Neuroimaging in Schizophrenia: Misattributions and Religious Delusions
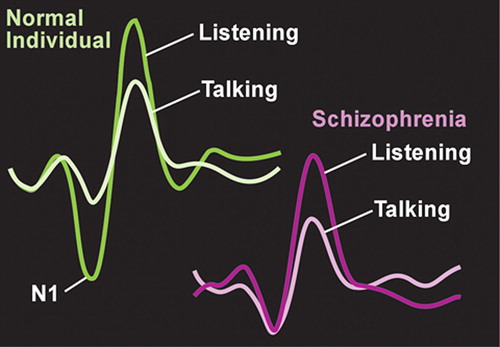
It has been shown that internal self-monitoring dampens neuronal responses to actions that are self-generated. Normal healthy individuals report stronger somatosensory sensations (e.g., tickle) when touched by another compared to their own touch, and much less activity is evoked in the primary sensory cortex by self-touch than by other-touch. 11 Psychiatric patients (e.g., with schizophrenia, bipolar affective disorder) who reported auditory hallucinations and/or passivity experiences rated both self-touch and other-touch as similarly intense. 12 The authors of this study suggest that these results support a breakdown in self-monitoring in this population. Electroencephalographic (EEG) and event-related potential (ERP) studies have shown that in normal healthy individuals there is less activity in the auditory cortex during both speaking out loud and inner speech compared with listening to speech. This results from an inhibitory influence (“corollary discharge”) from the area of frontal cortex generating speech onto auditory cortex. In subjects with schizophrenia, the clear difference in auditory cortex activity between the perception of self-generated speech and other-generated speech is not seen ( Figure 1 ). 1 In addition, this study found that measures of coherence of electrical activity between frontal and temporal speech-related areas were high during talking in normal healthy individuals, but not in subjects with schizophrenia. The authors of this study noted that these results suggest that speech-generating frontal areas are not “alerting” temporal areas that the speech is self-generated. As a result, the patient misattributes inner speech to external sources, and experiences an auditory hallucination. Supporting this interpretation are studies that have found that patients with schizophrenia, particularly those who currently have auditory hallucinations, are more likely to misattribute the source of speech (self/other/unsure) during a verbal self-monitoring task. 13
Recent studies support the therapeutic efficacy of low-level, repetitive transcranial magnetic stimulation (rTMS) in the region of the temporoparietal junction for auditory hallucinations. 14 , 15 This type of stimulation is believed to decrease neuronal excitability, and thus might be acting by supplementing the impaired “corollary discharge” in these patients. This interpretation is supported by a case study in which fluorodeoxyglucose positron emission tomography (FDG-PET) was acquired prior to and during treatment with rTMS in a patient with auditory hallucinations resistant to both drug therapy and electroconvulsive treatments. 16 rTMS was applied to the region of increased metabolic activity in the temporal cortex on the baseline PET scan. The hyperactivity of this region was decreased on the follow-up PET scans at 2 and 5 weeks of treatment. In a different study, rTMS to the temporoparietal area decreased auditory hallucinations and improved performance on a verbal self-monitoring task, suggesting a relationship between defective source monitoring and auditory hallucinations. 17
As noted above, patients with schizophrenia appear to have an impaired sense of individual agency such that they misattribute the source of self-generated thoughts or actions. One approach to delineating areas of the brain important for the sense of agency has been to compare the areas activated when an individual believes they control an action (e.g., movement of a circle on a computer screen) with the areas activated when control is attributed to another. Using event-related functional magnetic resonance imaging (fMRI), a study of normal healthy individuals found that activation of the anterior insula was associated with attribution of an action to self, whereas inferior parietal lobe activation was associated with attributing the action to another ( Figure 2 ). 2 When the visual feedback provided during a task was distorted to manipulate subjects’ attribution of control (self, self-distorted, other), rCBF (H 2 O 15 -PET) increased in the inferior parietal lobe with increasing distortion of movement. 18 Conversely, rCBF increased in the anterior insula with decreasing distortion of movement. This clear separation was not found in patients with schizophrenia who were experiencing first-rank symptoms, such as auditory hallucinations or delusions of control. 19 There were no areas in which rCBF covaried with the degree of movement distortion. However, when the extreme conditions were compared, increased rCBF was found in the inferior parietal lobe. This was also positively correlated with the severity of first-rank symptoms. Comparison of the patients with normal healthy individuals revealed higher than normal rCBF in this area, even in the 0° (undistorted) condition. The authors of this study noted that this high level of parietal activation during action that is clearly self-generated may result in a predisposition to misattribute the source of control.
