HIV Proviral DNA Associated With Decreased Neuropsychological Function
In this exploratory study, we analyzed an expanded data set to explore the association of HIV DNA with specific neuropsychological deficits. The objectives were to assess the relationship of baseline HIV DNA to baseline and yearly neuropsychological deficits for 2 years, and to determine whether baseline HIV DNA predicts subsequent neuropsychological deficits. Based on our earlier observation suggesting that HIV DNA may be predominant in circulating monocytes/macrophages, we hypothesized that HIV DNA levels and neuropsychological deficits would be transient and both would co-vary over time. 7
METHOD
Cohort and Clinical Data
Cases analyzed from the Hawaii Aging with HIV Cohort (HAHC) for this study matched those previously reported. 7 From the previously reported 49 subjects, 44 individuals completed at least two annual follow-up visits and comprised the cohort for the current study. The current study was limited to those reported in our earlier study 7 (i.e., those with HAD and normal cognition), because the intent of this study was to follow up and extend the analyses of individuals enrolled in the cohort over subsequent years. The original report studied randomly selected individuals enrolled in the HAHC and diagnosed with HAD and normal cognition but did not include those with intermediate neuropsychological deficits. 7 The rationale was to try to assess the value of HIV DNA on extreme opposite ends of neurocognitive diagnoses, HAD and normal cognition, which resulted in a skewed percentage of HAD cases. In actuality, the number of HAD cases in the HAHC is better represented in a recent analysis of HAD cases in older (25.2%) and younger (13.7%) individuals. 12 The diagnosis of HAD was made after individual cases were discussed in a consensus conference involving two neurologists, two neuropsychologists, and a geriatrician. 12 HIV dementia cases were defined with the American Academy of Neurology criteria using all clinical data obtained during the study visit. 8 A diagnosis of HIV dementia required an abnormality in at least two cognitive domains, including attention/concentration, speed of information processing, abstraction/reasoning, visuospatial skills, memory/learning and speech/language, and either an abnormality in motor function or a decline in motivation/emotional control.
The HAHC protocol, assessment battery, and demographic characteristics of the individuals are described elsewhere. 7 , 12 Briefly, the HAHC is a collaboration between the University of Hawaii and Johns Hopkins University to evaluate older (50 years of age or higher) and younger (20 to 39 years of age) HIV-1-seropositive individuals. Major exclusion criteria included the following: major psychiatric disorder including bipolar disorder, schizophrenia, and active major depression; head injury with loss of consciousness for more than 1 hour; CNS opportunistic infection; learning disability; and neurological disease, such as major stroke, multiple sclerosis, or delirium. English was the primary language for all individuals. Broad community-based recruitment techniques were implemented, including recruitment from AIDS service organizations, through advertisement in local newspapers, referrals from community physician clinics, and participation in local HIV/AIDS events. Participants were recruited from all major islands of Hawaii and were living in Hawaii.
Participant evaluations included demographics, medical history, the macroneurological examination as used in the Adult AIDS Clinical Trials Group (including the United Parkinson’s Disease Rating Scale to examine for extrapyramidal signs), medication/adherence history, DSM-IV-based substance abuse/dependence inventory, immunologic and virologic laboratory tests, and neuropsychological testing. 13 , 14 The 80-minute neuropsychological test battery assessed multiple cognitive domains affected by HIV-1 and included the following: Choice and Sequential Reaction Time from the California Computerized Assessment Package (CalCAP), the Rey Auditory Verbal Learning Test (RAVLT), Rey Osterreith Complex Figure (RCF) Copy and Recall, Trail Making Test, Parts A and B, Wechsler Adult Intelligence Scale–Revised (WAIS-R) Digit Symbol, Grooved Pegboard (dominant and nondominant hands), Verbal Fluency Test (FAS), Animal Naming, Boston Naming Test (BNT), the WAIS-R Digit Span (Forward and Backward), and Timed Gait. Depression symptomatology was assessed using the Beck Depression Inventory (BDI). This neuropsychological test battery was adapted from that used in the NorthEast AIDS Dementia (NEAD) cohort. 15
