Personality Disorder Symptomatology and Neuropsychological Functioning in Closed Head Injury
Increased incidence of personality disorder is common in head-injured samples, 6 provided that one ignores DSM-IV criteria that the pattern of behavior not be due to the effects of a general medical condition (e.g., traumatic brain injury). Personality disorder diagnoses have been made in 23% of TBI patients at 30-year follow-up, with avoidant, paranoid, and schizoid as the most common diagnoses. 7 Although a distinct neuropsychological profile for the personality disorders has yet to emerge, the bulk of the evidence supports a neurobehavioral basis for borderline, schizotypal, and antisocial personality pathology. 8 – 11 Despite findings revealing a decrement in frontal lobe function associated with several forms of personality disorder symptomatology, 12 little is known about the relations between other domains of neuropsychological functioning and personality pathology in patients who sustain TBI.
There is good reason to suspect that personality disorder traits are differentially associated with neurocognitive function and that certain personality patterns might actually be adaptive in some neuropsychological domains while others might be detrimental to neurocognitive functioning. Some evidence indicates that although histrionic and narcissistic personality features are associated with poorer performance on measures of attention and executive function in patients with schizophrenia, higher levels of these traits are also associated with enhanced facial affective recognition. 13 Additionally, obsessive-compulsive personality features have been found to be correlated with enhanced visual attention to small details of stimuli. 14 Thus, although some evidence highlights the relevance of neurocognitive status to personality functioning, no systematic investigation of these associations exists.
This is one of the first investigations examining the relations between personality disorder traits and neuropsychological functioning in patients who have experienced a closed head injury and thus is largely explorative. The purpose of the present study is to examine the relations among personality disorder traits and neuropsychological function in order to determine which forms of personality disorder symptomatology might be associated with deficits in neurocognitive function. Consistent with a conceptualization of personality disorders as extreme forms of normal personality traits 15 wherein one might expect that links between normal variation in personality and neuropsychological function would exist across multiple personality disorder domains, we hypothesize that neurocognitive function will be related to a wide range of personality disorder traits rather than the few personality disorders previously studied. The present examination allows for a sound test of these hypotheses given a wide range of variation in personality and neuropsychological function, from normal to abnormal, in a sample of head-injured adults. Furthermore, whereas certain personality traits might be linked to poorer neuropsychological function, other traits might actually be associated with higher functioning in certain neuropsychological domains.
METHOD
Subjects
We selected 161 outpatients referred for neuropsychological evaluation from archival data. Criteria for inclusion in the study were having sustained a mild closed head injury, completed a standard battery of neuropsychological tests, and obtained a valid profile on the Millon Clinical Multiaxial Inventory–III (MCMI–III). 16 Subjects were excluded if they failed the Test of Memory Malingering, 17 a neuropsychological symptom validity indicator. Subjects reported closed head injuries related to motor vehicle accidents (N=122), blows to the head (N=18), falls (N=16), physical assaults (N=4), and plane crashes (N=1). The mean age of the subjects was 39.91 (SD=12.32) years, including 91 men and 70 women. The ethnic composition of the sample was as follows: Caucasian (87.0%), African American (9.3%), Hispanic (1.9%), Native American (0.6%), and Other (1.2%). The subjects are primarily right-handed (87.0%) and have a mean level of education of 13.58 (SD=2.39) years. Forty-eight percent of the sample reported no current medication use, while current use of at least one of the following medications was reported: tricyclic antidepressants (N=12), serotonin specific reuptake inhibitors (N=20), other antidepressants (N=13), benzodiazepines (N=16), other sedative hypnotics or anxiolytics (N=7), antipsychotics (N=4), antiparkinsonian agents (N=1), stimulants (N=4), antimanic agents (N=9), pain medications (N=40), and other (N=46).
