Frontal White Matter Integrity in Borderline Personality Disorder With Self-Injurious Behavior
Imaging studies of individuals with borderline personality disorder are few. 5 Early studies using computed tomographic imaging reported no gross abnormalities, no difference in ventricle-brain ratio, and no evidence of frontal lobe atrophy in borderline personality disorder patients compared with healthy subjects. 6 A recent magnetic resonance image (MRI) volumetry study found evidence of significantly reduced volumes of left orbitofrontal and right anterior cingulate cortex in borderline personality disorder subjects compared with healthy subjects. 7 Positron emission tomography studies of borderline personality disorder have resulted in conflicting results of both hypo- and hyper- frontal metabolism. 8 – 10 A functional MRI study showed greater activation in the amygdala, and medial and inferolateral prefrontal cortex in borderline personality disorder subjects compared with healthy subjects, reflecting more intense emotional responses to stressors. 11
Diffusion tensor imaging (DTI) is an MRI technique that measures the magnitude and direction of water diffusion in brain tissue. DTI data can be visualized in a variety of ways, including two-dimensional maps of the scalar parameters: a) trace, a measure of the magnitude of water diffusion in each image voxel; and b) fractional anisotropy (FA), a measure of the extent to which water diffusion in each voxel is directionally restricted. Typically, in regions of compromised white matter integrity, trace values are higher and FA values lower than in normal white matter, presumably owing to axonal degeneration. 12
Prior DTI studies have demonstrated an association between impulsivity and the reduction of microstructural integrity of frontal white matter systems. For example, Hoptman et al. 4 , 13 found that lower FA (i.e., axonal disorganization) in the right inferior frontal white matter was associated with greater impulsivity in schizophrenia. Additionally, our own work using DTI has shown decreases in the structural integrity of frontal but not posterior white matter in patients with kleptomania. 14
Because frontal brain circuits, particularly the orbitofrontal circuit, are important in behavioral regulation, 15 we hypothesized that individuals with borderline personality disorder who engage in self-injurious behavior would show compromised white matter integrity (i.e., increased trace and decreased FA) in inferior frontal regions, but not in posterior regions, compared with a healthy comparison group using DTI. We also hypothesized that anterior white matter integrity measured by trace and FA would be correlated with executive functions but not with cognitive functions, such as naming and basic visuospatial perceptual ability, which are thought to be more dependent on posterior cortical regions.
METHOD
We recruited 10 women with borderline personality disorder self-injurious behavior (mean age=34.1 [SD=10.8]; range=18 to 51; all right-handed) from an outpatient clinic. The diagnosis was confirmed by the Structured Clinical Interview for DSM-IV Personality Disorders (SCID-II). 16 Because borderline personality disorder may have multiple domains with distinct neurobiological underpinnings, 17 we restricted our study to only on those borderline personality disorder subjects who reported an inability to control their impulses to self-injure. Therefore, inclusion criteria were: 1) borderline personality disorder as the primary psychiatric disorder; 2) self-injurious behavior (defined as impulsive acts of self-mutilation, such as cutting, head banging, or burning) at least once a week; and 3) urges to self-injure at least one time per week. Subjects rated intensity of urges to self-injure using a 10-point Likert scale (0=no urges; 10=incapacitating urges). We recruited 10 healthy, nonpsychiatric female subjects (mean age=32.8 [SD=9.5]; range=21 to 49; all right-handed) matched to the borderline personality disorder-self-injurious behavior group on key demographic variables from the community.
Exclusion criteria for all subjects included: 1) current or lifetime history of bipolar I or psychotic disorder based on the Structured Clinical Interview for DSM-IV (SCID); 18 2) a lifetime history of attention deficit hyperactivity disorder based on clinical interview; 3) a lifetime history of a DSM-IV impulse control disorder not elsewhere classified based on SCID-compatible modules; 4) a history of head injury or neurological disorder; and 5) a positive urine pregnancy test.
Subjects taking psychotropic medications were allowed to participate if the dose had been stable for at least 6 months prior to study entry and had not resulted in any subjective improvement in self-injurious urges or behavior.
Butler Hospital’s Institutional Review Board approved the study. After complete description of the study, subjects provided written informed consent.