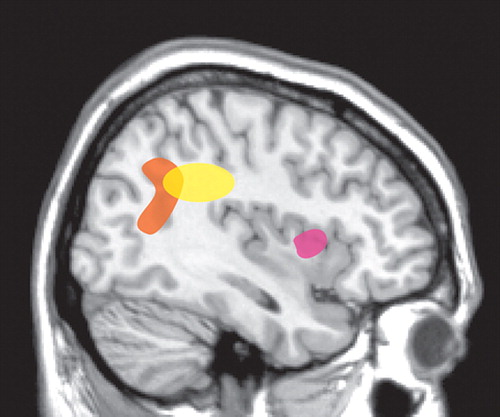
A study of the brain areas activated (H 2 O 15 -PET) when subjects manipulated a joystick found that patients with schizophrenia and active delusions of control over-activated areas in the inferior parietal and cingulate cortices and cerebellum compared with themselves when they had recovered from delusions of control and normal healthy individuals and schizophrenia patients who had never had delusions of control ( Figure 2 ). 3 This suggests that these areas of overactivation may relate more to “state” than to “trait.”
In addition, both patient groups failed to activate the prefrontal cortex during this task when compared with normal healthy individuals. The authors of this study commented that the inferior parietal area is important for spatial memory and orientation in space. Injury to this region has been implicated previously in giving rise to feelings of alienation, spatial dislocation, and control by external forces. Thus, misattribution of internally generated acts to external entities might be a result of impaired communication between frontal brain areas initiating action and parietal regions monitoring movement in space. Interestingly, the severity of first-rank symptoms correlated positively with rCBF (O-PET) in the parietal cortex (BA 7/40) and inversely with rCBF in the posterior cingulate cortex (BA 30) when scans were acquired under resting (eyes closed) conditions. 20
Both electrophysiological and anatomical studies suggest that long-range functional connectivity between cerebral regions is altered in patients with schizophrenia compared with normal healthy individuals. 21 , 22 Diffusion tensor imaging-based measurements provide a way to directly assess white matter integrity. Local changes in the structural integrity of white matter are believed to indicate areas of altered connectivity. There has been considerable variation in the results of diffusion tensor imaging studies of schizophrenia, some of which are likely to be due to methodological differences. 23 Most studies have been of medicated patients with chronic schizophrenia, which may also contribute to variability. 24 , 25 In addition, a recent study 26 assessed fractional anisotropy and mean diffusivity in specific white matter tracts as a function of age in patients with schizophrenia and age-matched healthy individuals. They concluded that there are age-related differences in brain maturation between these groups, particularly in the fronto-temporal pathways, which complicate comparisons.
