Proton MRS and Neuropsychological Correlates in AIDS Dementia Complex: Evidence of Subcortical Specificity
Consistent with the clinical expression of cognitive symptoms, pathological abnormalities associated with HIV are usually confined to the deep gray and white matter, but as discussed below, may also be seen in the cortex. The severity of clinical disease generally correlates most strongly with structural and functional abnormalities in the basal ganglia and white matter and is influenced by the expression of a variety of host and viral factors in these regions. 11 – 13 Structural and functional neuroimaging studies have shown that alterations in subcortical volume or metabolism, particularly in the basal ganglia, are strongly related to cognitive dysfunction. 14
Despite the converging evidence in support of a subcortical profile associated with HIV, there is neuropathological evidence that cortical abnormalities also exist among infected patients. 15 – 19 Reductions in cortical synaptic density have been reported at different stages of HIV infection, 20 and cortical dendritic branching is reduced among individuals with mild HIV-associated cognitive motor disorder. 21 These findings question the specificity of subcortical abnormalities in HIV as they relate to cognitive function.
Studies show that infiltration of the brain by infected macrophages is associated with an inflammatory cascade mediated by the release of host- (e.g., cytokines, oxygen free radicals) and viral-related factors (e.g., tat, gp120) that disrupt neural function. 22 , 23 This neurochemical cascade is associated with metabolic abnormalities that can be detected and directly measured in brain tissue by proton magnetic resonance spectroscopy (MRS). Proton MRS provides a robust method to examine such neurochemical changes at the regional and cellular level. Previous studies using MRS have focused on several specific brain metabolites including choline, myoinositol, and N-acetylaspartate. Choline and myoinositol are believed to represent markers of gliosis, whereas N-acetylaspartate is a putative marker of mature neurons. 24 Studies of HIV patients have revealed significant elevations in choline and myoinositol in subjects with mild cognitive impairment and significant reductions in N-acetylaspartate in the cortex of individuals with more severe neuropsychological impairment. 24 – 38
We formed an HIV MRS consortium to further assess the in vivo effects of HIV infection on regional brain function. The consortium developed a multicenter protocol that can reliably generate metabolite data at a short echo time, allowing for measurement of the myoinositol peak from three brain regions, specifically, the basal ganglia, frontal white matter, and parietal cortex. Initial outcomes from the consortium revealed that ADC patients exhibited significantly lower levels of N-acetylaspartate/creatine in the frontal white matter compared with the nondemented HIV-positive individuals and significantly increased levels of choline/creatine and myoinositol/creatine in the basal ganglia and frontal white matter compared with HIV seronegative subjects. 38 By contrast, nondemented HIV-positive individuals showed a significantly elevated myoinositol/creatine in the frontal white matter compared to seronegative subjects, and this was the sole metabolite abnormality.
The purpose of the present study was to examine relationships between metabolite abnormalities in the basal ganglia and frontal white matter and neuropsychological performance in patients with HIV in the context of antiretroviral therapy. We examined the specificity of relationships between MRS indices obtained from the basal ganglia and frontal white matter and neuropsychological performance by determining whether neuropsychological performance correlated with MRS indices obtained in the cortex. The parietal cortex was selected as a nonspecific cortical site, since, based on previous studies, 38 this site is believed to be less involved in HIV and therefore would serve as a general index of cortical health. We predicted that neuropsychological function would correlate strongly with levels of specific metabolites in the basal ganglia, subcortical white matter, or both. In contrast, we hypothesized that neuropsychological function would not strongly correlate with metabolite levels in the parietal cortex.