Neuropsychological Testing
All subjects underwent a standard neuropsychological evaluation. The neuropsychological battery included tests of skills in eight broad domains: attention, executive function, language, motor, speeded processing, visuospatial, and verbal and visual memory. Attention was assessed using the Stroop Color and Word Test 18 and the Wechsler Adult Intelligence Scale–III (WAIS–III) 19 Digit Span. Executive function was measured with the Controlled Oral Word Association Test (COWAT) and Trail Making Test Part B. Assessments of verbal memory were conducted with the California Verbal Learning Test (I or II) 20 and Wechsler Memory Scale–III (WMS–III) 21 Logical Memory (immediate and delayed recall), while visual memory was examined using WMS–III Visual Reproductions (immediate and delayed recall) and Rey-Osterrieth Complex Figure Test (ROCFT) delayed recall conditions.
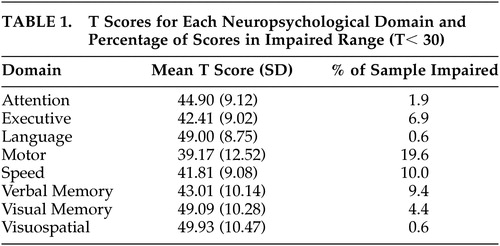
Motor skills were examined with the Finger Oscillation Test, and processing speed was assessed with the Trail Making Test Part A, Stroop Words and Colors, and WAIS–III Digit Symbol. The Boston Naming Test 22 and WAIS–III Vocabulary, Comprehension, and Similarities subtests were used to gauge language abilities. Visual and spatial abilities were assessed with the ROCFT copy condition, Tactual Performance Test total time, and WAIS–III Block Design. Mean T scores and percentage of the sample falling within the impaired range (T<30) are presented in Table 1 .
 |
Personality Testing
Participants completed the MCMI–III, 16 a 175-item self-report inventory designed to measure DSM-IV personality disorders and Axis I clinical syndromes. In addition to scales for depressive, aggressive/sadistic, passive-aggressive, and self-defeating/masochistic disorders, the MCMI–III contains scales for each of the DSM-IV personality disorders. Scales for Axis I conditions include dysthymia, somatoform disorder, anxiety disorder, posttraumatic stress disorder, thought disorder, mania, and drug and alcohol dependence. The MCMI–III scales are based on base rate scores that attempt to anchor cutoff scores for each scale in accordance with the prevalence of that trait in the psychiatric population. A Base Rate score greater than or equal to 80 represents the highest scoring 10% of the patient population; a base rate of 75 to 84 represents the next 15%; and a base rate of less than 35 represents the lowest 15% of the population. 16 The mean base rate for the nonpatient population is 35, while a base rate of 75 is commonly used for determining whether a scale is elevated. 23
Statistical Analyses
Scores for all neuropsychological tests were age and demographically (where available) corrected and transformed to T scores. The scores for each test were then aggregated to yield a mean T score for each participant within each of the relevant neuropsychological domains. Two-tailed Pearson correlations were used to examine the relations among 14 personality disorder scales and eight neuropsychological domains, yielding a total of 112 correlations. Because of the exploratory nature of the present study, a more conservative level of type I error (p<0.01) was adopted for each correlation to reduce the probability of identifying a spurious correlation but maintain sufficient statistical power. Based on this level of type I error, however, it should be noted that at least one of the findings is likely to be significant based on chance. Post-hoc correlations were used to examine whether the pattern of relations in whole-group analyses was maintained across gender. A series of multiple linear regression analyses were then conducted to determine which neuropsychological domains were most strongly associated with each personality disorder scale. Two separate linear regression analyses were conducted, the first with the eight neuropsychological domains and the second including Scale D (dysthymia) to examine the differential contributions of neurocognitive status and depressive symptoms to personality disorder symptomatology. Lastly, a canonical correlation was conducted to examine the overall multivariate shared relationship between personality pathology and neurocognition. 24
