Delta Power in Sleep in Relation to Neuropsychological Performance in Healthy Subjects and Schizophrenia Patients
In addition to its relationship to sleep homeostasis, delta wave activity is considered an important aspect of sleep in the link to cognitive performance. Bodizs et al. 4 found an association between temporo-lateral low delta (<1.25 Hz) activity during sleep and visual memory performance in epileptic patients. Anderson and Horne 5 described correlations between frontal low delta (<1 Hz) sleep EEG and neuropsychological tests specific to the prefrontal cortex. Further, a local increase in delta activity (1 to 4 Hz) during sleep after learning correlated with improved performance in an implicit learning paradigm the next morning. 6
Schizophrenia is characterized by psychotic and deficit symptoms, such as cognitive impairments. We recently described a correlation of visuospatial memory impairments and a decrease in the amount of slow wave sleep (SWS) in schizophrenia. 7 Furthermore, in addition to disturbances, especially of non-REM (rapid eye movement) sleep, 8 a decrease in delta (0.5–4 Hz) activity during sleep has been reported. 9 Therefore, we performed delta power analyses in sleep in relation to neuropsychological tasks in healthy subjects and schizophrenia patients. We hypothesized that delta activity is associated with neuropsychological performance in healthy subjects and that this link might be impaired in schizophrenia. Because cognitive symptoms play a major role in quality of life and predict functional outcome in schizophrenia, 10 research in this field is essential and important.
METHOD
Subjects
The original sample comprised 17 schizophrenia inpatients (seven women) diagnosed according to the International Classification of Diseases (subtypes: paranoid [N=15], hebephrenic [N=1], undifferentiated [N=1]) and 17 healthy subjects matched for age, sex and years of education according to the German school system. In one patient only two EEG channels were admitted, so he had to be excluded from spectral analysis. All patients were on a stable regimen of the antipsychotic amisulpride (median dose=600mg, range=100 to 800mg). Three patients received a concomitant sedative medication with zolpidem (10mg/day; two patients) or diazepam (7.5mg/day; one patient). Any participant with a relevant additional medical condition was excluded by medical history, physical examination, and routine laboratory investigation, including urinary drug screening. All participants gave their informed written consent. The study was approved by the local ethics committee and conforms to the Declaration of Helsinki, Finland.
Procedure
Each subject spent two nights in our sleep laboratory. The first night was used for adaptation to the conditions in the sleep laboratory. Sleep was recorded between lights off (regulated by the subjects themselves between 10 p.m. and 12 p.m.) and lights on (at 6:45 a.m.). Prior to polysomnography of the second night and the next morning at 7:30 a.m., we performed neuropsychological tasks. Sleep was recorded employing standard procedures, and the following parameters were measured: Electroencephalographic activity (EEG: Fz, F3, F4, F7, F8, C3, C4, T3, T4, T5, T6, Pz, P3, P4, O1, O2 according to the 10–20 system), electrooculographic activity (EOG) and submental electromyographic activity (EMG). Recordings were visually scored according to standard criteria 11 by a trained rater under blind conditions. These results are described elsewhere. 7
For spectral analysis all EEG electrodes mentioned above were referenced against Fpz. The fast Fourier transform (FFT) algorithm was used, and the truncating error was reduced by applying a Hanning window. The EEG signals were A/D converted at 100 samples per second with a 12 bit resolution. Only artifact-free epochs of 30-second duration were analyzed. The log-transformed absolute power values of the delta range (0.5–4 Hz) of each sleep stage and of all channels were used for further analysis.