METHOD
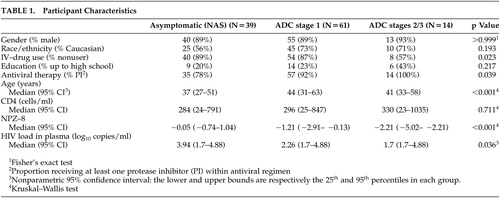
We recruited participants as part of the AIDS Clinical Trials Group (ACTG) study 301, a phase II trial of memantine for ADC and peripheral neuropathy and its proton MRS substudy, ACTG 700. They were enrolled between December 1997 and December 1999 at eight sites in the United States (Massachusetts General Hospital; University of California, Los Angeles; University of Pennsylvania; University of Washington, University of Rochester, Mt. Sinai Medical Center; University of Texas; and University of California, San Francisco). The study cohort comprised 61 participants diagnosed with ADC and 39 HIV positive neurologically asymptomatic subjects ( Table 1 ). The entry criteria for the ADC group included clinical diagnosis of ADC (Memorial Sloan Kettering Classification [MSKC] Stage I or greater) and performance on the cognitive battery that was at least 1 SD point below age- and education-specific normative data on two independent tests, or more than 2 SDs in at least one test (see below). Individuals who received an MSKC stage score of 0 were classified as neurologically asymptomatic. The majority of the participants at entry were receiving highly active antiretroviral therapy (HAART), consisting of at least one protease inhibitor. All information we report in the present study was obtained at the baseline assessment.
 |
Neuropsychological Assessment
A 40-minute neuropsychological test battery was administered to all participants. The battery included the Rey Auditory Verbal Learning Test; 39 Timed Gait; Grooved Pegboard, dominant and nondominant hands; 40 Symbol Digit Modalities Test; 41 Trail-Making Test; 42 Stroop Color Interference Test; 43 and the CalCAP, simple and sequential reaction time. 44 These measures have been used extensively since 1986 in the longitudinal Multicenter AIDS Cohort Study (MACS). 45 In addition, the measures are sensitive to the key cognitive signs of ADC (e.g., gross and fine motor slowing, slowed information processing) and are known to discriminate AIDS from nonAIDS cases and HIV-associated cognitive impairment from nonimpairment. 45 – 50
A composite score composed of eight neuropsychological test scores (NPZ-8) was derived by aggregating eight age- and education-adjusted individual neuropsychological scores from the above tests. Standardization of the scores was based on normative data from the MACS, which are available from over 1,000 HIV-negative comparison subjects. The standard scores were defined so that positive values indicate better performance compared with the corresponding age- and education-adjusted norm, while negative values indicate poorer performance. Scores close to zero indicate average performance. The utility of the NPZ-8 index score in the assessment of cognitive impairment at baseline has been documented in several clinical trials of ADC, and a close correlation has been found with the ADC stage as defined by the MSKC classification. 45 , 47 , 48
To further examine the relationship of specific domains of neuropsychological test performance (e.g., processing speed, fine motor coordination, psychomotor speed) to regional levels of brain metabolites, we categorized all of the neuropsychological tests into functional groupings based on previously reported factor analyses. 49 , 50 The six categories included: gross motor functioning (Timed Gait), fine motor functioning (Grooved Pegboard, dominant and nondominant hands), psychomotor functioning (Trail-Making Test and Symbol Digit), speed of cognitive processing (CalCAP Reaction Time tests), frontal systems functioning (Stroop Color Interference Test), and verbal memory (Auditory Verbal Learning Test, sum of trials 1 to 5).
Proton Magnetic Resonance Spectroscopy ( 1 H-MRS)
To ensure that data were acquired uniformly across all sites, all MRS/MRI exams were performed on Signa 1.5T MR imagers (GE Medical Systems, Milwaukee, Wisc.), operating with system 5.6 or higher and using the standard GE quadrature head coil. The imaging study protocol, detailing all parameter settings under operator control, was prepared and distributed by the central site. Spectra were localized in the parietal cortex, frontal white matter and basal ganglia. Images, raw spectroscopic data, and films showing voxel placement were transferred electronically to Massachusetts General Hospital. Raw data sets were analyzed using the commercial software package SageIDL (GE Medical Systems, Milwaukee). Intersite reliability was established in a multicenter validation study as described by Lee et al. 38
Statistical Procedures
We compared neuropsychological test performance between subgroups by the Wilcoxon-Mann-Whitney (WMW) rank sum test and assessed correlation between pairs of variables via the Spearman correlation coefficient and the partial Spearman correlation with an adjustment made for the age of each subject. We reported p values below 0.05 and between 0.05 and 0.10. In addition, we performed a Bonferroni adjustment to correct for multiple comparisons. Generally, five metabolite ratios were measured in the three brain regions, resulting in 15 comparisons. After adjustment, p values below 0.0033 (0.05/15) were considered significant at the 5% level, while p values between 0.003 and 0.0067 (0.10/15) were considered significant between the 5% and 10% level.