Assessments
We evaluated subjects at entry into the study by the Structured Clinical Interview for DSM-IV (SCID) 18 and SCID-compatible modules for impulse control disorders. 19 Self-injurious behavior was assessed with a semistructured phenomenological questionnaire evaluating types of self-injurious behaviors, frequency of behaviors, and related emotions before, during, and after self-injury. In addition, depressive symptoms were assessed using the Hamilton Depression Rating Scale (HAM-D), a valid and reliable 17-item, clinician-administered rating scale evaluating the severity of depressive symptoms. 20
Neuropsychological Battery
Borderline personality disorder-self-injurious behavior subjects underwent a battery of neuropsychological tests that emphasized executive functions. Two of the 10 subjects refused to undergo neuropsychological testing due to time constraints. The battery was administered by a clinical neuropsychologist. The tester was aware of the subjects’ diagnoses. Testing duration was approximately 1.5 to 2 hours. Control subjects were not administered the battery as normative means based on gender and age have been published.
Procedures
MRI scans were obtained on a 1.5T Siemens Symphony scanner using a volume head coil. A standard localizer was obtained followed by a 3D T1 MPRAGE (one acquisition, sagittal) as follows: 0.85 mm slices, no gap, 176 slices, 256×256 matrix, 21.7×21.7 cm FOV, TR=1900, TE=4.31 msec, TI=1100, NEX=1, and flip angle=15; acquisition time=8.08 minutes. Coregistered sagittal double spin-echo, echo-planar diffusion-weighted images were collected based on Siemens’ MDDW protocol as follows: three acquisitions with offset in slice direction by 0.0 mm, 1.7 mm, and 3.4 mm; 5 mm thick slices; 0.1 mm interslice spacing; 30 slices per acquisition; 128×128 matrix, 21.7 cm×21.7 cm FOV (interleaving during postprocessing provides true 1.7 mm 3 resolution images), TR=7200, TE=156. Bipolar diffusion gradients were applied in 12 noncollinear diffusion directions with 2 b magnitudes: 0, 1000 mm/s 2 , NEX=3, no partial echoes. A double-echo sequence was used that effectively cancels eddy current effects. 21 The entire brain was imaged. Time per acquisition=4:48 minutes. We used a vacu-pillow and head cushions to minimize subject movement during scanning.
All three offset diffusion scans were up-sampled to 0.85 mm 3 isotropic voxels for analysis. Scalar maps of trace and FA were produced using custom software. 22 An additional T2-weighted image (I0) without diffusion encoding (b=0) inherently coregistered with the trace and FA images was also produced.
Image Analysis
DTI data on one borderline personality disorder-self-injurious behavior subject and three comparison subjects could not be analyzed due to motion artifact. The final sample included nine borderline personality disorder-self-injurious behavior patients and seven comparison subjects. The mean age was 33.7 (SD=11.3) years (range: 18 to 51 years) in the borderline personality disorder-self-injurious behavior group and 31.1 (SD=10.6) years (range: 21 to 49 years) in the comparison subjects. This difference in age was not statistically significant (t=−0.455, p=0.656); skewness and kurtosis were within expectations for the age distribution for either group.
Experienced raters (S.C. and T.B.K.), blind to group assignment, analyzed images using Analyze AVW software (v. 5.0 & 6.0). 23 The MPRAGE images were manually corrected for head rotation and resliced along the AC-PC line. The transform matrix was applied to the DTI FA map volumes with manual adjustment and then this adjusted matrix was applied without further adjustment to the remaining DTI maps (trace and b=0). Four standard sized square (5 mm×5 mm voxel) regions of interest (ROIs) were placed bilaterally in anterior and posterior white matter on each of four axial slices based on a previously published method 3 for a total of 16 regions per subject. The most inferior slice was identified in sagittal view and was located at the inferior border of the rostrum of the corpus callosum. The remaining three slices were those falling three, six, and nine slices superior to the first. A prespecified coordinate-based algorithm was designed to guide ROI placement such that anterior ROIs would be placed anterior and slightly lateral to the anterior horns of the lateral ventricles on the three superior slices, and anterior and medial to the Sylvian fissure on the most inferior slice, and posterior ROIs would be placed lateral to the posterior horns of the lateral ventricles. Adjustments in final ROI placement were made to accommodate individual differences in brain anatomy. All ROIs were placed on the b=0 image without reference to the trace and FA images and transferred without further adjustment to the inherently coregistered trace and FA images for measurement.