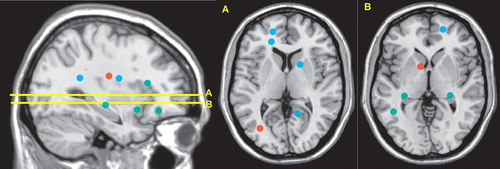
Several recent studies have used diffusion tensor imaging in patients with schizophrenia during their first episode in order to avoid the confounding effects of disease progression and medications. One utilized very strong gradients (maximum b= 14,000 s/mm 2 ) in an effort to more clearly isolate the intra-axonal compartment. 27 All patients were within 1 month of initial hospitalization. Histogram analysis identified fewer pixels consistent with white matter in the patient group, suggesting an overall decrease in the white matter compartment. Symptom severity was negatively correlated with this measure (more severe symptoms, less white matter). Two studies used voxel-based analysis in order to localize areas of abnormality ( Figure 3 ). 4 , 5 In one, average illness duration was 116 days (range=14 to 270). Intervoxel coherence maps were constructed to identify clusters (minimum of six voxels) of altered white matter. Three clusters of increased intervoxel coherence and 11 clusters of decreased intervoxel coherence were identified in the subcortical white matter, corpus callosum, and internal capsule. In the other, average illness duration was 10.3 months (range=6 to 24). 5 Fractional anisotropy maps were constructed to identify clusters (minimum of 30 voxels) of altered white matter. Eleven clusters of decreased fractional anisotropy were identified in the subcortical white matter and cerebral peduncles. Thus, all three studies support altered white matter connectivity in schizophrenia, with some overlap of areas.
One of the perplexing aspects of schizophrenia, which has a strong genetic component, is its continual existence in the human species. The presence of schizophrenia in groups that are known to have been genetically isolated for a very long time (e.g., Australian aborigines) suggests that its origin may be very far back in time. A great variety of hypotheses have been developed related to evolutionary pressures that might have maintained susceptibility genes for schizophrenia in the human gene pool, either as a byproduct of selection for other traits or due to adaptive advantages. 28 – 30 Several of these contend that schizophrenia, as a uniquely human brain disorder, could most logically be linked to characteristics that separate the human species from other animals, such as intelligence, language, and highly developed social cognition.
Two such theories posit the evolutionary advantages of having a few individuals prone to delusions/hallucinations by linking schizophrenia with a uniquely human cultural trait—religion. 30 Both are based on perceived similarities between schizophrenia and shamanism. One focuses on the evolutionary adaptive benefit to tribal units of spiritual ceremonies, and the value of having at least one individual within the group prone to “spirit possession” to serve as shaman. 31 The other concentrates on the charismatic traits of schizophrenia-prone individuals, and their possible important role as leaders providing a mechanism for splitting tribal communities that are growing too large. 30
Most of what is known about the neural substrates of religious experiences is based on clinical observations. 32 Only a few studies have explored the brain areas that are active during a religious, spiritual or mystical experience. In one, Carmelite nuns were instructed to “remember and relive the most intense mystical experience ever felt in their lives.” 33 The brain areas activated while they reexperienced this mystical state include medial orbital, medial prefrontal, anterior cingulate, and inferior and superior parietal cortices. In another study, Franciscan nuns were imaged during religious meditation that had the goal of “opening themselves to being in the presence of God.” 34 Increased blood flow was found in the prefrontal, inferior frontal, and inferior parietal cortices. In addition, there was an inverse correlation between cerebral blood flow in prefrontal and superior parietal cortices. In the third study, members of an evangelical fundamentalist community were imaged during religious meditation. 35 Areas activated included the dorsolateral prefrontal cortex, dorsomedial prefrontal cortex, medial parietal cortex, and cerebellum. No blood flow changes were found in the orbitofrontal cortex or amygdala.
One group has done extensive research exploring induction of a quasi-religious experience, that of the experience of the sensed presence of a “sentient being.” 36 They have found that application of weak (1 to 5 micro tesla) complex magnetic field stimulation to the temporoparietal area reliably evokes such experiences in normal individuals. The proposed mechanism is that the sense of self and sense of other depend upon the left and right hemispheres, respectively, and that any process that disrupts the normal reciprocal inhibition between the hemispheres should increase the incidence of a sensed presence. Two case reports are interesting in this context. In one, a patient with uncontrolled epilepsy who experienced a “sensed presence” during the aura had markedly increased perfusion in the frontoparietal region. The authors of this article postulated that the left temporal spike spread to the right-sided mirror area to create the clinical picture. 37 In the other, a patient with schizophrenia with active “florid religious delusions” had increased perfusion in frontal and temporal regions that normalized during remission of symptoms. 38 The authors of this case report note significance to the lateralization of these changes to the left side.
