Association Between Facial Emotion Recognition and Odor Identification in Schizophrenia
Several recent studies have examined the overlap between emotion and olfactory processing and reported differences in hedonic appraisal of odors. Crespo-Facorro et al. 8 found that schizophrenia patients demonstrated impairment in the subjective experience of a pleasant, but not unpleasant, odor. In another study, 9 patients showed normal ratings of odor intensity but deficits in pleasantness, familiarity, and edibility across different odors. Our group 10 examined intensity and hedonic judgments in persons with schizophrenia and matched comparison subjects to determine whether these functions were differentially impaired. Schizophrenia subjects, specifically men, demonstrated decreased ability to attach the appropriate hedonic valence to a pleasant odor, despite giving intact intensity ratings.
The purpose of this study was to investigate whether abilities of facial emotion recognition and olfactory identification exhibit altered associations in persons with schizophrenia relative to healthy comparison subjects. The presence of such associations may further inform us about laterality of brain processing of emotion and olfactory stimuli and the effect of schizophrenia on these separate processes that are governed by similar neural substrates.
METHOD
Subjects
Nineteen patients with schizophrenia (13 men, six women) and 14 healthy volunteers (eight men, six women) were recruited from the Schizophrenia Research Center at the University of Pennsylvania Medical Center. Patients were older than healthy comparison subjects (mean ages of 34.3 [SD=8.4] years and 27.4 [SD=6.9] years, respectively; F [1, 31]=6.3, p=0.017). Age was subsequently used as a covariate in all analyses. Subjects did not differ with regard to sex (chi-square=0.44, df=1, p=0.50) or smoking history (chi-square=1.5, df=1, p=0.21). There were 16 outpatients and three inpatients from an acute short-term psychiatric unit, who were close to discharge when tested. All patients met DSM-IV criteria for schizophrenia, with no other concurrent diagnoses. Healthy volunteers were free of any Axis I diagnosis, Axis II Cluster A (i.e., schizotypal, schizoid, or paranoid) personality disorder, and family history of psychiatric illness. Subjects were excluded if they had a history of neurological disorder, including head trauma with loss of consciousness, history of substance abuse or dependence (as assessed by history, record review, and serum toxicology), any medical condition that might alter cerebral functioning, a recent respiratory infection or any other condition that could affect olfactory functioning (e.g., common cold or allergies). Written informed consent was obtained after the procedures had been fully explained. Sixteen of 19 schizophrenia participants completed both testing within 3 months and all within 12 months.
Facial Emotion Recognition Assessment
All subjects were administered the Emotion Recognition Test–40 Faces version (ER-40), 11 a standardized test of facial emotion recognition ability. The ER-40 includes 40 color photographs of facial expressions of four universal emotions (happy, sad, angry, and fearful) balanced for poser’s gender, age and ethnicity, including four low-intensity and four high-intensity facial expressions of each emotion, plus eight neutral facial expressions. The stimuli are presented on a computer screen in randomized order, and the participant is asked to identify the expressed emotion from five possible choices.
Assessment of Olfactory Performance
All tests of olfaction were administered unirhinally (i.e., each nostril separately). Prior to presentation of the test stimuli, the nostril not being assessed was occluded with Durapore™ tape (3M Corporation, Minneapolis, MN) fitted tightly over the edge of the nostrils and columna. This procedure effectively isolates the nostril being examined and prevents retronasal airflow. Olfactory tests, University of Pennsylvania Smell Identification Test (UPSIT) booklets, and nostril presentation were systematically counterbalanced among subjects.
Odor Identification
Odor identification skills were assessed with the UPSIT. 12 The UPSIT is a standardized, four-alternative, forced-choice test of olfactory identification comprising four booklets that contain 10 odorants apiece, one odorant per page. The stimuli are embedded in “scratch and sniff” microcapsules fixed and positioned on strips at the bottom of each page. Two booklets of the test were administered to the left nostril, and two to the right nostril.
Odor Detection Threshold Sensitivity
Subjects received a single staircase, forced-choice odor detection threshold test to estimate basal detection sensitivity to phenylethyl alcohol (PEA: Gold Label Grade; Aldrich Chemical Co., Milwaukee), a compound with low trigeminal stimulation properties. 13 The geometric mean of the last four staircase reversal points of a total of seven served as the estimate of threshold sensitivity.
Data Analysis
Performances between patients and healthy volunteers on PEA detection threshold sensitivity, UPSIT, and ER-40 were compared using multivariate analysis of covariance (ANCOVA), with age as a covariate. Spearman correlations were calculated to assess relationships between performance scores on the ER-40 and UPSIT and PEA performance.
RESULTS
Schizophrenia patients showed poorer overall facial emotion recognition skills (F [1, 28]=6.3, p=0.015) including fearful (F [1, 28]=6.0, p=0.02) and neutral (F [1, 28]=9.4, p=0.004) expressions, but not happy, sad, or angry faces (all p values>0.22). Patients and healthy subjects did not differ in odor detection thresholds or identification ability, with no main effects of diagnosis, sex, or interactions evident (all p values>0.09).
