Predictors of Subjective Memory Complaint in Cognitively Normal Relatives of Patients with Alzheimer’s Disease
Previous literature on subjective memory complaint has focused upon either healthy volunteers or population-based cohorts. To our knowledge, subjective memory complaint in the relatives of patients with Alzheimer’s disease has not been previously examined. Because cognitively healthy relatives of patients with Alzheimer’s disease are at higher risk for developing Alzheimer’s disease compared with non-relatives, 7 they provide a unique opportunity to further understand this phenomenon. The goal of this study was to identify the factors associated with subjective memory complaint among cognitively healthy first-degree relatives of Alzheimer’s disease patients.
METHOD
Setting and Subjects
Data for this study were collected as part of the MIRAGE study (Multi-Institutional Research in Alzheimer Genetic Epidemiology), a family-based study of genetic and environmental risk factors for Alzheimer’s disease. The starting point for recruitment of MIRAGE families is the proband: a living individual with probable or definite Alzheimer’s disease as defined by the National Institute of Neurological and Communication Disorders and Stroke (NINCDS)/Alzheimer’s Disease and Related Disorders Association (ADRDA) research criteria. 9 Data were collected between May 1996 and March 2002 at 15 medical centers in the United States, Germany, and Canada, and this analysis utilizes 1,203 first-degree relatives and 296 spouses of Alzheimer’s disease patients who were 50 years of age or older and had no history of cognitive impairment. In order to validate the reports of healthy cognitive status, the modified Telephone Instrument for Cognitive Status (mTICS) 10 , 11 was administered to 1,223 of these participants. Using a cutoff score of ≥27 to define those with healthy cognition, only three individuals (0.2%) were questionable and thus were excluded from the analysis. Multiple informants (medical records including autopsy reports, death certificates, and nursing home records) were used where available to supplement information on both probands and first-degree relatives. The details of the MIRAGE study design, data collection procedures, and protocols for obtaining family histories have been reported elsewhere. 7 , 12 – 15
Data Collection
A structured questionnaire administered by trained staff was used to collect information about demographics, family, medical and social history, including one question to elicit subjective memory complaint. The subjective memory complaint question was, “Do you have trouble remembering things from one second to the next?” Data on other potential predictors also were collected. These included history of depression symptoms and history of diabetes or heart disease. History of depression symptoms was defined as an affirmative response to the question, “Aside from normal reaction to bereavement (death in family) or the impact of a physical/medical condition, has there been a period of weeks to several months (or longer) when you were unable to perform social and occupational functions normally because of depression?” 15 Age was treated as a continuous variable. Ethnicity was categorized as African-American, defined by self-report, or not categorized at all. The relationship of the participant to the Alzheimer’s disease patient was characterized as first-degree kinship (e.g., parent, sibling, or child) or spouse.
Data Analysis
Multivariate models for assessing predictors of subjective memory complaint were evaluated using generalized estimating equations (GEE) with a logit link to account for the family correlation structure of the data set. 16 In the multivariate model, the dependent variable was subjective memory complaint and the independent variables were relationship to the proband, history of depression symptoms, and history of diabetes or heart disease. The element of confounding was evaluated by assessing models with different combinations of covariates and examining the change in association with subjective memory complaint. Odds ratios with 95% confidence intervals (CI) were derived. All analyses were performed using SAS system, v8.2 (SAS Institute, Cary, NC), with PROC GENMOD for GEE modeling.
RESULTS
Table 1 shows the characteristics of the 1,499 participants according to response to the subjective memory complaint question. One hundred thirty-four (8.9%) cognitively healthy relatives (this figure includes both first-degree relatives and spouses) answered “yes” to the subjective memory complaint question. Participants who endorsed subjective memory complaint were similar in mean age and sex distribution to those who did not.
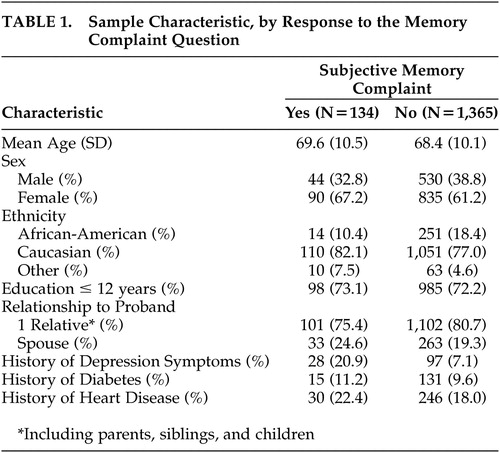 |
Multivariate analysis ( Table 2 ) revealed that first-degree relatives were significantly more likely to report subjective memory complaint than spouses (odds ratio=1.9, 95% CI 1.3, 3.0). Relatives with a history of depression symptoms were more likely to report subjective memory complaint than those without such history (odds ratio=3.5, 95% CI 2.1, 5.7). A history of either diabetes or heart disease was not significantly associated with subjective memory complaint.