RESULTS
Demographics (age, education, race/ethnicity) as well as HIV-related characteristics (CD4 count, type of antiviral therapy) are shown in Table 1 . Of note is the relatively high median CD4 count among all HIV-positive patients. Further, the median plasma viral load was significantly higher among cognitively intact individuals, possibly reflecting less frequent use of combined therapies in this group. Median NPZ-8 scores are presented in Table 1 . As expected, more severely impaired participants (ADC stages 2 and 3) scored lower than individuals with mild ADC (ADC stage 1) (WMW p<0.001). The most impaired group (ADC Stages 2 and 3) showed an NPZ-8 mean more than 2 SDs below the norm (below the third percentile).
Association Between NPZ-8 and Cerebral Metabolites
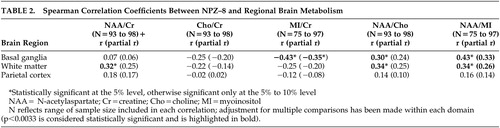
To identify the metabolic substrates that underlie neuropsychological performance ( Table 2 ), we examined associations between NPZ-8 and specific regional metabolite ratios. Individuals with higher scores on the NPZ-8 exhibited significantly higher levels of N-acetylaspartate/creatine in the frontal white matter. Conversely, individuals with lower scores on the NPZ-8 exhibited higher levels of myoinositol/creatine in the basal ganglia. Significant associations between the NPZ-8 score and N-acetylaspartate/choline, and N-acetylaspartate/myoinositol were also observed, a finding likely due to the relationships between the NPZ-8 and both N-acetylaspartate and myoinositol levels. Associations between metabolites in the parietal cortex and the NPZ-8 battery were not statistically significant. After adjusting for the age of the subject ( Table 2 , numbers in parentheses) the results were the same as described above with one notable exception: adjusting for age attenuated the correlation between NPZ-8 and levels of N-acetylaspartate/creatine in the frontal white matter.
 |
Associations Between Individual Neuropsychological Measures and Cerebral Metabolites
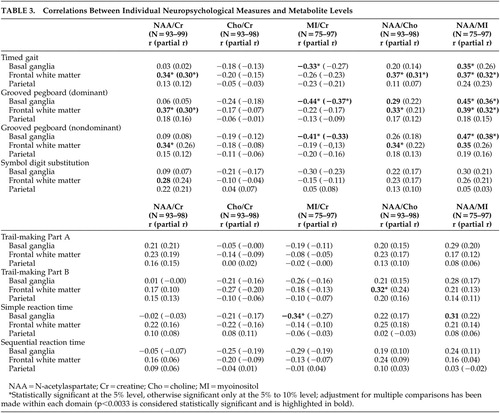
As shown in Table 3 , poorer performance on Timed Gait and Grooved Pegboard with dominant and nondominant hands was associated with higher levels of myoinositol/creatine in the basal ganglia, and lower levels of N-acetylaspartate/myoinositol in the basal ganglia. Poorer performances on Timed Gait and Grooved Pegboard dominant hand were also associated with lower levels of N-acetylaspartate/creatine, N-acetylaspartate/choline, and N-acetylaspartate/myoinositol in the frontal white matter. Further, individuals with lower scores on the Trail-Making Test, Part B, exhibited lower levels of N-acetylaspartate/choline in the frontal white matter, while poorer performances on Simple Reaction Time correlated with higher levels of myoinositol/creatine in the basal ganglia. Finally, poorer performance on Symbol Digit correlated with lower levels of N-acetylaspartate/creatine in the frontal white matter. No significant correlations were found between MRS indices and performances on the Trail-Making Test, Part A, or the Sequential Reaction Time test. Adjustment for age through partial correlations ( Table 3 ) produced similar results with the analysis as described above, though a few correlations were weakened.