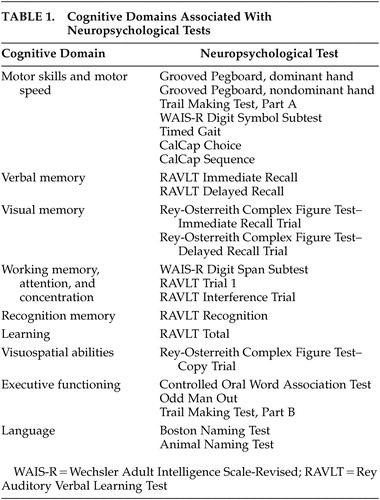
Various normative data were required due to the use of a comprehensive test battery and the inclusion of both younger and older subjects. The normative data were selected as the best possible dataset for this population with a long history of applications in HIV-1 research (e.g., Multicenter AIDS Cohort Study [MACS]). Application of these norms was guided by the clinical neuropsychologists on the team. Normative neuropsychological data for individuals with a high school or greater education were derived from the MACS consisting of 733 HIV-1-seronegative subjects with risk profiles similar to the Hawaii cohort. For individuals with less than a high school education, normative neuropsychological data from the AIDS Link to IV Experience study (N=150) were used. 16 These two normative sets have few individuals over 54 years old. Thus, for individuals over 54, alternative published normative data were used. 17 , 18 Normative data for the RCF were taken from alternative published norms for individuals over 59 years old and the main MACS normative set for individuals under 60 years old. 19 A similar battery with a large overlap in norms was shown to be appropriate for HIV-1-infected individuals of similar ethnic diversity. 20 All test results were transformed to z scores using appropriate age and education-matched normative data sets. Scores for cognitive domains (i.e., motor skills/motor speed; verbal memory; visual memory; working memory, attention, and concentration; learning; recognition memory; visuospatial abilities; executive functioning; language) were calculated by averaging the z scores of the neuropsychological tests corresponding to the domains they were intended to measure ( Table 1 ).
 |
Plasma HIV-1 RNA levels (VL) and CD4 cell counts were performed by a certified clinical laboratory, as previously reported. 7
Specimens and HIV DNA Assay
HIV DNA, normalized to the number of copies of HIV-1 proviral DNA per 10 6 cells, was measured as previously reported from peripheral blood mononuclear cells. 7 The assays were set up with independent standard curves measuring the relative ratios of HIV DNA copy number to cellular equivalent genomic DNA along with appropriate positive and negative controls and reported as HIV DNA copy per 10 6 cells. 7
Statistical Analysis
We examined the association between HIV DNA and each of the nine neuropsychological deficits in a series of multilevel longitudinal regression models. The level one variable in the model included initial neuropsychological deficits and annual rates of change. Given the exploratory aim of this study, p values were not adjusted for multiple endpoints and thus were construed to be complementary to the regression coefficients’ indications of the association strength for each neuropsychological deficit rather than strict hypothesis tests. All analyses were conducted using SAS® v9.1 PROC MIXED specifying maximum likelihood as the estimation method and an unstructured covariance matrix. The temporal variability within each deficit was estimated in two ways: 1) fixed effect for time, and 2) probability of the residual variance of each individual’s trajectory around the overall mean trajectory. We examined the association between HIV DNA and baseline neuropsychological deficit and annual rate of change of each deficit, as indicated by the TYPE III fixed effect for HIV DNA after it was added to the unconditional growth model. To determine whether the obtained estimates were attributable to other patient characteristics, we added age, ethnicity and premorbid IQ. To determine whether associations between HIV DNA and each deficit were present in individuals with undetectable plasma HIV RNA levels, we repeated the univariate analyses using only those with undetectable levels.
RESULTS
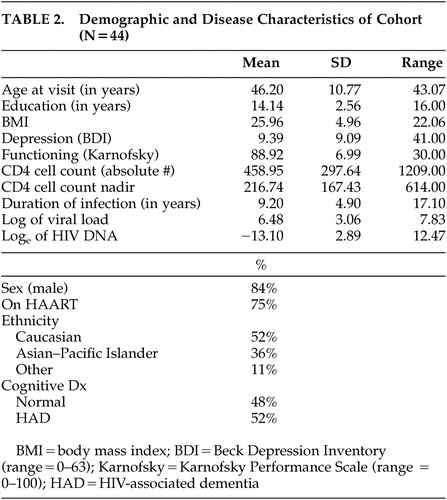
Characteristics and demographics of the subjects are summarized in Table 2 . The mean age at entry was 46.2 (SD=10.77) years with the mean number of years of education being 14.14 (SD=2.56). The mean BDI score was 9.39 (SD=9.09) and the mean Karnofsky score was 88.92 (SD=6.99). The mean duration of HIV-1 infection as self-reported was 9.2 (SD=4.9) years with nadir CD4 cell count reported as 217 cells/μL (SD=167.43).