RESULTS
Table 2 shows the correlation matrix for the MCMI–III personality disorder scales and neuropsychological domains. Linear regression analyses were conducted for each of the personality disorder scales with the eight neuropsychological domains as predictors. A significant association was observed between language and schizoid symptomatology (β=−0.323, t [8, 143]=−3.37, p=0.001), while executive function (β=−0.224, t [8, 143]=−2.20, p=0.029) and language (β=−0.350, t [8, 143]=−3.83, p<0.001) were significant predictors of avoidant (AVD) symptomatology. For dependent (DEP), depressive (DPR), antisocial (ANT), obsessive-compulsive (COM), and self-defeating/masochistic (MAS) scales, language was the sole predictor of these traits [DEP β=−0.304, t (8, 143)= −3.20, p=0.002; DPR β=−0.382, t (8, 143)= −4.03, p<0.001; ANT β=−0.212, t (8, 143)=−2.06, p=0.04; COM β=0.219, t (8, 143)=0.212, p=0.04; MAS β=−0.273, t (8, 143)=−2.94, p=0.004]. Motor skills [β=0.205, t (8, 143)=2.29, p=0.02] and language [β=0.217, t (8, 143)=2.31, p=0.02] were significant predictors of scores on the histrionic scale, whereas processing speed [β=0.244, t (8, 143)=2.13, p=0.04] was the only neuropsychological domain associated with narcissism in the regression model. Both executive function [β=−0.274, t (8, 143)=−2.61, p=0.01] and language [β=−0.274, t (8, 143)=−2.61, p=0.01] were significant predictors of aggressive/sadistic traits. For passive-aggressive traits, attention [β=0.214, t (8, 143)= 2.14, p=0.03], executive function [β=−0.316, t (8, 143)=−3.15, p=0.002], and language [β=−0.358, t (8, 143)= −4.00, p<0.001] were significantly associated with this scale.
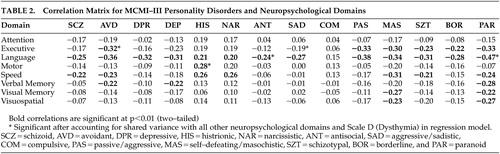 |
Linear regression analyses were also conducted on what Millon conceived as the severe personality pathologies, schizotypal (SZT), borderline (BOR), and paranoid (PAR). Language significantly predicted scores on each of these personality scales [SZT β=−0.291, t (8, 143)=−3.08, p=0.002; BOR β=−0.265, t (8, 143)=−2.76, p=0.006; PAR β=−0.404, t (8, 143)=−4.56, p<0.001].
When Scale D (dysthymia) was included in the regression model, a number of associations between neuropsychological domains and personality disorder traits were rendered nonsignificant. With the exception of the antisocial scale, the significant effect of language was removed for each of the following scales when Scale D was included in the regression model: schizoid, dependent, depressive, antisocial, obsessive-compulsive, self-defeating/masochistic, schizotypal, and borderline. Similarly, processing speed no longer predicted narcissistic traits when Scale D was included in the regression model. A number of neuropsychological domains remained significantly associated with personality disorder traits even after accounting for shared variance with other neuropsychological domains and Scale D ( Table 2 ). Executive function remained a significant predictor of avoidant and passive-aggressive traits when Scale D was included in the regression model, as did motor skills for the histrionic scale and language for aggressive/sadistic and paranoid scales.
We conducted a canonical correlation analysis using the eight neuropsychological domains as predictors of the 14 personality scales to evaluate the multivariate shared relationship between the two sets of variables. The analysis generated seven functions with squared canonical correlations of 0.255, 0.177, 0.122, 0.096, 0.070, 0.045, and 0.013 for each successive function. Using Wilks’ λ=0.427, F (98, 837.44)=1.23, p=0.07, the full model across all functions was not statistically significant. It should be noted that the canonical correlation is underpowered, necessitating an n to k ratio of 20:1, which is not satisfied by the sample size of the present study. However, given that Wilks’ lambda represents the variance unexplained by the model, 1 – λ represents an r 2 metric of the full model’s effect size. Thus, the r 2 type effect size was 0.573 for the set of seven canonical functions, which indicates that the full model explained approximately 57% of the variance shared between the variable sets.
