapy/treatment (pharmacotherapy of childhood depression). J Clin Child Psychol 2001; 30 : 88–97 Google Scholar
35. Melia KR, Duman RS: Involvement of corticotropin-releasing factor in chronic stress regulation of the brain noradrenergic system. Proc Natl Acad Sci U S A; 1991; 88:8382–8386Google Scholar
36. Uno H, Tarara R, Else JG, et al: Hippocampal damage associated with prolonged and fatal stress in primates. J Neurosci 1989; 9 : 1705–1711 Google Scholar
37. Luine V, Villegas M, Martinez C, et al: Repeated stress causes reversible impairments of spatial memory performance. Brain Res 1994; 639 : 167–170 Google Scholar
38. Bremner JD: Long-term effects of childhood abuse on brain and neurobiology. Child Adolesc Psychiatr Clin N Am 2003; 12 : 271–292 Google Scholar
39. Teicher MH, Andersen SL, Polcari A, et al: The neurobiological consequences of early stress and childhood maltreatment. Neurosci Biobehav Rev 2003; 27 : 33–44 Google Scholar
40. Herman JP, Cullinan WE: Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci 1997; 20 : 78–84 Google Scholar
41. Amaral DG, Corbett BA: The amygdala, autism and anxiety. Novartis Found Symp 2003; 251 : 177–187; discussion 187–197, 281–297 Google Scholar
42. Pavic L, Gregurek R, Petrovic R, et al: Alterations in brain activation in posttraumatic stress disorder patients with severe hyperarousal symptoms and impulsive aggressiveness. Eur Arch Psychiatry Clin Neurosci 2003; 253 : 80–83 Google Scholar
43. Dunn JD, Whitener J: Plasma corticosterone responses to electrical stimulation of the amygdaloid complex: cytoarchitectural specificity. Neuroendocrinology 1986; 42 : 211–217 Google Scholar
44. Takao K, Nagatani T, Kitamura Y, et al: Effects of corticosterone on 5-HT1A and 5-HT2 receptor binding and on the receptor-mediated behavioral responses of rats. Eur J Pharmacol 1997; 333 : 123–128 Google Scholar
45. Gingrich JA, Hen R: Dissecting the role of the serotonin system in neuropsychiatric disorders using knockout mice. Psychopharmacology (Berl) 2001; 155 : 1–10 Google Scholar
46. Donnelly CL: Pharmacologic treatment approaches for children and adolescents with posttraumatic stress disorder. Child Adolesc Psychiatr Clin N Am 2003; 12 : 251–269 Google Scholar
47. Abercrombie ED, Jacobs BL: Single-unit response of noradrenergic neurons in the locus coeruleus of freely moving cats: II: adaptation to chronically presented stressful stimuli. J Neurosci 1987; 7 : 2844–2848 Google Scholar
48. Lewis DO, Moy E, Jackson LD, et al: Biopsychosocial characteristics of children who later murder: a prospective study. Am J Psychiatry 1985; 142 : 1161–1167 Google Scholar
49. Hogarty GE, Flesher S, Ulrich R, et al: Cognitive enhancement therapy for schizophrenia: effects of a 2-year randomized trial on cognition and behavior. Arch Gen Psychiatry 2004; 61 : 866–876 Google Scholar
50. Foley SR, Kelly BD, Clarke M, et al: Incidence and clinical correlates of aggression and violence at presentation in patients with first episode psychosis. Schizophr Res 2005; 72 : 161–168 Google Scholar
51. Moran P, Walsh E, Tyrer P, et al: Impact of comorbid personality disorder on violence in psychosis: report from the UK700 trial. Br J Psychiatry 2003; 182 : 129–134 Google Scholar
52. Nelson MD, Saykin AJ, Flashman LA, et al: Hippocampal volume reduction in schizophrenia as assessed by magnetic resonance imaging: a meta-analytic study. Arch Gen Psychiatry 1998; 55 : 433–440 Google Scholar
53. Pearlson GD, Marsh L: Structural brain imaging in schizophrenia: a selective review. Biol Psychiatry 1999; 46 : 627–649 Google Scholar