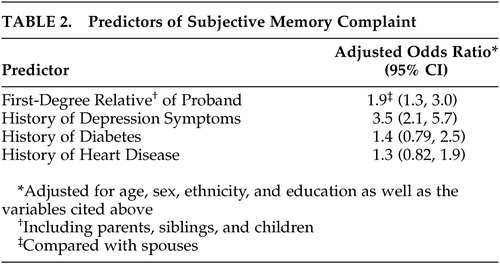 |
DISCUSSION
Both first-degree biological kinship and depression symptoms were predictors of subjective memory complaint in cognitively healthy family members of patients with Alzheimer’s disease. First-degree relatives were nearly twice as likely to report subjective memory complaint, and relatives reporting a history of depression symptoms were more than three times as likely to report subjective memory complaint. Subjective memory complaint has been associated with increased risk of subsequent dementia in some, but not all studies. In a large sample of healthy elderly Dutch, subjective memory complaint was significantly associated with the development of dementia within 3 years. 17 This association was stronger in those subjects who had both memory complaints and poor memory performance. In an Australian community sample, subjective memory complaint did not appear to predict cognitive change or the development of dementia over nearly 4 years of follow-up, once anxiety and depression symptoms were taken into account. 18 However, we found the opposite results when we reanalyzed their data with more sophisticated modeling techniques. 19 A recent longitudinal study used a single question to assess memory complaints with an average follow-up of 3.2 years and found that subjective memory complaint was a relatively strong predictor of incident Alzheimer’s disease in older persons in whom cognitive impairment was not yet apparent. 20 In a review of clinical and population-based studies, Jonker et al. 21 reported an association between memory complaints and memory impairment in elderly subjects, after adjustment for depressive symptomatology.
In several community-based studies, subjective memory complaint has been found to be associated with depression symptoms, 22 , 23 and in the MIRAGE data set (the same data set utilized for this study), depression symptoms occurring even 25 years prior to dementia were associated with an increased risk of developing Alzheimer’s disease. 15 These reports suggest that depression symptoms cannot only be part of the preclinical phase of Alzheimer’s disease, but may also be an early risk factor for later development of Alzheimer’s disease.
Our participants were only evaluated at one point in time; thus, we cannot comment on the predictive value of subjective memory complaint. However, we have shown two significant associations with subjective memory complaint among relatives of patients with Alzheimer’s disease. Consistent with some of the cross-sectional studies above, subjective memory complaint among cognitively healthy relatives of Alzheimer’s disease patients was associated with a prior history of depression symptoms. Since memory complaint (with or without memory impairment) is a common symptom of depression, 24 , 25 this suggests that subjective memory complaint in the presence of healthy cognition should trigger an evaluation for depression.
Subjective memory complaint was also more common among first-degree biological relatives of Alzheimer’s disease patients than among their spouses. The interpretation of this is unclear. One explanation is that since biological relatives of Alzheimer’s disease patients are at higher risk of developing Alzheimer’s disease than are spouses of Alzheimer’s disease patients, 7 the higher proportion of subjective memory complaint could reflect the sub-clinical manifestation of Alzheimer’s disease. Alternatively, since Alzheimer’s disease is now widely recognized as having a heritable component, 7 , 26 biological relatives may be more sensitized to memory lapses than spouses and may report them more frequently.
Our study is limited by the cross-sectional nature of the data collection, as noted above. Cognitive testing to confirm self-report of normal cognition was only available in a subset of the participants; however, the correlation between self-report and telephone cognitive testing was very high. The use of a single-item, self-reported measure to examine a complex construct, such as depression, may not be accurate enough to avoid misclassifications. Moreover, we have emphasized that we assessed “depression symptoms” rather than depression. Nonetheless, previous research on depression screening has found that a two-item measure, 27 or even a single question, 28 can be an effective screen for depression. In our study, relatively few participants endorsed subjective memory complaint compared with population prevalence studies that have reported a prevalence of 25% or more. 29 Self-selection may have resulted in a healthier sample with fewer complaints of memory problems than in the general population. However, to our knowledge this is the first study to examine the predictors of subjective memory complaints in cognitively healthy relatives of Alzheimer’s disease patients. Longitudinal studies of relatives are needed to define the association between subjective memory complaint and Alzheimer’s disease in this at-risk population.
Based on what is known about the predictors of subjective memory complaints among cognitively healthy relatives of patients with Alzheimer’s disease, when family members present such complaints, clinicians should evaluate them for memory impairment, since memory complaints may herald the onset of Alzheimer’s disease. If objective memory testing reveals no impairment, then depression should be considered as an alternative explanation for the complaint.