Because personality disorders are unevenly distributed across gender, 25 separate post hoc correlation analyses were conducted for men and women in order to examine the role of gender as a potential moderating variable. Many of the correlations for both men and women were attenuated when examining individual correlations by gender. Although a handful of correlations were no longer statistically significant (p<0.01) relative to whole-group analyses, the most prominent findings were in the domain of language: for women, only passive-aggressive, self-defeating/masochistic, schizotypal, and paranoid traits were associated with language skills, while nearly all of the associations between language and personality disorder traits were maintained for men, with the exception of antisocial and aggressive/sadistic traits. Similarly, for speeded processing, only self-defeating/masochistic traits were associated with this neuropsychological domain for women, whereas for men the schizoid, avoidant, self-defeating/masochistic, and schizotypal traits remained statistically significant. Furthermore, while verbal memory was uncorrelated with personality disorder traits for women, men maintained a significant association with paranoid traits in addition to narcissistic, passive-aggressive, and schizotypal traits.
DISCUSSION
Consistent with findings in a healthy adult sample, 12 neuropsychological functioning was associated with many personality disorder traits rather than isolated personality disorder symptomatologies. Gender differences in these associations were apparent in a number of domains, particularly language skills, speeded processing, and verbal memory. The most robust correlations were observed for men, suggesting that there may be key ways in which men and women differ in certain personality traits and that these differences may be associated with unique neurocognitive underpinnings. Generally, however, associations present in the entire sample tended to be maintained, albeit at a smaller magnitude, when gender was examined as a moderating variable. The personality disorder scales that had the broadest set of correlations with the neuropsychological domains were avoidant, dependent, self-defeating/masochistic, schizotypal, and paranoid, all of which were correlated with at least three of the eight domains. Of these personality pathologies, schizotypal personality disorders solely have received extensive neuropsychological examination in the literature.
For those personality disorders generally considered within the schizophrenia spectrum, deficits were apparent within specific neuropsychological domains even after accounting for shared variance with depressive symptomatology. While avoidant traits were associated with reduced executive function and paranoid traits with language deficits, no neurocognitive functions were associated with schizoid symptomatology after accounting for depressive symptoms. Further evaluation of executive function deficits in patients with avoidant traits may be warranted given the absence of any data implicating executive dysfunction in association with avoidant symptomatology in the literature.
We observed a dissociation in language and executive function for the two scales indicative of psychopathic attitudes and behavior. The antisocial scale, which emphasizes social mistrust, behavioral acting-out, and social independence, 23 , 26 was associated with language deficits, whereas the aggressive/sadistic scale, which focuses more on emotional acting-out, strong-willed determination, and defensive aggression, 23 , 26 was linked more strongly to deficiencies in executive function. These data are consistent with findings of weak associations between antisocial personality traits and executive function but robust links between psychopathic behavior and executive dysfunction. 11 Thus, overt hostile and aggressive behavior, as represented more by the aggressive/sadistic scale, may be more indicative of organic frontal lobe impairments (e.g., disinhibition), while an antisocial personality and associated attitudes, as indicated more by the antisocial scale, may be linked more strongly to deficits in language skills.
The present investigation provides evidence of a unique set of relations between neuropsychological functioning and passive-aggressive (or negativistic personality disorder) symptomatology, a disorder listed in DSM-IV for further study. Though executive function and language were associated with passive-aggressive traits, when depressive symptomatology was accounted for in the regression model, executive function remained the sole neuropsychological domain significantly associated with passive-aggressive traits. The passive-aggressive scale had the largest standardized regression weight associated with executive function deficits and was one of the few personality disorder traits—along with avoidant and aggressive/sadistic—linked to executive dysfunction after accounting for depressive symptomatology and shared variance with other neuropsychological domains. These findings suggest that passive-aggressive traits may be a more severe form of personality pathology with substantial deficits in higher-order regulatory and supervisory functions subserved primarily by the frontal lobes. 27 Of course, this assertion ought to be confirmed with a cohort of diagnosed passive-aggressive patients with no history of neurological insult.