 |
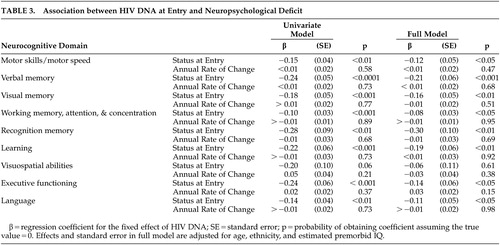
The decrement in baseline of each neuropsychological deficit associated with HIV DNA is plotted in Figure 1 , and adjusted for age, ethnicity, and estimated premorbid IQ, where ethnicity and IQ are not confounded with cognitive diagnosis. For each unit increase in log e of HIV DNA, there is a decrease in neuropsychological deficit. Neuropsychological deficits were significantly associated with baseline HIV DNA in all deficits except for one: visuospatial abilities (regression coefficients range from −0.27 to −0.10, p<0.01) ( Table 3 ). The effect of HIV DNA on neuropsychological deficits remained significant after adjusting for age, ethnicity, and estimated premorbid IQ. When analyses were repeated using only individuals with undetectable plasma HIV-1 RNA levels, the association between neuropsychological deficits and baseline HIV DNA remained significant (N=26, p<0.01). The effect for time in the unconditional growth model was significant in only two of the nine domains: visual memory (regression coefficient=0.18, SE=0.04, p<0.0001) and executive functioning (regression coefficient=0.22, SE=0.04, p<0.0001). However, the variance component for residual variance in change over time was significant for only one domain: motor skills and motor speed (z=2.48. p<0.01). In this exploratory analysis, baseline HIV DNA baseline was not associated with neuropsychological deficit change over the subsequent 2 years.
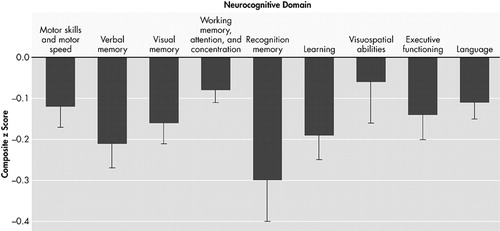
Error bars represent Standard Error for regression coefficient in a multilevel longitudinal model. Effects and standard errors are adjusted for age, ethnicity, and estimated premorbid IQ. No significant effects were observed for annual rate of change.
 |
DISCUSSION
Much progress has been made in understanding the pathogenesis of HAD, but the mechanisms leading to disease progression are still not completely delineated. 21 – 24 We previously reported that high HIV DNA levels were associated with HAD and hypothesized that HIV DNA, independent of plasma HIV-1 RNA levels, was involved in HAD pathogenesis. 7 In our current exploratory study, we expanded our prior results with a more complete dataset using less restrictive endpoints. We demonstrated that HIV DNA was significantly associated with baseline neuropsychological deficits in all but one domain (visuospatial abilities), but did not predict subsequent changes in neuropsychological deficits. Although not shown yet, we hypothesize that HIV DNA levels and neuropsychological deficits are transient and both factors may co-vary over time; however, the limitations of this exploratory study cannot conclusively rule out any potential role for HIV DNA as a predictive marker. The lack of predictive value is best understood against the backdrop of minimal temporal variation that was available for the analysis. Only two deficits (visual memory and executive functioning) showed a significant effect for time in the unconditional growth models, indicating that the average trajectory over 2 years was fairly flat. The time effect alone can be assessed on the outcome without any additional predictors.
In this model, the coefficients indicate the amount of change in the outcome that is attributable to a unit of change in the predictor. The direction of change is indicated by the sign of the beta. In this case, the positive sign of the betas indicate improved performance in the two outcomes, which could possibly be due to a practice effect. The fact that only these two outcomes were significant suggests that there may be too little temporal variability in the data for the question of HIV DNA’s impact on subsequent change to be adequately posed.