Neuropsychological Tasks
We used Trail Making Tests A and B for testing attention, cognitive flexibility and psychomotor speed in the evening and in the morning. 12 Declarative memory was assessed by the nonverbal Rey-Osterrieth Complex Figure Test (copy in the evening and announced recall in the morning). 13 For testing procedural learning we used a mirror-tracing skill. Subjects were to trace carefully inside the doubled lines of a triangle (for warming up, data not evaluated) and a star in the evening and in the morning. Direct visual access to the platform with the figures was prevented by a 30- by 22-cm board. The figures could only be seen via a 26- by 18-cm mirror. We assessed the overnight improvement (ratio of drawing time in the morning to drawing time in the evening). Aspects of spatial memory were measured by a test established by Smith and Milner. 14 Eleven toys are placed on a board. In the evening the only task is to guess on the retail price of each item. In the morning the task is to correctly replace all 11 items on the empty board. The distance between the assigned and original position of the objects is measured, and all numbers are added up. For measurement of executive function we performed the Tower of Hanoi (four disk version). To assess verbal intelligence, we used the Mehrfach-Wortschatz-Intelligenz-Test (Multiple Choice Word Fluency Test; MWT-B). 15
Statistics
As the data do not constantly meet criteria for normal distribution, we used nonparametric statistics and provide median and range as descriptives. A Mann-Whitney test (two-tailed) was used to compare healthy subjects and patients. The relationship between sleep and neuropsychological parameters was examined by nonparametric correlations (Spearman-Rho, two-tailed). The level of significance was set at 5%. No adjustment of the error probabilities for multiple testing was performed because of the explorative nature of the study. Therefore, all results have been treated with particular caution and have been considered for their consistency with other results.
RESULTS
Delta Power in Healthy Subjects Versus Schizophrenia Patients
There were no significant differences between healthy subjects and schizophrenia patients regarding age (31 years [24 to 43 years] versus 31 years [22 to 44 years], respectively), premorbid verbal intelligence (107% [92% to 136%] versus 112% [97% to 143%], respectively) and education (13 years [9 to 13 years] versus 12 years [9 to 13 years], respectively; always median and range). The median of the Positive and Negative Syndrome Scale (PANSS) total score 16 was 77 (range=52 to 112). In almost all neuropsychological tasks patients performed significantly worse in the morning and in the evening; only mirror tracing revealed no significant differences between patients and comparison subjects. 7 Delta power in SWS was significantly reduced in schizophrenia patients in comparison to matched healthy subjects in temporal and occipital channels ( Table 1 ). There were no significant differences of delta power in Stage 2 sleep and REM sleep in patients versus comparison subjects.
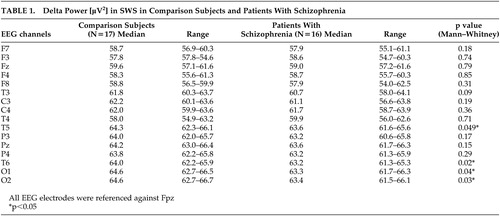 |
Interrelationship Between Delta Power and Neuropsychological Performance in Healthy Subjects
There were no significant correlations between performance of any neuropsychological task and age, verbal intelligence, or education level. As shown in Table 2 , all significant correlations in healthy subjects were seen in this way: the higher the delta power in sleep, the better the waking performance or overnight improvement in different neuropsychological tasks. A highly significant correlation was seen between recall of the Rey figure and delta power in SWS in a left frontal channel. A high number of significant correlations was found between neuropsychological tasks and delta power in SWS. There was a lower number regarding Stage 2 sleep and only few concerning REM sleep. Delta power in SWS or Stage 2 sleep was mainly significantly correlated with performance in tests carried out in the morning. Most of the significant correlations were found in frontal channels.
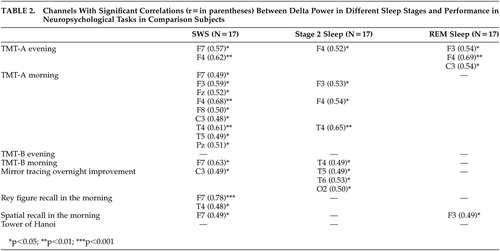 |
Interrelationship Between Delta Power and Neuropsychological Performance in Schizophrenia Patients and Healthy Comparison Subjects
There were no significant correlations between age, total PANSS score or dosage of amisulpride and performance in any neuropsychological task in schizophrenia patients. Similar to the results of healthy subjects, patients with schizophrenia showed the highest number of significant correlations in neuropsychological performance with delta power in SWS. There were only few significant correlations of neuropsychological performance and delta power in Stage 2 sleep and REM sleep ( Table 3 ).