Histrionic and narcissistic traits seem to be associated with enhanced functioning in a number of neurocognitive domains, which is consistent with the finding that higher scores on the hysteria scale of the Minnesota Multiphasic Personality Inventory–2 (MMPI–2) are associated with greater intellectual ability. 28 With the level of depression accounted for, motor skills were significantly associated with scores on the histrionic scale. Similarly, narcissism scores were related to higher levels of speeded processing, but this association was eliminated when depressive symptomatology was included in the regression model. Poor performance on measures of motor skills and processing speed are reflective of frontal deficits and more diffuse cerebral impairments. The associations between these neuropsychological domains and histrionic and narcissistic scores are positive, suggesting that these personality traits are associated with more intact frontal and generalized cerebral function.
Though normal variation in these traits may be neurocognitively advantageous, more extreme expressions of these traits may be neuropsychologically detrimental. Examining potential moderating factors that might affect these findings is also warranted. For instance, differentiating between the more maladaptive “vulnerable” versus the more adaptive “grandiose” narcissist 29 in neuropsychological domains might be revealing.
Executive function, speeded processing, and language skills were the primary neuropsychological domains implicated in personality disorder traits. Executive function and speeded processing are skills primarily subserved by the frontal lobes, suggesting that many forms of personality disorder symptomatology might at least partly be represented by pathological behavioral manifestations of frontal lobe dysfunction within this head-injured sample. Language skills, as presently defined, consist of expressive word knowledge, abstract reasoning, naming, commonsense reasoning, and social judgment. These also rely heavily upon anterior rather than posterior brain regions. On the whole, these findings implicate a more diffuse and generalized disturbance of personality which may be mediated by the disruption of frontal-subcortical circuits that govern the appropriate inhibition and expression of behavior and emotion, which collectively might be referred to as a disordered personality.
A consistent finding across several of the personality disorder scales was that many of the associations between personality traits and neuropsychological domains were rendered nonsignificant when shared variance with depressive symptomatology was taken into account. We conducted post hoc analyses to determine whether years of education might mitigate the relationship between language and depression. Education level, however, did not attenuate the association between language skills and depressive symptomatology.
Another postulation is that the relation between language task performance and elevations on the personality disorder scales might be mediated by depressive symptomatology, wherein greater levels of negative affect might impede performance on cognitive tasks as a result of depressed mood while also predisposing one to report more symptoms of disordered personality. However, language functions are generally preserved for depressed persons, 30 arguing against a specific decrement in performance on language tasks resulting from low mood.
It also may be possible that compromised temporal regions of the brain bring about greater levels of depressive and personality disorder symptomatology in tandem with poor performance on tests of language. Indeed, comorbidity of depression and personality disorders is pervasive, 31 left compared with right temporal lobectomy is associated with greater levels of negative affect, 32 and depression is more common in left- versus right-sided stroke. 33 Furthermore, one of the primary circuits postulated to be involved in secondary depression is a basotemporal-limbic pathway that links the orbitofrontal cortex and anterior temporal cortex through the uncinate fasciculus. 30 If this is the case, then the findings observed in this investigation suggest that dysfunction of temporal regions of the brain might bring about pronounced depressive symptomatology but only moderate levels of personality pathology, with the former overshadowing the latter. Findings in accordance with these results were obtained in a sample of healthy adults in which levels of negative affect apparently overshadowed the associations between frontal lobe-mediated tasks and several MMPI–2 personality disorder scales. 12 Support for temporal lobe dysfunction in personality disorders is also provided by functional neuroimaging examinations of borderline personality disorder in which abnormalities of temporal regions have been observed in positron emission tomography studies. 34 , 35
The multivariate shared relationship between personality disorder symptomatology and neuropsychological domains is considerable. Though the canonical correlation between the two sets of variables was not statistically significant, 57% of the variance was shared across these variable sets. Interestingly, attachment style has been shown to share approximately 56% of the variance in MCMI–III personality scales. 24 Evidently, brain function is as pertinent as some of the more venerable and extensively studied psychodynamic variables to the study of personality pathology.