 |
Associations Between Functional Domains and Cerebral Metabolites
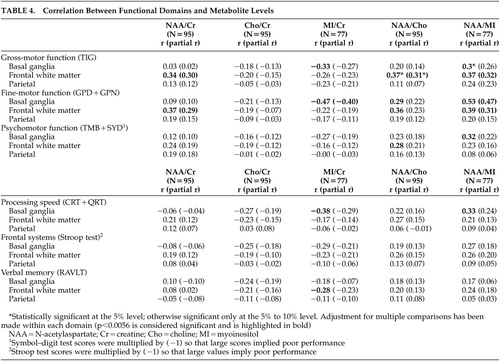
To further understand the associations between specific cognitive functions and brain metabolites, we categorized each of the neuropsychological tests into functional domains based on a factor analysis of the test battery ( Table 4 ). Poorer performance in the gross motor function domain was associated with higher levels of myoinositol/creatine in the basal ganglia and lower levels of N-acetylaspartate/myoinositol in the basal ganglia. In addition, poorer performance in this domain was associated with lower levels of N-acetylaspartate/creatine, N-acetylaspartate/choline, and N-acetylaspartate/myoinositol in the frontal white matter. Poorer performance in the fine motor domain was correlated with higher levels of myoinositol/creatine in the basal ganglia and lower levels of N-acetylaspartate/myoinositol in the basal ganglia. Poorer performance in this domain was also significantly correlated with lower levels of N-acetylaspartate/creatine, N-acetylaspartate/choline, and N-acetylaspartate/myoinositol in the frontal white matter. Psychomotor function was associated with N-acetylaspartate/myoinositol in the basal ganglia and N-acetylaspartate/choline in the frontal white matter and reduced processing speed was associated with higher levels of myoinositol/creatine in the basal ganglia. No significant relationships were evident between the frontal systems and verbal memory domains and MRS indices. In addition, as described above, there were no significant correlations between functional domains and individual metabolites measured in the parietal cortex. After adjustment for age through partial correlations ( Table 4 , numbers in parentheses), the relationships between myoinositol/creatine in the basal ganglia and Timed Gait were reduced, but other relationships between myoinositol/creatine in the basal ganglia and N-acetylaspartate/creatine in the frontal white matter remained significantly associated with neuropsychological performance.
 |
DISCUSSION
We undertook this study to examine the relationship between in vivo brain metabolism as measured by MRS and neuropsychological domains of cognitive function previously shown to be affected among HIV-infected patients. Strong relationships were found between neuropsychological performance and MRS indices of increased gliosis and reduced mature neurons in the subcortical regions, whereas no significant relationships were identified between these measures in the parietal cortex.
A number of previous studies have reported significant relationships between brain metabolite levels and cognitive performance in HIV. Rottenberg et al. 51 showed that decreases in glucose metabolism in the subcortical regions as measured by positron emission tomography correlated with measures of motor function, such as the Grooved Pegboard, while cortical metabolic abnormalities predicted performance on tests of executive function. López-Villegas et al. 33 applied high-resolution spectroscopy in the frontal gray and white matter in 15 HIV-positive subjects and found significant correlations between an aggregate MRS score based on an increase in myoinositol/creatine and a decrease in N-acetylaspartate/creatine and performance on Grooved Pegboard, finger tapping, Trail-Making Test, Part A, and Digit Symbol. Using a multivoxel imaging approach, Meyerhoff et al. 52 reported elevated choline in subcortical brain regions among cognitively intact individuals, and reduced N-acetylaspartate in subcortical brain regions among individuals with severe cognitive impairments. Specific relationships were observed between N-acetylaspartate levels in the subcortical brain regions and performances on measures of abstraction, immediate and delayed memory, verbal fluency, and fine motor dexterity. Finally, Chang et al. 53 reported significant relationships between elevated myoinositol to creatine levels in the frontal white matter and performance on executive tasks among HIV-positive individuals naïve to HAART.