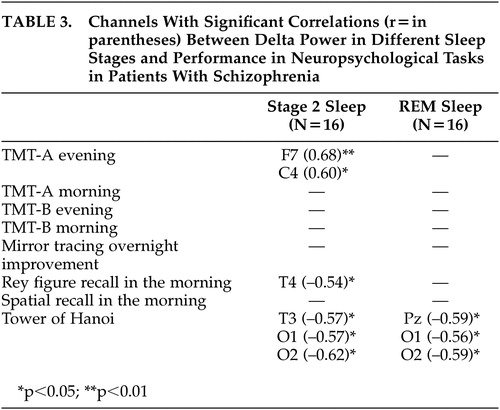 |
Because five of 16 patients with schizophrenia showed a duration of SWS of less than 4 minutes, only 11 patients (all with duration of SWS of longer than 18 minutes) were included in the correlation analyses of delta power in SWS compared with 11 healthy subjects matched for age, sex, and education level ( Table 4 ). For SWS in both groups significant positive correlations were found with TMT-A, in comparison subjects mainly with morning execution and in schizophrenia patients mainly with performance in the evening. Whereas a predominance of significant positive correlations between neuropsychological performance and delta power in frontal channels was seen in healthy subjects, this was not so in schizophrenia patients. In contrast to healthy subjects, schizophrenia patients’ better performance on the Tower of Hanoi puzzle correlated with a lower delta power in different sleep stages.
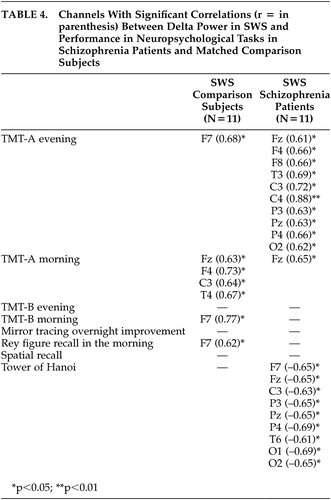 |
DISCUSSION
To our knowledge, this is the first report studying the interrelationship of delta power in sleep and neuropsychological performance in healthy subjects and schizophrenia patients . For healthy subjects, the main findings were significant positive correlations between performance in declarative memory, procedural learning, and attention/cognitive flexibility with delta power, especially in SWS and in frontal channels. In schizophrenia patients, significant positive correlations were only seen between delta power and performance in attention/cognitive flexibility. In addition, significant negative correlations were found to exist between performance in executive functions and delta power in sleep.
The findings of significant correlations between delta power in sleep and performance in neuropsychological tasks in healthy subjects are in line with recent published studies. Power in the delta or low delta range was also associated with performance in recall of the Rey figure in epileptic patients, 4 with performance in tests specific to the prefrontal cortex in older people 5 and with improvement in a procedural learning task in young healthy subjects. 6 Although Anderson and Horne 5 reported a strong link between frontal low delta power in non-REM sleep and a Tower test in old healthy subjects, we did not find this in young healthy subjects. This might be due to a different test procedure, because only a three-disk version applied to schizophrenia patients was preferentially sensitive to frontal lobe lesions, while the four-disk version used in our study was sensitive to basal ganglia disease. 17
Because slow wave activity is the basis of non-REM sleep, it is not surprising that an association of performance in neuropsychological tasks was mainly found with delta power in SWS and Stage 2 sleep, but rarely in REM sleep. The highly significant correlation of performance in recall of the Rey figure and delta power in SWS in healthy subjects supports earlier studies that emphasize the importance of SWS versus REM sleep in the consolidation of declarative memory. 18 The association of improvement of performance in a mirror tracing skill with delta power in SWS and Stage 2 sleep confirms the importance of non-REM sleep also in consolidation of procedural memory. 3 The predominance of significant results in frontal channels in healthy subjects in our study is in line with other studies emphasizing the importance of frontal regions for performance of the Trail Making Test 19 and for declarative memory processes. 20 , 21