The present study highlights the need to develop a comprehensive understanding of the neuropsychological and neuroanatomical underpinnings of the personality disorders and personality disorder traits, as has already been initiated with borderline, 8 , 9 schizotypal, 10 and antisocial personality disorders. 11 Particular emphasis might be placed on avoidant, aggressive/sadistic, and passive-aggressive personality disorders, traits of which were shown to be associated with demonstrable deficits in executive function, the latter two of which are not currently listed on Axis II of DSM-IV. A neurobehavioral basis for these and nearly all of the personality disorders seems tenable, likely because the processes governing normal and disordered expressions of personality are themselves dimensional and mediated by dynamic neural systems that function along continua. Indeed, a neurobehavioral basis for the personality disorders calls into question the distinction of personality disorders from Axis I conditions based solely on an argument of biological etiology. 36
Because the patients in this study were not necessarily personality disordered but instead had sustained a mild closed head injury, the implications of these findings for psychiatric patients should be interpreted with caution. There may or may not be deficits in neuropsychological functioning unique to individuals who are diagnosed with a personality disorder, particularly if there is no history of brain compromise.
However, these findings indeed shed light on potential neurocognitive deficits that may be involved in specific personality disorders that have not received extensive evaluation in the neuropsychological literature. Knowledge of those cognitive dysfunctions that might be pervasive among the personality disorders or strongly associated with specific traits would be crucial to consider when formulating treatments for personality disorder patients, particularly for psychopharmacological agents with known impact on specific cognitive functions.
In addition, the statistical analyses implemented in the present study assume a linear relationship between personality traits and neurocognitive functioning when, in fact, a nonlinear relationship may predominate. It may be the case that extreme levels of a particular trait in association with a diagnosable personality disorder may be reflected in neurocognitive dysfunction, whereas moderate levels of some traits may be associated with enhanced neurocognitive function. In investigations with suitably large sample sizes it will be necessary to use nonlinear approaches to further explore these relationships. In addition, it should be noted that the findings of the present investigation are indeed tentative given that most of the correlations between personality disorder scales and neuropsychological domains do not meet statistical significance after a Bonferroni correction.
To investigate the potentially causal role of brain injury on personality disorders, prospective research designs would be advantageous not only to determine which personality disorders occur most frequently following TBI but also to examine the influence of TBI severity and localization of insult on personality pathology. Compared with epidemiological studies that have investigated personality functioning among persons reporting a history of TBI, 3 prospective longitudinal designs would allow for a determination of whether differences in personality profiles among head-injured and non-head-injured persons are due to the presence of certain personality traits that tend to predispose one to TBI or whether neurological insult itself causes personality disorders. It may also be the case that certain predisposing personality traits act as risk factors for engaging in high-risk activities, and should a TBI occur, those and likely most personality traits may become more extreme and inflexible, crossing the boundary from personality style to disorder.