The 16 ROIs were placed by each rater on each of the 16 brain volumes (nine patients, seven comparison subjects), for a total of 16 measurements per ROI per rater (a total of 256 ROIs placed per rater). Across the 16 regions, interrater reliability, measured as the intraclass correlation coefficient (ICC) between two raters, ranged from 0.71 to 0.98 for trace with only two regions falling below 0.80; and ranged from 0.78 to 0.98 for FA with only one region falling below 0.80. To optimize measurement reliability, we omitted those individual FA and trace measurements that differed more than 15% between the raters. After removal of these individual measurements, ICC improved to 0.84 or greater for 15 of the 16 trace ROIs and was 0.76 for the remaining region; ICC improved to 0.86 or greater for the 16 FA ROIs. To further improve reliability, we used the mean of the two raters’ values for trace and FA for each of the 16 regions in the analysis. For the group analysis, the 16 ROIs (i.e., right and left anterior and posterior regions on each of four slices) were summed across slices so that each subject had four trace and four FA measurements (i.e., right and left anterior and right and left posterior).
Cognitive Testing
Borderline personality disorder-self-injurious behavior subjects underwent a battery of neuropsychological tests that emphasized executive functions: Behavioral Dyscontrol Scale (BDS) 24 for executive functioning; Trail-Making Test, Parts A and B (TMT-A and -B) 25 for executive functioning, sequencing; psychomotor processing speed, cognitive set switching; Controlled Oral Word Association Test (COWAT) 26 for executive functioning, lexical fluency; Stroop Color-Word Test (Stroop) 27 for response inhibition; Wisconsin Card Sorting Test (WCST) 28 for executive functioning, mental flexibility; Boston Naming Test (BNT) 29 for language, confrontational naming; Judgment of Line Orientation (JLO) 30 for visuospatial perception; Hopkins Verbal Learning Test–Revised (HVLT-R) 31 for memory, verbal list learning and recall.
Data Analysis
Student’s t tests and two-group one-way ANOVA were used to examine group differences in background variables. Group differences in trace and FA were analyzed in separate (2×2) repeated measures (one for trace and one for FA) ANOVA with group (borderline personality disorder versus comparison) as the between-groups factor and region (anterior-posterior) as the within-groups factor. Follow-up analysis of simple effects was performed using one-way ANOVA. We did not use age as a covariate in our repeated measures analyses given that the means and frequency distributions of age in the two groups were highly comparable. There were no right versus left hemisphere differences in trace or FA in either group, with the exception of significantly greater posterior FA on the left in the borderline personality disorder-self-injurious behavior group only ( F [1, 70]=4.21, p=0.04). However, since we did not pose a hemispheric lateralization hypothesis, we summed anterior and posterior ROIs across hemispheres. The relationship between anterior and posterior trace and FA values and performance on cognitive tests was assessed in the borderline personality disorder-self-injurious behavior group using Pearson bivariate correlations. We used age- and education-corrected T scores for this analysis and since our hypothesis was directional, we used a one-tailed test. We used an alpha level of p<0.05; we did not adjust the alpha level to reflect multiple comparisons because this is the first study of this topic and is therefore exploratory.
RESULTS
The demographics of the borderline personality disorder-self-injurious behavior and comparison groups are presented in Table 1 . The borderline personality disorder-self-injurious behavior group was significantly more likely to have graduated from college and have a history of major depressive disorder, and higher scores on the HAM-D. The groups did not differ significantly on other demographic variables.