The present study extends the above findings by identifying the specificity of MRS/cognitive relationships across brain regions. In our study, impaired gross motor and fine motor skills were associated with increases in myoinositol/creatine, a presumed marker of gliosis, in the basal ganglia and with decreases in N-acetylaspartate/creatine, a marker of mature neurons, in the frontal white matter. It is of interest that we did not observe significant relationships between N-acetylaspartate/creatine levels in the basal ganglia and neuropsychological function. Studies conducted prior to the widespread use of HAART reported reduced N-acetylaspartate/creatine levels in the frontal gray matter among patients with ADC, 33 and one might expect similar results in the basal ganglia given the anatomical connections between these regions of the brain. However, N-acetylaspartate measured in the subcortical gray matter appears relatively intact in the earliest stages of neuropsychological compromise associated with HIV. For example, Chang et al. 36 reported no significant alterations in N-acetylaspartate in the basal ganglia among patients with mild neuropsychological dysfunction, and Meyerhoff et al. 52 observed significant reductions in N-acetylaspartate in the subcortical gray matter only among patients with severe neuropsychological impairments.
Most patients in the current study received an ADC stage score of 1, consistent with mild to moderate neuropsychological impairment. As such, our findings suggest that increasing inflammatory change or membrane damage in the basal ganglia contributes to neuropsychological impairment independent of the effects of a decrease in N-acetylaspartate/creatine, at least among patients with less severe ADC who are on stable treatment. Future studies will be needed to examine these MRS indices in multiple subcortical and cortical regions (including the frontal lobe gray matter) in order to delineate the relationships between changes in metabolite ratios and neuropsychological function across these defined brain circuits. In addition, examination of metabolite concentrations in these brain regions may be useful as previous work has demonstrated alterations in multiple metabolites in patients with HIV, including the reference metabolite creatine, and these alterations could influence the ratio values and possibly mask the effects of changes in individual metabolites (e.g., reductions in N-acetylaspartate). 36 , 53
Surprisingly, as both choline and myoinositol are markers of glial cell function and choline/creatine is increased in HIV-infected individuals, we found only weak associations with choline/creatine in the frontal white matter and basal ganglia and neuropsychological performance. Changes in myoinositol may thus reflect metabolic events not measured by choline. Together these findings suggest that abnormalities in myoinositol/creatine and N-acetylaspartate/creatine may provide the primary metabolic substrates underlying neuropsychological dysfunction among HIV-infected individuals. The observation that correlations were generally higher with measures of pure motor function and processing speed as opposed to psychomotor function and verbal memory further support earlier observations that impairment in these domains comprises one of the more salient manifestations of ADC. 2 – 11
The current findings support the hypothesis that HIV-associated cognitive impairment is associated with dysfunction of subcortical brain structures and white matter. 2 – 11 Assuming that any disruption of subcortical function results in concomitant disruption of frontal-subcortical circuits, it is reasonable to conclude that frontal measures, including measures of psychomotor function, would tend to correlate both with basal ganglia dysfunction (the presumed site of damage) as well as interconnected white matter. In fact, psychomotor function as measured by Symbol Digit and Trail-Making Test, Part B correlated with metabolites in the basal ganglia and frontal white matter.
The relative lack of correlations between neuropsychological function and MRS indices in the parietal cortex is consistent with models of HIV-related brain injury. 14 , 15 The most parsimonious explanation for this observation is that cortical abnormalities may be most significant among individuals with more advanced cognitive impairment. Alternatively, certain functional relationships may be localized to cortical regions other than the parietal cortex. The neuropsychological battery administered in the present study was not particularly sensitive to cognitive functions mediated by the parietal cortex, and therefore the lack of significant associations between the MRS indices from the parietal cortex and neuropsychological performance may reflect this bias. However, the parietal cortex is involved in attentional processes, among other tasks, and there is evidence of disrupted attentional networks among patients with HIV. 54 Future studies that employ more selective measures of attention will be required to define these relationships with greater certainty. Nevertheless, to the extent that brain metabolite abnormalities in the parietal cortex represent general cortical disease involvement, the absence of a significant relationship between MRS indices in the parietal cortex and neuropsychological performance emphasize the specificity of subcortical brain regions to neuropsychological impairment in HIV. As noted previously, this issue will be clarified with greater certainty in future studies that examine MRS indices in multiple brain regions, including the frontal gray matter.