Findings from the present investigation and a healthy adult sample 12 implicate more global associations between personality disorder traits and neuropsychological functioning, suggesting that each of these psychological domains exists along a continuum wherein disruptions in brain functioning would likely result in an exacerbation of both premorbid personality traits and neuropsychological functioning. Evidence from a large sample of TBI patients certainly indicates that shifts toward more extreme expressions of normal personality traits occur following TBI. 37
Combined approaches integrating personality, neuropsychological, and functional neuroimaging methods are important for characterizing the neural bases of normal and disordered personality. Though neuropsychological and neuroimaging studies have provided a wealth of evidence implicating multiple brain regions and neural systems involved in several forms of personality pathology, the results generally possess limited clinical utility. 9 One means by which to overcome the weak clinical utility of these findings is to enhance the ecological validity of neurocognitive investigations of personality disorders. Neuroimaging techniques, particularly those that employ ecologically valid interpersonal paradigms, as are afforded by such emerging technologies as functional near infrared spectroscopy, 38 and in the context of a multitrait-multimethod framework, 39 will likely provide useful insights into the neurodynamics of the personality disorders.
1. McAllister TW: Neuropsychiatric sequelae of head injuries. Psychiatr Clin N Am 1992; 15:395–413Google Scholar
2. Anstey KJ, Butterworth P, Jorm AF, et al: A population survey found an association between self-reports of traumatic brain injury and increased psychiatric symptoms. J Clin Epidemiol 2004; 7:1202–1209Google Scholar
3. Silver JM, Kramer R, Greenwald S, et al: The association between head injuries and psychiatric disorders: findings from the New Haven NIMH Epidemiologic Catchment Area Study. Brain Inj 2001; 15:935–945Google Scholar
4. van Reekum R, Cohen T, Wong J: Can traumatic brain injury cause psychiatric disorders? J Neuropsychiatry Clin Neurosci 2000; 12:316–327Google Scholar
5. Castano B, Gonzalez G, Andres P, et al: Comparative study of psychiatric disorders in general traumatism and brain injured patients. Actas Esp Psiquiatr 2005; 33:96–101Google Scholar
6. Stong C: Personality disorders following brain injury–a common problem requiring comprehensive care. Neurol Rev 2004; 53–54Google Scholar
7. Koponen S, Taiminen T, Portin R, et al: Axis I and II psychiatric disorders after traumatic brain injury: a 30-year follow-up study. Am J Psychiatry 2002; 159:1315–1321Google Scholar
8. Swirsky-Sacchetti T, Gorton G, Samuel S, et al: Neuropsychological function in borderline personality disorder. J Clin Psychol 1993; 49:385–396Google Scholar
9. Ruocco AC: The neuropsychology of borderline personality disorder: a meta-analysis and review. Psychiatry Res (in press)Google Scholar
10. Voglmaier MM, Seidman LJ, Niznikiewicz MA, et al: A comparative profile analysis of neuropsychological function in men and women with schizotypal personality disorder. Schizophr Res 2005; 74:43–49Google Scholar
11. Morgan AB, Lilienfeld SO: A meta-analytic review of the relation between antisocial behavior and neuropsychological measures of executive function. Clin Psychol Rev 2000; 20:113–156Google Scholar
12. Ruocco AC, Trobst KK: Frontal lobe functioning and personality disorder symptomatology. Arch Clin Neuropsychol 2003; 18:737Google Scholar
13. Lysaker PH, Wickett AM, Lancaster RS, et al: Neurocognitive deficits and history of childhood abuse in schizophrenia spectrum disorders: associations with cluster B personality traits. Schizophr Res 2004; 68:87–94Google Scholar
14. Yovel I, Revelle W, Mineka S: Who sees trees before forest? the obsessive-compulsive style of visual attention. Psychol Sci 2005; 16:123–129Google Scholar