54. Lipska BK, Weinberger DR: To model a psychiatric disorder in animals: schizophrenia as a reality test. Neuropsychopharmacology 2000; 23 : 223–239 Google Scholar
55. Heckers S, Rauch SL, Goff D, et al: Impaired recruitment of the hippocampus during conscious recollection in schizophrenia. Nat Neurosci 1998; 1 : 318–323 Google Scholar
56. Weinberger DR, Berman KF, Suddath R, et al: Evidence of dysfunction of a prefrontal-limbic network in schizophrenia: a magnetic resonance imaging and regional cerebral blood flow study of discordant monozygotic twins. Am J Psychiatry 1992; 149 : 890–897 Google Scholar
57. Combs DR, Penn DL: The role of subclinical paranoia on social perception and behavior. Schizophr Res 2004; 69 : 93–104 Google Scholar
58. Lindstrom LH, Gefvert O, Hagberg G, et al: Increased dopamine synthesis rate in medial prefrontal cortex and striatum in schizophrenia indicated by L-(beta-11C) DOPA and PET. Biol Psychiatry 1999; 46 : 681–688 Google Scholar
59. Breier A, Su TP, Saunders R, et al: Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations: evidence from a novel positron emission tomography method. Proc Natl Acad Sci U S A 1997; 94 : 2569–2574 Google Scholar
60. Carlsson A, Waters N, Holm-Waters S, et al: Interactions between monoamines, glutamate, and GABA in schizophrenia: new evidence. Annu Rev Pharmacol Toxicol 2001; 41 : 237–260 Google Scholar
61. Berridge KC, Robinson TE: What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev 1998; 28 : 309–369 Google Scholar
62. Kapur S: Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry 2003; 160 : 13–23 Google Scholar
63. Lehman AF, Lieberman JA, Dixon LB, et al: Practice guideline for the treatment of patients with schizophrenia. Am J Psychiatry 2004; 161 : 1–56 Google Scholar
64. Vitaro F, Brendgen M, Tremblay RE: Reactively and proactively aggressive children: antecedent and subsequent characteristics. J Child Psychol Psychiatry 2002; 43:495–505Google Scholar
65. Raine A, Meloy JR, Bihrle S, et al: Reduced prefrontal and increased subcortical brain functioning assessed using positron emission tomography in predatory and affective murderers. Behav Sci Law 1998; 16 : 319–332 Google Scholar
66. Raine A, Lencz T, Bihrle S, et al: Reduced prefrontal gray matter volume and reduced autonomic activity in antisocial personality disorder. Arch Gen Psychiatry 2000; 57 : 119–127; discussion 128–129 Google Scholar
67. Smith DA, Flynn JP: Afferent projections to quiet attack sites in cat hypothalamus. Brain Res 1980; 194 : 29–40 Google Scholar
68. Shaikh MB, Lu CL, MacGregor M, et al: Dopaminergic regulation of quiet biting attack behavior in the cat. Brain Res Bull 1991; 27 : 725–730 Google Scholar
69. Schultz W, Dayan P, Montague PR: A neural substrate of prediction and reward. Science 1997; 275 : 1593–1599 Google Scholar
70. Henggeler SW, Schoenwald SK, Rowland MD, et al: Serious Emotional Disturbance in Children and Adolescents: Multisystemic Therapy. New York, Guilford, 2002Google Scholar
71. Kazdin AE: Treatments for aggressive and antisocial children. Child Adolesc Psychiatr Clin N Am 2000; 9 : 841–858 Google Scholar
72. Pliszka SR: Psychiatric comorbidities in children with attention deficit hyperactivity disorder: implications for management. Paediatr Drugs 2003; 5 : 741–750 Google Scholar
73. McDougle CJ, Stigler KA, Posey DJ: Treatment of aggression in children and adolescents with autism and conduct disorder. J Clin Psychiatry 2003; 64(suppl 4) : 16–25 Google Scholar