Previous studies have revealed that the expression of host factors (inducible nitric oxide synthase, TNF-α) and viral proteins in the basal ganglia are predictive of earlier stages of ADC, while increasing expression of these factors in both the basal ganglia and white matter correlate with more advanced stages of cognitive impairment. 14 , 15 It is of interest then that performance in only three domains of function, fine motor (Grooved Pegboard), gross motor function (Timed Gait) and information processing speed (CalCAP), was strongly associated with myoinositol/creatine in the basal ganglia, whereas fine and gross motor function in addition to psychomotor function (Trail-Making Test, Part B and Symbol Digit) correlated with metabolite changes in the frontal white matter. Metabolite changes in the basal ganglia may thus provide a sensitive marker of early neuropsychological impairment, while the subtests related to these changes may prove useful in its assessment.
Overall, our results reinforce the notion that HIV-associated neuropsychological impairment is associated with region-specific changes in the brain. Future studies will need to determine the long-term benefits of HAART on cerebral and cognitive function in the setting of chronic HIV infection. Additional studies are needed to elucidate the mechanisms that underlie the evolution of these neural changes in the setting of HAART and advanced disease, as well as the possibility to improve cognitive function with adjunctive pharmacological agents.
1 . Janssen RS, Cornblath DR, Epstein LG, et al: Nomenclature and research case definitions for neurological manifestions of human immunodeficiency virus type-1 (HIV-1) infection, report from the American Academy of Neurology AIDS Task Force. Neurology 1991; 41:778–785Google Scholar
2 . VanGorp WG, Satz P, Hinkin C, et al: The neuropsychological aspects of HIV-1 spectrum disease. Psychiatr Med 1989; 7:59–78Google Scholar
3 . Paul R, Cohen R, Stern R: Neuropsychiatric and neurobehavioral functioning in human immunodeficiency virus. CNS Spectr 2003; 7:860–866Google Scholar
4 . Navia BA, Jordan BD, Price RW: The AIDS dementia complex, I: clinical features. Ann Neurol 1986; 19:517–524Google Scholar
5 . Heaton RK, Grant I, Butters N, et al: The HNRC 500–Neuropsychology of HIV infection at different disease stages. J Int Neuropsychol Soc 1995; 1:231–251Google Scholar
6 . Miller EN, Satz P, Visscher BV: Computerized and conventional neuropsychological assessment of HIV-1 infected homosexual men. Neurology 1991; 41:1608–1616Google Scholar
7 . Martin EM, Pitrak DL, Pursell KJ, et al: Information processing and antiretroviral therapy in HIV-1 infection. J Int Neuropsychol Soc 1998; 4:329–335Google Scholar
8 . Wilkie FL, Morgan R, Fletcher MA, et al: Cognition and immune function in HIV-1 infection. AIDS 1992; 6:977–981Google Scholar
9 . Tross S, Price RW, Navia BA, et al: Neuropsychological characterization of the AIDS dementia complex: preliminary report. AIDS 1988; 2:81–88Google Scholar
10 . Sidtis JJ: Evaluation of the AIDS dementia complex in adults. Res Publ Assoc Res Nerv Ment Dis 1994; 72:273–287Google Scholar
11 . Navia BA, Choline ES, Petito CK, et al: The AIDS dementia complex, II: neuropathology. Ann Neurol 1986; 19:525–535Google Scholar
12 . Glass JD, Wesselingh SL, Selnes OA, et al: Clinical-neuropathologic correlation in HIV-associated dementia. Ann Neurol 1993; 43:2230–2237Google Scholar
13 . Everall IP, Luthert PJ, Lantos PL: A review of neuronal damage in human immunodeficiency virus infection: its assessment, possible mechanism and relationship to dementia. J Neuropathol Exp Neurol 1993; 52:561–566Google Scholar
14 . Navia BA, Gonzalez RG: Functional imaging and metabolic pathology of the HIV-1-infected brain. Neuroimaging Clin N Am 1997; 7:431–435Google Scholar