In conclusion, these preliminary imaging studies in patients with schizophrenia support the theory that the misattribution of self-generated thoughts or actions to outside entities/forces may contribute to the psychotic state. The inferior parietal cortex has been implicated. The few studies that have been completed in healthy individuals suggest that this region may also be important to normal religious experience.
1. Ford JM, Mathalon DH: Electrophysiological evidence of corollary discharge dysfunction in schizophrenia during talking and thinking. J Psychiatr Res 2004; 38:37–46Google Scholar
2. Farrer C, Frith CD: Experiencing oneself versus another person as being the cause of an action: the neural correlates of the experience of agency. Neuroimage 2002; 15:596–603Google Scholar
3. Spence SA, Brooks DJ, Hirsch SR, et al: A PET study of voluntary movement in schizophrenic patients experiencing passivity phenomena (delusions of alien control). Brain 1997; 120:1997–2011Google Scholar
4. Federspiel A, Begre S, Kiefer C, et al: Alterations of white matter connectivity in first episode schizophrenia. Neurobiol Dis 2006; 22:270–709Google Scholar
5. Hao Y, Liu Z, Jiang T, et al: White matter integrity of the whole brain is disrupted in first-episode schizophrenia. Neuroreport 2006; 17:23–26Google Scholar
6. Schneider K: Klinische Psychopathologie. Stuttgart, Thieme Verlag, 1955Google Scholar
7. Feinberg I, Guazzelli M: Schizophrenia—a disorder of the corollary discharge systems that integrate the motor systems of thought with the sensory systems of consciousness. Br J Psychiatry 1999; 174:196–204Google Scholar
8. Frith C: The neural basis of hallucinations and delusions. C R Biol 2005; 328:169–175Google Scholar
9. Frith C: The self in action: lessons from delusions of control. Conscious Cogn 2005; 14:752–770Google Scholar
10. Pacherie E, Green M, Bayne T: Phenomenology and delusions: who put the “alien” in alien control? Conscious Cogn 2006; 15:566–577Google Scholar
11. Blakemore SJ, Wolpert DM, Frith CD: Central cancellation of self-produced tickle sensation. Nat Neurosci 1998; 1:635–640Google Scholar
12. Blakemore SJ, Smith J, Steel R, et al: The perception of self-produced sensory stimuli in patients with auditory hallucinations and passivity experiences: evidence for a breakdown in self-monitoring. Psychol Med 2000; 30:1131–1139Google Scholar
13. Johns LC, Gregg L, Allen P, et al: Impaired verbal self-monitoring in psychosis: effects of state, trait and diagnosis. Psychol Med 2006; 4:465–474Google Scholar
14. Hoffman RE, Gueorguieva R, Hawkins KA, et al: Temporoparietal transcranial magnetic stimulation for auditory hallucinations: safety, efficacy and moderators in a 50 patient sample. Biol Psychiatry 2005; 548:97–104Google Scholar
15. Poulet E, Brunelin J, Bediou B et al: Slow transcranial magnetic stimulation can rapidly reduce resistant auditory hallucinations in schizophrenia. Biol Psychiatry 2005; 15:188–191Google Scholar
16. Langguth B, Eichhammer P, Zowe M, et al: Neuronavigated transcranial magnetic stimulation and auditory hallucinations in a schizophrenic patient: monitoring of neurobiological effects. Schizophr Res 2006; 84:185–186Google Scholar
17. Bruneline J, Poulet E, Bediou B, et al: Low frequency repetitive transcranial magnetic stimulation improves source monitoring deficit in hallucinating patients with schizophrenia. Schizophr Res 2006; 81:41–45Google Scholar