74. Klein RG, Abikoff H, Klass E, et al: Clinical efficacy of methylphenidate in conduct disorder with and without attention deficit hyperactivity disorder. Arch Gen Psychiatry 1997; 54 : 1073–1080 Google Scholar
75. Connor DF, Barkley RA, Davis HT: A pilot study of methylphenidate, clonidine, or the combination in ADHD comorbid with aggressive oppositional defiant or conduct disorder. Clin Pediatr (Phila) 2000; 39 : 15–25 Google Scholar
76. Steketee JD: Neurotransmitter systems of the medial prefrontal cortex: potential role in sensitization to psychostimulants. Brain Res Brain Res Rev 2003; 41 : 203–228 Google Scholar
77. Volkow ND, Fowler JS, Wang G, et al: Mechanism of action of methylphenidate: insights from PET imaging studies. J Atten Disord 2002; 6(suppl 1):31–43Google Scholar
78. Durston S: A review of the biological bases of ADHD: what have we learned from imaging studies? Ment Retard Dev Disabil Res Rev 2003; 9 : 184–195 Google Scholar
79. Castellanos FX: Toward a pathophysiology of attention-deficit/hyperactivity disorder. Clin Pediatr (Phila) 1997; 36 : 381–393 Google Scholar
80. Arnsten AFT: Dopaminergic and noradrenergic influences on cognitive functions mediated by prefrontal cortex, in Stimulant Drugs and ADHD: Basic and Clinical Neuroscience. Edited by Solanto MV, Arnsten FT. London, Oxford University Press, 2001, pp 185–208Google Scholar
81. Carlson GA, Mick E: Drug-induced disinhibition in psychiatrically hospitalized children. J Child Adolesc Psychopharmacol 2003; 13 : 153–163 Google Scholar
82. Coccaro EF, Kavoussi RJ, Hauger RL: Serotonin function and antiaggressive response to fluoxetine: a pilot study. Biol Psychiatry 1997; 42 : 546–552 Google Scholar
83. Kavoussi RJ, Coccaro EF: Divalproex sodium for impulsive aggressive behavior in patients with personality disorder. J Clin Psychiatry 1998; 59 : 676–680 Google Scholar
84. Bel N, Artigas F: Chronic treatment with fluvoxamine increases extracellular serotonin in frontal cortex but not in raphe nuclei. Synapse 1993; 15 : 243–245 Google Scholar
85. Wegerer V, Moll GH, Bagli M, et al: Persistently increased density of serotonin transporters in the frontal cortex of rats treated with fluoxetine during early juvenile life. J Child Adolesc Psychopharmacol 1999; 9 : 13–24; discussion 25–26 Google Scholar
86. Meyer JH, Kapur S, Eisfeld B, et al: The effect of paroxetine on 5-HT(2A) receptors in depression: an [(18)F]setoperone PET imaging study. Am J Psychiatry 2001; 158 : 78–85 Google Scholar
87. Drevets WC: Prefrontal cortical-amygdalar metabolism in major depression. Ann N Y Acad Sci 1999; 877 : 614–637 Google Scholar
88. van Praag HM, Asnis GM, Kahn RS, et al: Monoamines and abnormal behaviour: a multi-aminergic perspective. Br J Psychiatry 1990; 157 : 723–734 Google Scholar
89. Konradi C, Heckers S: Antipsychotic drugs and neuroplasticity: insights into the treatment and neurobiology of schizophrenia. Biol Psychiatry 2001; 50 : 729–742 Google Scholar
90. Liddle PF, Lane CJ, Ngan ET: Immediate effects of risperidone on cortico-striato-thalamic loops and the hippocampus. Br J Psychiatry 2000; 177 : 402–407 Google Scholar
91. Cohen BM, Cherkerzian S, Ma J, et al: Cells in midline thalamus, central amygdala, and nucleus accumbens responding specifically to antipsychotic drugs. Psychopharmacology (Berl) 2003; 167 : 403–410 Google Scholar
92. McElroy SL: Recognition and treatment of DSM-IV intermittent explosive disorder. J Clin Psychiatry 1999; 60(suppl 15):12–16Google Scholar
93. Hollander E, Dolgoff-Kaspar R, Cartwright C, et al: An open trial of divalproex sodium in autism spectrum disorders. J Clin Psychiatry 2001; 62 : 530–534 Google Scholar