15 . Masliah E, Achim C, Ge N: Spectrum of human immunodeficiency virus-associated neocortical damage. Ann Neurol 1992; 32:321–329Google Scholar
16 . Wiley CA, Masliah E, Morey M, et al: Neocortical damage during HIV infection. Ann Neurol 1991; 29:651–657Google Scholar
17 . Zheng J, Thylin MR, Cotter RL, et al: HIV-1 infected and immune competent mononuclear phagocytes induce quantitative alterations in neuronal dendritic arbor: relevance for HIV-1-associated dementia. Neurotox Res 2001; 3:443–459Google Scholar
18 . Weis S, Haug H, Budka H: Neuronal damage in the cerebral cortex of AIDS brains: a morphometric study. Acta Neuropathol (Berl) 1993; 85:185–189Google Scholar
19 . Masliah E, Achim CL, Ge N, et al: Spectrum of human immunodeficiency virus-associated neocortical damage. Ann Neurol 1992; 32:321–329Google Scholar
20 . Everall IP, Heaton RK, Marcotte TD, et al: Cortical synaptic density is reduced in mild to moderate human immunodeficiency virus neuropsychological disorder: HNRC group. HIV Neurobehavioral Res Center Brain Pathol 1999; 9:209–217Google Scholar
21 . Masliah E, Heaton RK, Marcotte TD, et al: Dendritic injury is a pathological substrate for human immunodeficiency virus-related cognitive disorders: HNRC Group, The HIV Neurobehavioral Research Center. Ann Neurol 1997; 42:963–972Google Scholar
22 . Wiley CACA, Achim CL, Christopherson C, et al: HIV mediates a productive infection of the brain. AIDS 1999; 13:2055–2059Google Scholar
23 . Anderson E, Zink W, Xiong H, et al: HIV-1-associated dementia: a metabolic encephalopathy perpetrated by virus-infected and immune-competent mononuclear phagocytes. J Acquir Immune Defic Syndr 2002; 31:S43–54Google Scholar
24 . Chong WK, Sweeney B, Wilkinson ID, et al: Proton spectroscopy of the brain in HIV infection: correlation with clinical, immunologic and MR imaging findings. Radiology 1993; 188:119–124Google Scholar
25 . Meyerhoff DJ, MacKay S, Bachman L, et al: Reduced brain N acetylaspartate suggests neuronal loss in cognitively impaired immunodeficiency virus-seropositive individuals: in vivo 1 H magnetic resonance spectroscopic imaging. Neurology 1993; 43:509–515 Google Scholar
26 . Jarvik JG, Lenkinski RE, Grossman RI, et al: Proton MR spectroscopy of HIV-infected patients: characterization of abnormalities with imaging and clinical correlation. Radiology 1993; 186:739–744Google Scholar
27 . McConnell JR, Swindells S, Ong CS, et al: Prospective utility of cerebral proton magnetic resonance spectroscopy in monitoring HIV infection and its associated neurological impairment. AIDS Res Hum Retroviruses 1994; 8:977–982Google Scholar
28 . Barker PB, Lee RR, McArthur JC: AIDS dementia complex: evaluation with proton MR spectroscopic imaging. Radiology 1995; 195:58–64Google Scholar
29 . Jarvik JG, Lenkinski RE, Saykin AJ, et al: Proton spectroscopy in asymptomatic HIV infected adults: initial results in a prospective cohort study. J Acquir Immune Defic Syndr Hum Retrovirol 1996; 13:247–253Google Scholar
30 . Laubenberger J, Haussinger D, Bayer S, et al: HIV-related metabolic abnormalities in the brain: depiction with proton MR spectroscopy with short echo times. Radiology 1996; 199:805–810Google Scholar
31 . Tracey I, Carr CA, Guimaraes AR, et al: Brain choline-containing compounds are elevated in HIV-positive patients before the onset of AIDS dementia complex: a proton magnetic resonance spectroscopic study. Neurology 1996; 46:783–788Google Scholar
32 . Paley M, Cozzone P, Alonso J, et al: A multicenter proton magnetic spectroscopy study of neurological complications of AIDS. AIDS Res Hum Retroviruses 1996; 12:213–22Google Scholar
33 . López Villegas DR, Lenkinski RE, Frank I: Biochemical changes in the frontal lobe of HIV-infected individuals detected by magnetic resonance spectroscopy. Proc Natl Acad Sci U S A 1997; 94:9854–9859Google Scholar