1. O’Connor DW, Pollitt PA, Roth M, et al: Memory complaints and impairment in normal, depressed, and demented elderly persons identified in a community survey. Arch Gen Psychiatry 1990; 47:224–227Google Scholar
2. Bassett S, Folstein M: Memory complaint, memory performance, and psychiatric diagnosis: a community study. J Geriatr Psychol Neurol 1993; 6:105–111Google Scholar
3. Gagnon M, Dartigues JF, Mazaux JM, et al: Self-reported memory complaints and memory performance in elderly French community residents: results of the Paquid research program. Neuroepidemiology 1994; 13:145–154Google Scholar
4. Tobiansky R, Blizard R, Livingston G: The gospel Oak study stage IV: the clinical relevance of subjective memory impairment in older people. Psychol Med 1995; 25:779–786Google Scholar
5. Jonker C, Launder L, Hooijer C, et al: Memory complaints and memory impairment in older individuals. J Am Geriatr Soc 1996; 44:46–49Google Scholar
6. Blazer D, Hays J, Fillenbaum G, et al: Memory complaint as a predictor of cognitive decline. J Aging Health 1997; 9:171–184Google Scholar
7. Green RC, Cupples LA, Go R, et al: Risk of dementia among white and African American relatives of patients with Alzheimer’s disease. J Am Med Assoc 2002; 287:329–336Google Scholar
8. Green RC: Risk assessment for Alzheimer’s disease with genetic susceptibility testing: has the moment arrived? Alzheimer’s Care Quarterly 2002; 3:208–214Google Scholar
9. McKhann G, Drachman D, Folstein M, et al: Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA work group. Neurology 1984; 34:939–944Google Scholar
10. Brandt J, Spencer M, Folstein M: The telephone interview for cognitive status. Neuropsychiatry Neuropsychol Behav Neurol 1988; 1:111–117Google Scholar
11. Welsh KA, Breitner JCS, Magruder-Habib KM: Detection of dementia in the elderly using telephone screening of cognitive status. Neuropsychiatry Neuropsychol Behav Neurol 1993; 6:103–110Google Scholar
12. Farrer LA, Cupples LA, Blackburn S, et al: Interrater agreement for diagnosis of Alzheimer’s disease: the MIRAGE study. Neurology 1994; 44:652–656Google Scholar
13. Lautenschlager NT, Cupples LA, Rao VS, et al: Risk of dementia among relatives of Alzheimer’s disease patients in the MIRAGE study: what is in store for the oldest old? Neurology 1996; 46:641–650Google Scholar
14. Demissie S, Green RC, Mucci L, et al: Reliability of information collected by proxy in family studies of Alzheimer’s disease. Neuroepidemiology 2001; 20:105–111Google Scholar
15. Green RC, Cupples LA, Kurz A, et al: Depression as a risk factor for Alzheimer’s disease: the MIRAGE study. Arch Neurol 2003; 60:753–759Google Scholar
16. Zeger S, Liang K-Y: Longitudinal data analysis for discrete and continuous outcomes. Biometrics 1986; 42:121–130Google Scholar
17. Schmand B, Lindeboom J, Launer L, et al: What is a significant score change on the Mini-Mental State Examination? Int J Geriatr Psychiatry 1995; 10:411–414Google Scholar
18. Jorm AF, Christensen H, Korten AE: Do cognitive complaints either predict future cognitive decline or reflect past cognitive decline? a longitudinal study of an elderly community sample. Psychol Med 1997; 27:91–98Google Scholar
19. Jorm AF, Christensen H, Korten AE, et al: Memory complaints as a predictor of memory impairment in older people: a longitudinal analysis over 7–8 years. Psychol Med 2001; 31:441–449Google Scholar
20. Geerling M, Jonker C, Bouter L, et al: Association between memory complaints and incident Alzheimer’s disease in elderly people with normal baseline cognition. Am J Psychiatry 1999; 156:531–537Google Scholar
21. Jonker C, Geerlings MI, Schmand B: Are memory complaints predictive for dementia? a review of clinical and population-based studies. Int J Geriatr Psychiatry 2000; 15:983–991Google Scholar
22. Bolla K, Lindgren K, Bonaccorsy C, et al: Memory complaints in older adults: fact or fiction? Arch Neurol 1991; 48:61–64Google Scholar
23. Grut M, F JA, Fratiglioni L, et al: Memory complaints of elderly people in a population survey: variation according to dementia stage and depression. J Am Geriatr Soc 1993; 139:1295–1300Google Scholar
24. Grossman I, Kaufman AS, Mednitsky S, et al: Neurocognitive abilities for a clinically depressed sample versus a matched control group of normal individuals. Psychiatry Res 1994; 51:231–244Google Scholar
25. Brown RG, Scott LC, Bench CJ, et al: Cognitive function in depression: its relationship to the presence and severity of intellectual decline. Psychol Med 1994; 24:829–847Google Scholar
26. Farrer LA: Genetics and the dementia patient. Neurologist 1997; 3:13–30Google Scholar
27. Whooley MA, Avins AL, Miranda J, et al: Case-finding instruments for depression: two questions are as good as many. J Gen Intern Med 1997; 12:439–445Google Scholar
28. Mahoney J, Drinka TJK, Abler R, et al: Screening for depression: single question versus GDS. J Am Geriatr Soc 1994; 42:1006–1008Google Scholar
29. Dik MG, Jonker C, Comijs HC, et al: Memory complaints and APOE-epsilon4 accelerate cognitive decline in cognitively healthy elderly. Neurology 2001; 57:2217–2222Google Scholar