94. Steiner H, Saxena K, Chang K: Psychopharmacologic strategies for the treatment of aggression in juveniles. CNS Spectr 2003; 8 : 298–308 Google Scholar
95. Donovan SJ, Stewart JW, Nunes EV, et al: Divalproex treatment for youth with explosive temper and mood lability: a double-blind, placebo-controlled crossover design. Am J Psychiatry 2000; 157 : 818–820 Google Scholar
96. Hollander E, Tracy KA, Swann AC, et al: Divalproex in the treatment of impulsive aggression: efficacy in cluster B personality disorders. Neuropsychopharmacology 2003; 28 : 1186–1197 Google Scholar
97. Lindenmayer JP, Kotsaftis A: Use of sodium valproate in violent and aggressive behaviors: a critical review. J Clin Psychiatry 2000; 61 : 123–128 Google Scholar
98. Malone RP, Bennett DS, Luebbert JF, et al: Aggression classification and treatment response. Psychopharmacol Bull 1998; 34 : 41–45 Google Scholar
99. Manji HK, Moore GJ, Chen G: Clinical and preclinical evidence for the neurotrophic effects of mood stabilizers: implications for the pathophysiology and treatment of manic-depressive illness. Biol Psychiatry 2000; 48 : 740–754 Google Scholar
100. Moore GJ, Bebchuk JM, Wilds IB, et al: Lithium-induced increase in human brain grey matter. Lancet 2000; 356 : 1241–1242 Google Scholar
101. Pachet AK: Differential neuropychological performance of bipolar mood disorder patients treated with divalproex sodium or lithium carbonate. Dissertation Abstracts Int. 2000; 60Google Scholar
102. Puente RM: The use of carbamazepine in the treatment of behavioural disorders in children, in Epileptic Seizures-Behaviour-Pain. Edited by Bern BW. Switzerland, Hans Huber, 1976, pp 273–247Google Scholar
103. Cueva JE, Overall JE, Small AM, et al: Carbamazepine in aggressive children with conduct disorder: a double-blind and placebo-controlled study. J Am Acad Child Adolesc Psychiatry 1996; 35:480–490Google Scholar
104. Gaudino MP, Smith MJ, Matthews DT: Use of oxcarbazepine for treatment-resistant aggression. Psychiatr Serv 2003; 54:1166–1167Google Scholar
105. MacDonald E, Scheinin M: Distribution and pharmacology of alpha 2-adrenoceptors in the central nervous system. J Physiol Pharmacol 1995; 46:241–258Google Scholar
106. Arnsten AFT, Steere JC, Hunt RD: The contribution of alpha 2-noradrenergic mechanisms of prefrontal cortical cognitive function: potential significance for attention-deficit hyperactivity disorder. Arch Gen Psychiatry 1996; 53:448–455Google Scholar
107. Coull JT, Nobre AC, Frith CD: The noradrenergic alpha2 agonist clonidine modulates behavioural and neuroanatomical correlates of human attentional orienting and alerting. Cereb Cortex 2001; 11:73–84Google Scholar
108. Durlak JA, Fuhrman T, Lampman C: Effectiveness of cognitive-behavior therapy for maladapting children: a meta-analysis. Psychol Bull 1991; 110:204–214Google Scholar
109. Goldbeck L, Schmid K: Effectiveness of autogenic relaxation training on children and adolescents with behavioral and emotional problems. J Am Acad Child Adolesc Psychiatry 2003; 42:1046–1054Google Scholar
110. Kazdin AE, Whitley MK: Treatment of parental stress to enhance therapeutic change among children referred for aggressive and antisocial behavior. J Consult Clin Psychol 2003; 71 : 504–515 Google Scholar
111. Goldapple K, Segal Z, Garson C, et al: Modulation of cortical-limbic pathways in major depression: treatment-specific effects of cognitive behavior therapy. Arch Gen Psychiatry 2004; 61:34–41Google Scholar