It was demonstrated by intracellular recordings in cats that certain oscillations in SWS enhance the responsiveness of thalamic and cortical neurons and it was therefore assumed that SWS may consolidate memory traces acquired during wakefulness in corticothalamic networks. 22 The intense discharge of neocortical neurons during the depolarization phase of the slow oscillations might provide signals that are used in synaptic reorganization, plasticity and the consolidation of information acquired in waking. 22 , 23 Therefore, it was assumed that slow oscillations reflect a cortical reorganization of waking circuits 5 and might help synaptic consolidation. 6
In this study we analyzed the frequency range of 0.5 to 4 Hz, because in schizophrenia patients a decrease in classical delta activity in SWS was shown, 9 which was confirmed by our results. Regarding the known origin of delta waves within the thalamus and within the cortex, Keshavan et al. 9 assumed a thalamocortical dysfunction in schizophrenia. In line with this, a large number of papers are showing anatomical and functional alterations of the thalamus in schizophrenia. 21 , 24 , 25
Cognitive deficits, considered to be key features of schizophrenia since the first descriptions of this disorder, are often linked to prefrontal dysfunction, also a consistent finding in schizophrenia research. 21 , 26 , 27 According to our hypothesis, we confirmed the results of a decrease in delta power in SWS. Additionally, the associations between especially frontal delta power and neuropsychological morning performance in healthy subjects were not found in patients with schizophrenia. Because a decrease in delta power in patients was only seen in temporal and occipital, but not frontal channels, it might be speculated that the above-mentioned link between delta activity and synaptic consolidation and plasticity 22 , 23 was additionally disturbed in schizophrenia patients.
Our results concerning the schizophrenia subjects are of course limited by the fact that our patients were not drug-free. Systematic data on the impact of amisulpride on delta power in sleep in schizophrenia have not yet been published. But in our previous paper 7 we reported typical alterations, especially of non-REM sleep parameters in our patients on stable medication with amisulpride as known from the literature of drug-naïve patients. 8 Furthermore, we did not find significant correlations between the dosage of amisulpride and delta power in any EEG channel. Because our patients, as opposed to comparison subjects, also showed diminished delta power in SWS, as did drug-free patients, 9 our patients seem to be quite typical in this respect.
In conclusion, we found further indications for the importance of especially frontal delta oscillations for neuropsychological performance in healthy subjects. Correlations cannot delineate causal relationships, but a diminution of this link might indicate disturbances in thalamocortical and prefrontal networks in schizophrenia. Future studies need to confirm and to extend these pilot results in a greater number of subjects. It should be analyzed further if applications of delta activity-enhancing drugs, such as gaboxadol, 28 could yield positive correlations between delta power and neuropsychological performance in schizophrenia patients. Finally, we believe that spectral analysis of sleep EEGs might provide new insights into processes of cognitive dysfunctions in a complex disorder like schizophrenia.