The one deficit (motor skills and motor speed) that showed a significant residual variance component for change over time in unconditional growth models suggests that little variation was available to be explained by HIV DNA or other predictors added to the model. The residual variance is the variance that is not explained by the fixed and random effects in the mixed model. The mixed model allowed for testing of the residual variance of the trajectory of motor skills and motor speed to determine whether it was statistically different. Thus, the findings suggest the possibility that the lack of association may be partly a function of the small amount of change that was available to be explained to begin with, and that if the amount of change were increased, for instance, by prolonging the period of observation, the predictor would have a better chance of being associated with change over time.
The study is limited by including only subjects with normal cognition and HAD, excluding individuals with mild or intermediate neuropsychological deficits, thus limiting the generalizability of the results. 7 An additional limitation of interpreting the results of the study is the absence of comparable and adequate neuropsychological norms for the entire subject group, particularly the norms used for the older subjects in which data for comparable education levels were lacking. Thus, the results of this study should be taken in the context that various norms for the subject groups may place limitations on the comparable standard scores and diagnoses.
HIV DNA has been studied by other investigators 11 to assess HIV-1 disease progression and has been found to predict progression to AIDS. Rouzioux et al. 11 reported on a cohort of individuals who were not on antiretroviral treatment initially but subsequently were put on therapy; and demonstrated that HIV DNA was a marker for progression to AIDS, independent of plasma HIV-1 RNA levels. 11 In our previous study, to assess the association of HIV DNA with HAD, we used the AAN diagnostic criteria for HAD, which are based on combined functional impairments and neuropsychological deficits. 1 , 8 , 12 , 25 The HIV-1-associated cognitive deficits now observed in developed countries may be less severe and their trajectories more varied than the dementia label implies. Nevertheless, even in an era where viremia in developed countries is declining among HIV-1-infected individuals, HAD and milder neurocognitive diagnoses clearly carry clinically relevant functional implications.
In the current study, we looked specifically at neuropsychological deficits for associations with HIV DNA in an attempt to identify focal deficits that might be affected by HIV DNA. Because we believe that HIV DNA could potentially co-vary and affect neuropsychological deficits over time, future studies should focus on: increasing the amount of explainable temporal variation by analyzing neuropsychological deficits over a longer duration in order to assess the predictive value of HIV DNA on neuropsychological deficits; sampling older individuals whose rate of change over a given period is likely to be greater; and including subjects with intermediate neuropsychological deficits.
In conclusion, the results of this study suggest that the neuropsychological deficits associated with HIV DNA levels may be global in nature. Any forthcoming studies to assess the association between HIV DNA and subsequent neuropsychological deficits should require periods of observation in excess of 2 years to address the predictive value of HIV DNA. It is possible that the lack of association of baseline HIV DNA levels with subsequent neuropsychological deficits may suggest that HIV DNA and neuropsychological deficits co-vary over time. Similar to the scenario where plasma HIV-1 RNA levels fluctuate with nonadherence of HAART, HIV DNA copies in monocytes/macrophages may also change through some as-of-yet unidentified influence. Thus, if HIV DNA influences neuropsychological deficits, resulting in the two entities co-varying over time, this fact may need to be taken into consideration in developing future treatment or preventative strategies.