34 . Salvan AM, Vion-Dury J, Confort-Gouny S, et al: Brain proton magnetic resonance spectroscopy in HIV-related encephalopathy: identification of evolving metabolic patterns in relation to dementia and therapy. AIDS Res Hum Retroviruses 1997; 13:1055–1066Google Scholar
35 . Wilkinson I, Miller R, Miszkiel K, et al: Cerebral proton magnetic resonance spectroscopy in asymptomatic HIV infection. Acquir Immunodefic Syndr 1997; 11:289–295Google Scholar
36 . Chang L, Ernst T, Leonido-Yee M, et al: Cerebral metabolite abnormalities correlate with clinical severity of HIV-1 cognitive motor complex. Neurology 1999; 52:100–108Google Scholar
37 . Chang L, Ernst T, Leonido-Yee M, et al: Highly active antiretroviral therapy reverses brain metabolite abnormalities in mild HIV dementia. Neurology 1999; 53:782–789Google Scholar
38 . Lee PL, Yiannoutsos CT, Ernst T, et al: A multi-center 1 H MRS study of the AIDS dementia complex: validation and preliminary analysis. J Magn Reson Imaging 2003; 17:625–633 Google Scholar
39 . Rey A: L’examen clinique en psychologie. Paris, Presses Universitaires de France, 1964Google Scholar
40 . Kløve: Clinical neuropsychology, in The Medical Clinics of North America. Edited by Forster FM. New York, WB Saunders, 1963Google Scholar
41 . Smith A: Symbol Digit Modalities Test: Manual. Los Angeles, Western Psychological Services, 1973Google Scholar
42 . Reitan R: Validity of the Trail-Making Test as an indicator of organic brain damage. Percept Mot Skills 1958; 8:271–276Google Scholar
43 . Comalli PE, Wapner S, Werner H: Interference effects of Stroop Color Word test in childhood, adulthood, and aging. J Genet Psychol 1962; 100:47–53Google Scholar
44 . Miller EN: California Computerized Assessment Package (CalCAP). Los Angeles, Norland Software, 1990Google Scholar
45 . Sidtis JJ, Gatsonis C, Price RW, et al: Zidovudine treatment of the AIDS dementia complex: results of a placebo-controlled trial. Ann Neurol 1992; 33:343–349Google Scholar
46 . Miller EN, Selnes OA, McArthur JC, et al: Neuropsychological performance in HIV-1 infected homosexual men: the Multicenter AIDS Cohort Study (MACS). Neurology 1990; 40:197–203Google Scholar
47 . Price RW, Marlowe N, Glidden D, et al: Neurological outcomes in late HIV infection: adverse impact of neurological impairment on survival and protective effect of antiviral therapy: AIDS Clinical Trial Group and Neurological AIDS Research Consortium study team. AIDS 1999; 13:1677–1685Google Scholar
48 . Navia BA, Dafni U, Simpson D, et al: A phase I/II trial of nimodipine for HIV-related neurologic complications. Neurology 1998; 51:221–228Google Scholar
49 . Selnes OA, Miller EN: Development of a screening battery for HIV-related cognitive impairment: the MACS experience, in Neuropsychology of HIV Infection: Current Research and New Directions. Edited by Grant I, Martin A. New York, Oxford University Press, 1994, pp 176–187Google Scholar
50 . Miller EN, Satz P, Bing EG, et al: Methodological issues in the assessment of human immunodeficiency virus-related cognitive impairment. Arch Gen Psychiatry 1992; 49:686–688Google Scholar
51 . Rottenberg DA, Sidtis JJ, Strother SC, et al: Abnormal cerebral glucose metabolism in HIV-1 seropositive individuals with and without dementia. J Nucl Med 1996; 37:1133–1141Google Scholar
52 . Meyerhoff DJ, Bloomer C, Cardenas V, et al: Elevated subcortical choline metabolites in cognitively and clinically asymptomatic HIV+ patients. Neurology 1999; 52:995–1003Google Scholar
53 . Chang L, Ernst T, Witt MD, et al: Relationships among brain metabolites, cognitive function, and viral loads in antiretroviral-naive HIV patients. Neuroimage 2002; 17:1638–1648Google Scholar
54 . Chang L, Tomasi D, Yakupov R, et al: Adaptation of the attention network in human immunodeficiency virus brain injury. Ann Neurol 2004; 56:259–272Google Scholar