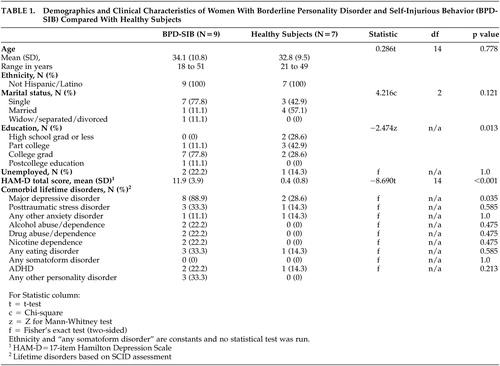 |
Mean self-injurious behavior onset was 16.7 (SD=4.1) years (range=9 to 23). Mean self-injurious behavior urge intensity was 7.40 (SD=1.58), and mean self-injurious behavior frequency was 3.40 (SD=1.17) times per week at the time of assessment. Eight subjects had been psychiatrically hospitalized due to self-injurious behavior, and three had required medical care (e.g., stitches, antibiotics). All subjects cut themselves, and six reported additional self-injurious behavior (burning [N=3]; head banging [N=2], beating self [N=1]). Lifetime comorbidity data are presented ( Table 1 ). Although only three of the borderline personality disorder-self-injurious behavior subjects met criteria for lifetime PTSD, all nine had histories of sexual abuse compared to none in the comparison group.
The nine borderline personality disorder-self-injurious behavior women had extensive treatment histories. All nine women had experienced at least 1 year of group dialectical behavioral therapy (DBT) plus therapy with an individual DBT counselor. Seven of the nine women had undergone 2 or more consecutive years of DBT groups. Mean onset of treatment was at 21.7 (SD=8.4) years (range=13 to 25). All of the women were currently taking at least one psychotropic medication (mean number of current psychotropic medications per subject was 4.0 (SD=1.3 [range=2 to 6]). Of the medications currently prescribed for the nine women, seven were taking at least one atypical antipsychotic, six were taking at least one antidepressant and at least one hypnotic, five were taking at least one mood stabilizer, and one was taking a stimulant.
DTI Results
Repeated measures ANOVA with diagnosis as the between-subjects factor and region (anterior-posterior) as the within-subjects factor revealed significant main effects of group ( F [1, 14]=7.81, p=0.014) and region ( F [1, 14]=14.19, p=0.002) . There was a significant group-by-region interaction effect ( F [1, 14]=7.30, p=0.017). Follow-up analysis of simple effects revealed that this interaction was driven by significantly higher anterior trace in the borderline personality disorder-self-injurious behavior group compared with the comparison group ( F [1, 14]=10.45, p=0.006); the groups did not differ significantly in posterior trace ( F [1, 14]=0.205, p=0.658) ( Table 2 ).
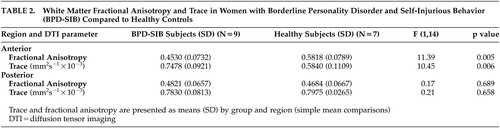 |
A second repeated measures ANOVA revealed nonsignificant trends for the main effects of group ( F [1, 14]=4.29, p=0.057) region ( F [1, 14]=3.48, p=0.083). There was a significant group-by-region interaction effect ( F [1, 14]=9.92, p=0.007). Follow-up analysis of simple effects revealed that this interaction was driven by significantly lower anterior FA in the borderline personality disorder-self-injurious behavior group compared with the comparison group ( F [1, 14]=11.39, p=0.005); the groups did not differ significantly in posterior FA ( F [1, 14]=0.167, p=0.689) ( Table 2 ).
Neither self-injurious behavior frequency nor self-injurious behavior urge intensity was significantly correlated with either anterior trace ([r=−0.193, p=0.620]; [r=−0.098, p=0.802], respectively) or anterior FA ([r=0.206, p=0.594]; [r=0.219, p=0.571], respectively).
Cognitive Results
Cognitive data were available for only eight of the nine borderline personality disorder-self-injurious behavior subjects with analyzable imaging data. Briefly, the means for all scores derived from the cognitive tests were within normal limits, with the exception of a number of categories completed on the WCST (mean=4.5 [SD=2.14]). For the correlation analysis, we chose only cognitive variables for which the range of the scores in the sample extended into the impaired range (i.e., T score ≥1 SD below the mean). Posterior trace was significantly correlated (p<0.05), with perseverative (r=0.646) and nonperseverative (r=0.627) responses on the WCST. Anterior trace and FA were significantly correlated (r=0.755 and –0.753, respectively, p<0.05), with true positive responses on the HVLT-R recognition recall trial.