1. Langford TD, Letendre SL, Larrea GJ, et al: Changing patterns in the neuropathogenesis of HIV during the HAART era. Brain Pathol 2003; 13:195–210Google Scholar
2. Albright AV, Soldan SS, Gonzalez-Scarano F: Pathogenesis of human immunodeficiency virus-induced neurological disease. J Neurovirol 2003; 9:222–227Google Scholar
3. Gartner S, Liu Y: Insights into the role of immune activation in HIV neuropathogenesis. J Neurovirol 2002; 8:69–75Google Scholar
4. Gendelman HE, Lipton SA, Tardieu M, et al: The neuropathogenesis of HIV-1 infection. J Leukoc Biol 1994; 56:389–398Google Scholar
5. Fischer-Smith T, Croul S, Sverstiuk AE, et al: CNS invasion by CD14+/CD16+ peripheral blood-derived monocytes in HIV dementia: perivascular accumulation and reservoir of HIV infection. J Neurovirol 2001; 7:528–541Google Scholar
6. Gelbard HA, James HJ, Sharer LR, et al: Apoptotic neurons in brains from paediatric patients with HIV-1 encephalitis and progressive encephalopathy. Neuropathol Appl Neurobiol 1995; 21:208–217Google Scholar
7. Shiramizu B, Gartner S, Williams A, et al: Circulating proviral HIV DNA and HIV-associated dementia. AIDS 2005; 19:45–52Google Scholar
8. Nomenclature and research case definitions for neurological manifestations of human immunodeficiency virus-type 1 (HIV-1) infection: Report of a Working Group of the American Academy of Neurology AIDS Task Force. Neurology 1991; 41:778–785Google Scholar
9. McArthur C: HIV dementia: an evolving disease. J Neuroimmunol 2004; 157:3–10Google Scholar
10. Brew BJ: Evidence for a change in AIDS dementia complex in the era of highly active antiretroviral therapy and the possibility of new forms of AIDS dementia complex. AIDS 2004; 18(suppl 1):75–78Google Scholar
11. Rouzioux C, Hubert JB, Burgard M, et al: Early levels of HIV-1 DNA in peripheral blood mononuclear cells are predictive of disease progression independently of HIV-1 RNA levels and CD4+ t cell counts. J Infect Dis 2005; 192:46–55Google Scholar
12. Valcour V, Shikuma C, Shiramizu B, et al: Higher frequency of dementia in older HIV+ individuals: the Hawaii Aging With HIV Cohort. Neurology 2004; 63:822–827Google Scholar
13. Gancher S: Scales for the assessment of movement disorders, in Handbook of Neurological Rating Scales. Edited by Herndon R. New York, Demos Vermande, 1997, pp 81–123Google Scholar
14. Schmitt FA, Bigley JW, McKinnis R, et al: Neuropsychological outcome of zidovudine (AZT) treatment of patients with AIDS and AIDS-related complex. N Engl J Med 1988; 319:1573–1578Google Scholar
15. Clinical confirmation of the American Academy of Neurology algorithm for HIV-1-associated cognitive/motor disorder: the Dana Consortium on Therapy for HIV Dementia and Related Cognitive Disorders. Neurology 1996; 47:1247–1253Google Scholar
16. Concha M, Selnes OA, McArthur JC, et al: Normative data for a brief neuropsychologic test battery in a cohort of injecting drug users. Int J Addict 1995; 30:823–841Google Scholar
17. Tombaugh TN, Kozak J, Rees L: Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Arch Clin Neuropsychol 1999; 14:167–177Google Scholar
18. Heaton RK, Avitable N, Grant I, et al: Further crossvalidation of regression-based neuropsychological norms with an update for the Boston Naming Test. J Clin Exp Neuropsychol 1999; 21:572–582Google Scholar
19. Chiulli S, Haaland K, LaRue A, et al: Impact of age on drawing the Rey-Osterrieth figure. Clin Neuropsychol 1995; 9:219–224Google Scholar
20. Robbins R, Rivera Mindt M, Heaton RK, et al: Are there ethnicity differences in the ability of neuropsychological test performance to predict functional impairment in HIV infection? in Thirty-Second Annual International Neuropsychological Society Conference. Baltimore, 2004Google Scholar
21. Sacktor NC, Bacellar H, Hoover DR, et al: Psychomotor slowing in HIV infection: a predictor of dementia, AIDS and death. J Neurovirol 1996; 2:404–410Google Scholar
22. Swindells S, Zheng J, Gendelman HE: HIV-associated dementia: new insights into disease pathogenesis and therapeutic interventions. AIDS Patient Care STDS 1999; 13:153–163Google Scholar
23. Pulliam L, Clarke JA, McGrath MS, et al: Monokine products as predictors of AIDS dementia. AIDS 1996; 10:1495–1500Google Scholar
24. Paul RH, Cohen RA, Stern RA: Neurocognitive manifestations of human immunodeficiency virus. CNS Spectr 2002; 7:860–866Google Scholar
25. McArthur JC, Haughey N, Gartner S, et al: Human immunodeficiency virus-associated dementia: an evolving disease. J Neurovirol 2003; 9:205–221Google Scholar