15. Costa PT, Widiger, TA (eds): Personality Disorders and the Five-Factor Model of Personality. Washington, DC, American Psychological Association, 1994Google Scholar
16. Millon T, Davis RD, Millon C: MCMI-III Manual, 2nd ed. Minneapolis, MN, National Computer Systems, 1997Google Scholar
17. Tombaugh TN: Test of Memory Malingering (TOMM). Toronto, ON, Multi-health Systems, 1996Google Scholar
18. Golden CJ: Stroop Colour and Word Test: A Manual for Clinical and Experimental Uses. Chicago, Ill, Stoelting Company, 1978Google Scholar
19. Wechsler D: Wechsler Adult Intelligence Scale, 3rd ed. San Antonio, Tex, Psychological Corporation, 1997Google Scholar
20. Delis DC, Kramer JH, Kaplan E, et al: California Verbal Learning Test. San Antonio, Tex, Psychological Corporation, 1987Google Scholar
21. Wechsler D: Wechsler Memory Scale, 3rd ed. New York, Psychological Corporation, 1997Google Scholar
22. Kaplan E, Goodglass H, Weintraub S: Boston Naming Test. Philadelphia, Penn, Lea & Febiger, 1978Google Scholar
23. Choca J: Interpretive Guide to the Millon Clinical Multiaxial Inventory. Washington, DC, American Psychological Association, 2004Google Scholar
24. Sherry A, Henson RK: Conducting and interpreting canonical correlation analysis in personality research: a user-friendly primer. J Pers Assess 2005; 84:37–48Google Scholar
25. Golomb M, Fava M, Abraham M, et al: Gender differences in personality disorders. Am J Psychiatry 1995; 152:579–582Google Scholar
26. Choca J, Retzlaff P, Strack S, et al: Factorial elements of the MCMI–II personality scales. J Personal Disord 1996; 10:377–383Google Scholar
27. Zillmer EA, Spiers MV: Principles of neuropsychology. Belmont, Calif, Wadsworth, 2001Google Scholar
28. Graham JR: MMPI-2: Assessing Personality and Psychopathology, 3rd ed. New York, Oxford University Press, 1999Google Scholar
29. Dickinson KA, Pincus AL: Interpersonal analysis of grandiose and vulnerable narcissism. J Personal Disord 2003; 17:188–207Google Scholar
30. Mayberg H: Depression and frontal-subcortical circuits: focus on prefrontal-limbic interactions, in Frontal-Subcortical Circuits in Psychiatric and Neurological Disorders. Edited by Lichter DG, Cummings JL. New York, Guilford, 2001, pp 177–206Google Scholar
31. Skodol AE, Stout RL, McGlashan TH, et al: Co-occurrence of mood and personality disorders: a report from the collaborative longitudinal personality disorders study (CLPS). Depress Anxiety 1999; 10:175–182Google Scholar
32. Burton LA, Labar D: Emotional status after right vs. left temporal lobectomy. Seizure 1999; 8:116–119Google Scholar
33. Narushima K, Kosier JT, Robinson RG: A reappraisal of poststroke depression, intra- and inter-hemispheric lesion location using meta-analysis. J Neuropsychiatry Clin Neurosci 2003; 15:422–430Google Scholar
34. Soloff PH, Meltzer CC, Greer PJ, et al: A fenfluramine-activated FDG-PET study of borderline personality disorder. Biol Psychiatry 2000; 47:540–547Google Scholar
35. Leyton M, Okazawa H, Diksic M, et al: Brain regional alpha-[11C]methyl-L-tryptophan trapping in impulsive subjects with borderline personality disorder. Am J Psychiatry 2001; 158:775–782Google Scholar
36. Ruocco AC: Reevaluating the distinction between Axis I and Axis II disorders: the case of borderline personality disorder. J Clin Psychol 2005; 61:1509–1523Google Scholar
37. Trobst KK: Personality change in traumatic brain injury. Paper presented at the fourth annual intramural scientific retreat of the National Institute on Aging, Lithicum Heights, Md, 1999Google Scholar
38. Irani F, Platek SM, Ruocco AC, et al: Applications of functional near infrared spectroscopy (fNIRS) to neurobehavioral disorders. Clin Neuropsychol (in press)Google Scholar
39. Campbell DT, Fiske DW: Convergent and discriminant validation by the multitrait-multimethod matrix. Psychol Bull 1959; 56:81–105Google Scholar