18. Farrer C, Franck N, Georgieff N, et al: Modulating the experience of agency: a positron emission tomography study. Neuroimage 2003; 18:324–333Google Scholar
19. Farrer C, Franck N, Frith CD, et al: Neural correlates of action attribution in schizophrenia. Psychiatry Res 2004; 131:31–44Google Scholar
20. Franck N, O’Leary DS, Flaum M, et al: Cerebral blood flow changes associated with Schneiderian first-rank symptoms in schizophrenia. J Neuropsychiatry 2002; 14:277–282Google Scholar
21. Walterfang M, Wood SJ, Velakoulis D, et al: Neuropathological, neurogenetic and neuroimaging evidence for white matter pathology in schizoprenia. Neurosci Biobehav Rev 2006; 30:918–948Google Scholar
22. Foucher JR, Luck D: Psychosis related to neurological conditions: pro and cons of the dis-/mis-connectivity modes of schizophrenia. Dialogues Clin Neurosci 2006; 8:17–27Google Scholar
23. Kanaan RA, Shergill SS, Barker GJ, et al: Tract-specific anisotropy measurements in diffusion tensor imaging. Psychiatry Res 2006; 14:73–82Google Scholar
24. Kubicki M, Westin CF, McCarley RW, et al: The application of DTI to investigate white matter abnormalities in schizophrenia. Ann N Y Acad Sci 2006; 1064:134–148Google Scholar
25. Kanaan RA, Kim JS, Kaufmann WE, et al: Diffusion tensor imaging in schizophrenia. Biol Psychiatry 2005; 58:921–929Google Scholar
26. Jones DK, Catani M, Pierpaoli C, et al: Age effects on diffusion tensor MRI tractography measures of frontal cortex in schizophrenia. Hum Brain Mapp 2006; 27:230–238Google Scholar
27. Mendelsohn A, Strous RD, Bleich MAY, et al: Regional axonal abnormalities in first episode schizophrenia: preliminary evidence base on high b-value diffusion-weighted imaging. Psychiatry Res 2006; 146:223–229Google Scholar
28. Burns JK: Psychosis: a costly byproduct of social brain evolution in Homo sapiens . Prog Neuropsychopharmacol Biol Psychiatry 2006; 30:797–814 Google Scholar
29. Brune M: Schizophrenia—an evolutionalry enigma? Neurosci Biobehav Rev 2004; 28:41–53Google Scholar
30. Polimeni J, Reiss JP: Evolutionary perspective on schizophrenia. Can J Psychiatry 2003; 48:34–39Google Scholar
31. Polimeni J, Reiss JP: How shamanism and group selection may reveal the origins of schizophrenia. Med Hypotheses 2002; 58:244–248Google Scholar
32. Saver JL, Rabin J: The neural substrates of religious experience. J Neuropsychiatry Clin Neurosci 1997; 3:498–510Google Scholar
33. Beauregard M, Paquette V: Neural correlates of a mystical experience in Carmelite nuns. Neurosci Lett 2006; 405:186–190Google Scholar
34. Newberg A, Pourdehnad M, Alavi A, et al: Cerebral blood flow during meditative prayer: preliminary findings and methodological issues. Percept Mot Skills 2003; 97:625–630 AzariGoogle Scholar
35. Azari NP, Nickel J, Wunderlich G, et al: Neural correlates of religious experience. Eur J Neurosci 2001; 13:1649–1652Google Scholar
36. St-Pierre LS, Persinger MA: Experimental facilitation of the sensed presence is predicted by the specific patterns of the applied magnetic fields, not by suggestibility: reanalyses of 19 experiments. Int J Neurosci 2006; 116:1079–1096Google Scholar
37. Landtblom AM: The “sensed presence”: an epileptic aura with religious overtones. Epilepsy Behav 2006; 9:186–188Google Scholar
38. Puri BK, Lekh SK, Nijran KS, et al: SPECT neuroimaging in schizophrenia with religious delusions. Int J Psychophysiol 2001; 40:143–148Google Scholar