DISCUSSION
These DTI results appear to support our hypothesis that patients with borderline personality disorder-self-injurious behavior exhibit compromised frontal white matter systems. These findings of compromised white matter microstructure in inferior frontal regions are consistent with results reported in other impulsive behaviors and with the hypothesis that impaired inferior frontal brain circuits underlie impulsive aggressive behaviors. 3 Compromised frontal white matter microstructure in patients with borderline personality disorder-self-injurious behavior may reflect an inability to balance the desire for immediate gratification from cutting with the recognition of the long-term consequences, an activity that may involve prefrontal cortical function. 32
The exploratory correlational analyses between DTI and cognitive variables in the borderline personality disorder-self-injurious behavior group yielded mixed pattern results vis-à-vis our executive cognitive hypothesis. In fact, higher posterior, not anterior, trace was associated with higher numbers of errors on the WCST, whereas higher anterior trace and lower FA was associated with better performance (i.e., higher numbers of true-positive responses) on a verbal recognition recall task. This latter finding is clearly the opposite of what was expected. These findings are inconsistent with other studies assessing neurocognitive functioning in borderline personality disorder. 33 , 34 Previous studies of neurocognitive functioning in borderline personality disorder have produced mixed results with some, 35 , 36 but not others 37 , 38 reporting distinct impairments relative to comparison subjects. Whether inconsistent results from previous studies are due to the possible heterogeneity of borderline personality disorder is unclear. Our sample was fairly homogenous, consisting only of female borderline personality disorder patients with urges to self-injure who were treatment-resistant. Although we had no cognitive data on the comparison subjects with which to compare, no robust pattern of neuropsychological impairment emerged when compared to test norms. In fact, as a group, the borderline personality disorder-self-injurious behavior participants performed within normal limits on virtually all tests thereby limiting the ability to identify significant associations. An alternative explanation is that executive cognitive functions and impulse control are subserved by distinct frontal regions—dorsolateral and orbital, respectively. Previous reports have demonstrated that cognitive deficit and disinhibition are dissociable in patients with focal frontal lesions. 39 , 40 Our analytical approach did not examine whether a similar dissociation exists in patients with borderline personality disorder-self-injurious behavior. Lastly, the very small size of our sample relative to the fairly large number of neuropsychological test scores raises questions about the reliability of these cognitive/imaging correlations.
The DTI findings of compromised inferior frontal microstructure may explain why certain therapies and medications have historically proven useful for borderline personality disorder behaviors. Psychotherapies that improve self-regulation 41 may counterbalance possible frontal deficits. Similarly, pharmacotherapies that increase inhibition, possibly through action in the prefrontal cortex, 42 may reduce self-injurious behavior in borderline personality disorder.
This is a preliminary analysis, and the findings should be interpreted cautiously. First, although our acquisition protocols control for eddy current and susceptibility artifacts, such effects may have affected our measurements, particularly in anterior regions where such artifacts tend to be greater. However, it is unlikely that such effects would have interacted systematically with subject group to produce a bias favoring our hypothesis. Second, we reduced the likelihood that motion artifact had a strong impact on the results by removing from the analysis cases with excessive motion. It is unlikely that subtle motion artifact (e.g., from physiological effects) in the retained cases would have produced systematic group bias in ROI measurements. Third, the sample size was small, and therefore replication in a larger sample is warranted. Fourth, the sample was limited to borderline personality disorder-self-injurious behavior subjects who were still engaging in self-injury at a late age despite an extensive treatment history. Therefore, these subjects appear to represent a largely treatment-resistant subset of borderline personality disorder subjects. These findings, therefore, may not generalize to all borderline personality disorder-self-injurious behavior subjects. Fifth, no comparison group of borderline personality disorder subjects without self-injurious behavior were examined. Only by using such a comparison group could we appreciate validity to the FA analysis, that is, whether disturbed frontal white matter is related to the borderline personality disorder diagnosis or the self-injurious behavior. Finally, the presence of co-occurring lifetime disorders may have contributed to the observed between-group differences in the frontal cortex. Without a comparison to another psychiatric sample with impulsivity, we cannot comment on how specific these findings are to this subject population. We did not control for age in our main analysis because the age distributions in the groups were highly similar. Moreover, there was no overall effect of age by region when age was added as a covariate in our main analysis (p=0.560; data not shown). These results lessen the likelihood that age was an important confounding factor in our results, but it cannot be ruled out in this small sample.