1. Maquet P: The role of sleep in learning and memory. Science 2001; 294:1048–1052Google Scholar
2. Smith C: Sleep states and memory processes in humans: procedural versus declarative memory systems. Sleep Med Rev 2001; 5:491–506Google Scholar
3. Stickgold R: Human studies on sleep and off-line memory reprocessing, in Sleep and Brain Plasticity. Edited by Maquet P, Smith C, Stickgold R. Oxford, Oxford University Press, 2003, pp 41–63Google Scholar
4. Bodizs R, Bekesy M, Szucs A, et al: Sleep-dependent hippocampal slow activity correlates with waking memory performance in humans. Neurobiol Learn Mem 2001; 78:441–457Google Scholar
5. Anderson C, Horne JA: Prefrontal cortex: links between low frequency delta EEG in sleep and neuropsychological performance in healthy, older people. Psychophysiology 2003; 40:349–357Google Scholar
6. Huber R, Ghilardi MF, Massimini M, et al: Local sleep and learning. Nature 2004; 430:78–81Google Scholar
7. Göder R, Boigs M, Braun S, et al: Impairment of visuospatial memory is associated with decreased slow wave sleep in schizophrenia. J Psychiatr Res 2004; 38:591–599Google Scholar
8. Monti JM, Monti D: Sleep in schizophrenia patients and the effects of antipsychotic drugs. Sleep Med Rev 2004; 8:133–148Google Scholar
9. Keshavan MS, Reynolds CF, Miewald BA, et al: Delta sleep deficits in schizophrenia. Arch Gen Psychiatry 1998; 55:443–448Google Scholar
10. Green MF, Kern RS, Braff DL, et al: Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr Bull 2000; 26:119–136Google Scholar
11. Rechtschaffen A, Kales A: A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Los Angeles, University of California, Brain Information Service, 1968Google Scholar
12. Reitan RM: Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills 1958; 8:271–276Google Scholar
13. Osterrieth PA: Le test de copie dune figure complexe. Archives de Psychologie 1944; 30:206–356Google Scholar
14. Smith ML, Milner B: The role of the right hippocampus in the recall of spatial location. Neuropsychologia 1981; 19:71–81Google Scholar
15. Lehrl S: Manual zum MWT-B. Erlangen, Perimed, 1977Google Scholar
16. Kay SR, Opler LA, Lindenmayer JP: The positive and negative syndrome scale (PANNS): rationale and standardisation. Br J Psychiatry 1998; 155:59–65Google Scholar
17. Goldberg TE, Saint-Cyr JA, Weinberger DR: Assessment of procedural learning and problem solving in schizophrenic patients by Tower of Hanoi type tasks. J Neuropsychiatry Clin Neurosci 1990; 2:165–173Google Scholar
18. Plihal W, Born J: Effects of early and late nocturnal sleep on declarative and procedural memory. J Cogn Neurosci 1997; 9:534–547Google Scholar
19. Segalowitz SJ, Unsal A, Dywan J: CNV evidence for the distinctiveness of frontal and posterior neural processes in a traumatic brain-injured population. J Clin Exp Neuropsychol 1992; 14:545–565Google Scholar
20. Nyberg L, McIntosh AR, Tulving E: Functional brain imaging of episodic and semantic memory with positron emission tomography. J Mol Med 1998; 76:48–53Google Scholar
21. Weiss AP, Heckers S: Neuroimaging of declarative memory in schizophrenia. Scand J Psychol 2001; 42:239–250Google Scholar
22. Steriade M, Timofeev I: Neuronal plasticity in thalamocortical networks during sleep and waking oscillations. Neuron 2003; 37:563–576Google Scholar
23. Steriade M, Timofeev I: Neuronal plasticity during sleep oscillations, in corticothalamic systems, in Sleep and Brain Plasticity. Edited by Maquet P, Smith C, Stickgold R. Oxford, Oxford University Press, 2003, pp 271–291Google Scholar
24. Danos P: Pathology of the thalamus and schizophrenia: an overview. Fortschr Neurol Psychiat 2004; 72:621–634Google Scholar
25. Clinton SM, Meador-Woodruff JH: Thalamic dysfunction in schizophrenia: neurochemical, neuropathological, vivo imaging abnormalities. Schizophr Res 2004; 69:237–253Google Scholar
26. Hofer A, Weiss EM: Advances in the neuroimaging of cognitive functions in schizophrenia. Curr Opin Psychiatry 2002; 15:3–7Google Scholar
27. Winterer G, Weinberger DR: Genes, dopamine and cortical signal-to-noise ratio in schizophrenia. Trends Neurosci 2004; 11:683–690Google Scholar
28. Mathias S, Steiger A, Lancel M: The GABA (A) agonist gaboxadol improves the quality of post-nap sleep. Psychopharmacology (Berl) 2001; 157:299–304Google Scholar