In the schizophrenia group, an association between ER-40 total score and right nostril UPSIT (r=0.46, p=0.049) but not left nostril UPSIT performance was observed. For this primary finding, we compared the effect sizes for the correlation between right nostril UPSIT performance and total ER-40 score for patients and comparison subjects. This contrast revealed that these effects were significantly different between patients and comparison subjects (Q 1 =8.4, p=0038). That is, the effect size for the relationship between right nostril UPSIT and total ER-40 score was quite large in patients (d + =1.88, 95% CI=1.12<δ<2.65) and significantly smaller in comparison subjects (d + =0.31, 95% CI=−0.44<δ<1.05). When broken down by individual emotion, the observed relationship between right nostril UPSIT performance and ER-40 total score appeared to be primarily mediated by the recognition of sad faces (r=0.53, p=0.018) ( Figure 1 ). There were no significant correlations between olfactory and emotion measures and positive and negative symptoms; however, the small sample size may have insufficient power for detection. No significant correlations were observed between ER-40 and UPSIT performance in healthy comparison subjects. No significant correlations were observed between odor detection thresholds and ER-40 scores in either group. Similarly, no sex-specific correlations with olfactory or facial emotion indices were observed.
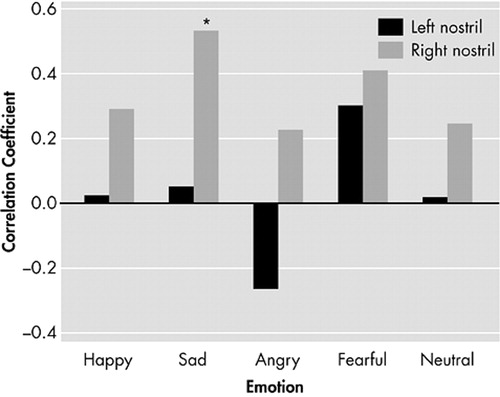
*p<0.05
CONCLUSIONS
These data support a relationship between abilities for recognition of facial emotions and odor identification in persons with schizophrenia, but not in healthy persons. Similarly, in a previous study we reported a selective association in schizophrenia involving emotion recognition and certain cognitive abilities that are mediated by fronto-temporal brain areas, including abstraction, attention, verbal and spatial memory, and language abilities. 14 The existence of a differential emotion recognition deficit remains controversial and has been supported in some older, but not more recent studies. Nevertheless, associations between emotion recognition and related but separate higher cortical functions of olfaction and cognition support the notion that emotion processing may be more selectively affected in schizophrenia.
The relationship of right nostril UPSIT performance with facial emotion recognition is consistent with the literature, showing an advantage for the right hemisphere in the processing of both olfactory and emotional stimuli. 5 There is also evidence suggesting that subjects with unilateral right amygdala damage show impairment in their ability to identify sad facial expressions. 15 In summary, our findings indicate that emotional processing, which utilizes similar neural substrates as olfactory processing, is more selectively affected in schizophrenia.
1. Edwards J, Jackson HJ, Pattison PE: Emotion recognition via facial expression and affective prosody in schizophrenia. Clin Psychol Rev 2002; 22:789–832Google Scholar
2. Kohler CG, Turner TH, Bilker WB, et al: Facial emotion recognition in schizophrenia: intensity effects and error pattern. Am J Psychiatry 2003; 140:1768–1774Google Scholar
3. Moberg PJ, Agrin R, Gur RE, et al: Olfactory dysfunction in schizophrenia: a qualitative and quantitative review. Neuropsychopharmacology 1999; 21:325–340Google Scholar
4. Pitkanen A, Pikkarainen M, Nurminen N, et al: Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat: a review. Ann N Y Acad Sci 2000; 911:369–391Google Scholar
5. Borod JC, Bloom RL, Brickman AM, et al: Emotional processing deficits in individuals with unilateral brain damage. Appl Neuropsychol 2002; 9:23–36Google Scholar
6. Phillips ML, Drevets WC, Rauch SL, et al: Neurobiology of emotion perception I: the neural basis of normal emotion perception. Biol Psychiatry 2003; 54:504–514Google Scholar
7. Royet JP, Plailly J: Lateralization of olfactory processes. Chem Senses 2004; 29:731–745Google Scholar
8. Crespo-Facorro B, Paradiso S, Andreasen NC, et al: Neural mechanisms of anhedonia in schizophrenia: a PET study of response to unpleasant and pleasant odors. JAMA 2001; 286:427–435Google Scholar
9. Hudry J, Saoud M, D’Amato T, et al: Ratings of different olfactory judgments in schizophrenia. Chem Senses 2002; 27:407–416Google Scholar
10. Moberg PJ, Arnold SE, Doty RL, et al: Impairment of odor hedonics in men with schizophrenia. Am J Psychiatry 2003; 160:1784–1789Google Scholar
11. Kohler CG, Anselmo-Gallagher G, Bilker W, et al: Emotion discrimination deficits in mild Alzheimer disease. Am J Geriatr Psychiatry 2005; 13:926–933Google Scholar
12. Doty RL, Shaman P, Dann M: Development of the University of Pennsylvania Smell Identification Test: a standardized microencapsulated test of olfactory function. Physiol Behav 1984; 32:489–502Google Scholar
13. Doty RL, McKeown DA, Lee WW, et al: A study of the test-retest reliability of ten olfactory tests. Chem Senses 1995; 20:645–656Google Scholar
14. Kohler CG, Bilker W, Hagendoorn M, et al: Emotion recognition deficit in schizophrenia: association with symptomatology and cognition. Biol Psychiatry 2000; 48:127–136Google Scholar
15. Ralph A, Tranel D: Impaired judgments of sadness but not happiness following bilateral amygdala damage. J Cogn Neurosci 2004; 16:453–462Google Scholar