Despite these limitations, including its restriction in sample size, this study demonstrated significant decreased white matter microstructural integrity in inferior frontal brain regions of women with borderline personality disorder-self-injurious behavior. To the extent that we sampled components of orbitofrontal white matter circuits, our results provide preliminary support for the hypothesis that orbitofrontal abnormalities may underlie some of the behavioral dysregulation in borderline personality disorder patients with self-injurious behavior. The study of a larger number of subjects with more in-depth assessments of impulsivity are the logical next steps for this research. It remains to be determined whether successful response to treatment in borderline personality disorder-self-injurious behavior individuals is dependent upon white matter integrity.
1 . Stanley B, Brodsky BS: Suicidal and self-injurious behavior in borderline personality disorder: a self-regulation model, in Understanding and Treating Borderline Personality Disorder: A Guide for Professionals and Families. Edited by Gunderson JG, Hoffman PD. Washington, DC, American Psychiatric Publishing, 2005, pp 43–63Google Scholar
2 . Best M, Williams JM, Coccaro EF: Evidence for a dysfunctional prefrontal circuit in patients with an impulsive aggressive disorder. Proc Nat Acad Sci USA 2002; 99:8448–8453Google Scholar
3 . Lim KO, Hedehus M, Moseley M, et al: Compromised white matter tract integrity in schizophrenia inferred from diffusion tensor imaging. Arch Gen Psychiatry 1999; 56:367–374Google Scholar
4 . Hoptman MJ, Volavka J, Johnson G, et al: Frontal white matter microstructure, aggression, and impulsivity in men with schizophrenia: a preliminary study. Biol Psychiatry 2002; 52:9–14Google Scholar
5 . Schmahl C, Bremner JD: Neuroimaging in borderline personality disorder. J Psychiatr Res 2006; 40:419–427Google Scholar
6 . Lucas PB, Gardner DL, Cowdry RW, et al: Cerebral structure in borderline personality disorder. Psychiatr Res 1989; 27:111–115Google Scholar
7 . Tebartz van Elst L, Hesslinger B, Thiel T, et al: Frontolimbic brain abnormalities in patients with borderline personality disorder: a volumetric magnetic resonance imaging study. Biol Psychiatry 2003; 54:163–171Google Scholar
8 . Goyer PF, Andreason PJ, Semple WE, et al: Positron-emission tomography and personality disorders. Neuropsychopharmacology 1994; 10:21–28Google Scholar
9 . Juengling FD, Schmahl C, Hesslinger B, et al: Positron emission tomography in female patients with borderline personality disorder. J Psychiatr Res 2003; 37:109–115Google Scholar
10 . Soloff PH, Meltzer CC, Becker C, et al: Impulsivity and prefrontal hypometabolism in borderline personality disorder. Psychiatr Res 2003; 123:153–163Google Scholar
11 . Herpertz SC, Dietrich TM, Wenning B, et al: Evidence of abnormal amygdale functioning in borderline personality disorder: a functional MRI study. Biol Psychiatry 2001; 50:292–298Google Scholar
12 . Beaulieu C, Does MD, Snyder RE, et al: Changes in water diffusion due to wallerian degeneration in peripheral nerve. Mag Res Med 1996; 36:627–631Google Scholar
13 . Hoptman MJ, Ardekani BA, Butler PD, et al: DTI and impulsivity in schizophrenia: a first voxelwise correlational analysis. Neuroreport 2004; 15:2467–2470Google Scholar
14 . Grant JE, Correia S, Brennan-Krohn T: White matter integrity in kleptomania: a pilot study. Psychiatr Res 2006; 147:233–237Google Scholar
15 . Mega MS, Cummings JL: Frontal-subcortical circuits and neuropsychiatric disorders. J Neuropsychiatr Clin Neuroscience 1994; 6:358–370Google Scholar
16 . First MB, Gibbon M, Spitzer RL, et al: Structured Clinical Interview for DSM-IV Axis II Personality Disorders (SCID-II): User’s Guide. Washington, DC, American Psychiatric Press, 1997Google Scholar
17 . Zanarini MC, Gunderson JG, Frankenburg FR, et al: The revised diagnostic interview for borderlines: discriminating borderline personality disorder from other axis II disorders. J Person Disord 1989; 3:10–18Google Scholar
18 . First MB, Spitzer RL, Gibbon M, et al: Structured Clinical Interview for DSM-IV - Patient Edition (SCID - I/P, Version 2.0). New York, Biometrics Research Department, NY State Psychiatric Institute, 1995Google Scholar
19 . Grant JE, Levine L, Kim D, et al: Impulse control disorders in adult psychiatric inpatients. Am J Psychiatry 2005; 162:2184–2188Google Scholar
20 . Hamilton M: A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56–62Google Scholar
21 . Reese TG, Heid O, Weisskoff RM, et al: Reduction of eddy-current-induced distortion in diffusion MRI using a twice-refocused spin echo. Mag Res Med 2003; 49:177–182Google Scholar
22 . Robb RA, Hanson DP: A software system for interactive and quantitative analysis of biomedical images, in 3D imaging in medicine. Hohne (Eds.). NATO ASI Series, F 1990; 60:333–361Google Scholar
23 . Basser P, Mattiello J, LeBihan D: MR diffusion tensor spectroscopy and imaging. Biophysics J 1994; 66:259–267Google Scholar
24 . Grigsby J, Kaye K, Robbins LJ: Reliabilities, norms and factor structure of the behavioral dyscontrol scale. Percept Motor Skills 1992; 74:883–892Google Scholar
25 . Reitan R: Validity of the Trail Making Test as an indicator of organic brain disease. Percept Motor Skills 1958; 8:271–276Google Scholar
26 . Benton AL, Hamsher KS: Multilingual aphasia examination. Iowa City, AJA Associates, 1989Google Scholar
27 . Stroop JR: Studies of interference in serial verbal reactions. J Exper Psychol 1935; 18:643–662Google Scholar
28 . Berg EA: A simple objective treatment for measuring flexibility in thinking. J Gen Psychol 1948; 39:15–22Google Scholar
29 . Kaplan E, Goodglass H, Weintraub S: Boston Naming Test. Philadelphia, Lee & Febiger, 1983Google Scholar
30 . Benton A, Varney N, Hamsher K: Visuospatial judgment, a clinical test. Arch Neurol 1978; 35:364–367Google Scholar
31 . Benedict RHB, Schretlen D, Groninger L, et al: Hopkins Verbal Learning Test–Revised: normative data and analysis of inter-form and test-retest reliability. Clin Neuropsychol 1998; 12:43–55Google Scholar
32 . Bechara A, Tranel D, Damasio H: Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain 2000; 123(Pt 11):2189–2202Google Scholar
33 . Ruocco AC: The neuropsychology of borderline personality disorder: a meta-analysis and review. Psychiatr Res 2005; 137:191–202Google Scholar
34 . LeGris J, van Reekum R: The neuropsychological correlates of borderline personality disorder and suicidal behaviour. Can J Psychiatry 2006; 51:131–142Google Scholar
35 . O’Leary KM, Brouwers P, Gardner DL, et al: Neuropsychological testing of patients with borderline personality disorder. Am J Psychiatry 1991; 148:106–111Google Scholar
36 . Judd PH, Ruff RM: Neuropsychological dysfunction in borderline personality disorder. J Person Disord 1993; 7:275–284Google Scholar
37 . Sprock J, Rader TJ, Kendall JP, et al: Neuropsychological functioning in patients with borderline personality disorder. J Clin Psychol 2000; 56:1587–1600Google Scholar
38 . Kunert HJ, Druecke HW, Sass H, et al: Frontal lobe dysfunctions in borderline personality disorder? neuropsychological findings. J Person Disord 2003; 17:497–509Google Scholar
39 . Eslinger PJ, Damasio AR: Severe disturbance of higher cognition after bilateral frontal lobe ablation: patient EVR. Neurology 1985; 35:1731–1741Google Scholar
40 . Malloy P, Bihrle A, Duffy J, et al: The orbitomedial frontal syndrome. Arch Clin Neuropsychol 1993; 8:185–201Google Scholar
41 . van den Bosch LM, Koeter MW, Stijnen T, et al: Sustained efficacy of dialectical behaviour therapy for borderline personality disorder. Behav Res Ther 2005; 43:1231–1241Google Scholar
42 . Markovitz PJ, Calabrese JR, Schulz SC, et al: Fluoxetine in the treatment of borderline and schizotypal personality disorders. Am J Psychiatry 1991; 148:1064–1067Google Scholar



