Within-Session Mood Changes From TMS in Depressed Patients
Target areas of repetitive TMS in the treatment of mood disorders are the left and the right prefrontal cortex. The selection of these areas is based on evidence from neuroimaging and neuropsychological studies that suggest that prefrontal cortical areas show significant abnormalities in mood disorders. 7 – 10
During treatment with TMS, magnetic pulses are applied to the scalp. These pulses induce an electrical current in the underlying cortical tissue which results in depolarization of cortical neurons. Indirectly TMS also has effects on related cortical and subcortical areas as evidenced by studies of the motor cortex. 11 , 12
TMS studies regarding mood disorders have focused largely on depressed patients during a course of TMS, which usually consists of multiple treatment sessions conducted over several weeks. In contrast, studies regarding immediate effects of TMS on mood and on neural networks have mostly assessed healthy subjects. Reasons for studying healthy subjects rather than depressed patients include concerns about symptom heterogeneity in depression and the impact of psychotropic medications. 13 , 14
Several studies assessed immediate mood effects with high frequency (≥10Hz) TMS in small groups of healthy volunteers. George et al., 15 Pascual-Leone et al., 16 and Dearing et al. 17 reported decreased scores in measures of happiness with visual analogue scales (VAS) after left-sided compared to right-sided prefrontal stimulation. Barrett et al. 14 reported lower mean affect ratings without significant differences in individual ratings assessing comfort, fatigue, irritation, mood, anxiety, and pain in 10 volunteers. Padberg et al. 18 described increased sadness over the left dorsolateral prefrontal cortex, but no significant differences between left- versus right-sided stimulation. Two sham-controlled studies 19 , 20 did not produce differences in immediate mood ratings between TMS and sham with sample sizes of 12 and 25 subjects. All of these studies represent small numbers of subjects, and results are not consistent. Furthermore, these results were based on VAS ratings in which relatively small differences may be statistically but not clinically significant.
Meanwhile, immediate effects of TMS on mood in depressed patients remain largely unknown. Szuba et al. 21 performed a randomized, double blind, 2-week trial of TMS to the left prefrontal cortex (10 Hz at 100% of motor threshold) compared to sham. Fourteen patients were studied using four different treatment conditions: “high dose” (twice daily active treatments); “intermediate dose” (once a day active treatments); “low dose” (every other day active treatments alternating with sham treatment days) and “placebo” (twice daily sham treatments). Immediate mood effects were assessed as differences between mood states directly before and after TMS. The results are based on data from 18 active versus 10 sham sessions. Significant improvements in the categories of depression, anxiety and anger were found in the Profile of Mood States (POMS) ratings.
More data regarding immediate mood effects of TMS in depressed patients are needed. This could be helpful in at least two ways. First, immediate mood changes may help predict response and tolerance in longer term treatment; this concept has been investigated in the case of seasonal affective disorder where early response to light therapy in part predicts long-term antidepressant effects. 22 Second, new paradigms for functional imaging and other neurophysiological research could be developed.
As part of a recently published controlled trial of TMS in treatment-resistant major depression, 23 we collected data of mood ratings with VAS evaluated immediately before and after TMS sessions.
Null-hypotheses were that there are: 1) no differences in VAS ratings immediately before and after active TMS; 2) no differences between immediate changes in the VAS ratings before and after treatment in the active and sham treatment groups; 3) no differences between immediate changes in the VAS ratings in the TMS responder versus nonresponder groups; 4) no differences in the pattern of immediate change in VAS ratings for the TMS versus sham groups across the four assessment points (e.g., no significant interaction between assessment period and treatment modality); and 5) no differences in the pattern of immediate change in VAS ratings for the TMS responder versus nonresponder groups across the four assessment points (e.g., no significant two-way interaction between assessment period and response).
METHOD
Details regarding the study methods are described elsewhere. 23 Following is a brief overview of study procedures. Approval from the University of Washington Human Subjects Review Committee was granted.
Patients
Subjects gave informed consent to participate in the study. Inclusion criteria for comprised between 21 and 65 years old and having had a current major depressive episode according to DSM-IV criteria. Hamilton Depression Rating Scale scores had to be 17 or higher (17-item HAM-D). Another criterion was treatment resistance as evidenced either by a failure in response or the inability to tolerate at least two antidepressant medication trials in the current or prior depressive episodes.
Exclusion criteria included previous TMS treatment, bipolar disorder, previous nonresponse to ECT as well as having had a current major depressive episode for longer than 5 years, active substance abuse or dependence within 2 years prior to the study, antisocial or borderline personality disorder, current suicidal ideation, seizure disorder, prior brain surgery, history of head injury or a major psychiatric or medical comorbidity. Subjects were encouraged but not required to discontinue antidepressant medication; however, they needed to be on a stable antidepressant dose for at least 4 weeks before initiation of TMS. Sixty-eight subjects were randomized; 35 in the TMS group, 33 in the sham group. They showed no significant demographic or clinical differences.
Study Design and Ratings
In the efficacy study, 23 15 treatments were administered over a 4-week period. Subjects and raters were blind to the treatment. Assessments for immediate mood changes were done before and after treatments T1, T5, T10, T15. Treatment response was defined as a decrease in HAM-D scores of ≥50% after T15.
Treatment subjects were presented a sheet with five 90 mm horizontal lines. Each line was headed with one of the categories sadness, anxiety, happiness, tiredness, pain/discomfort. On the left side the lines were marked “less than normal;” on the right side “more than normal.” Subjects were asked to indicate their current state in each category immediately before and after each treatment.
TMS Treatment
Treatments were performed at Harborview Medical Center in Seattle, Wash. We used a Dantec Magpro Stimulator (Medtronic, Inc.) with a 70 mm figure-eight coil. The site of TMS was 5 cm anterior of the scalp location for optimal stimulation of the right dorsal interosseus muscle. The resting motor threshold was determined before each session. Treatments were delivered at 110% of the estimated prefrontal threshold at 10Hz in 5-second trains. A total of 1600 pulses were administered during 32 trains with 25- to 30-second intertrain intervals. Active and sham treatments were administered at the same anatomical position, the coil being placed flat against the scalp for active treatment and 90° away from the scalp for sham.
Statistical Analysis
Statistical analyses were performed with SPSS 13.0. The level for significance was set at a two-tailed probability of less than 0.05. However, due to the five sets of dependent variables and analyses, we used a Bonferroni correction to the p value. Therefore, a p value of 0.01 was employed; however, p values less than 0.05 will be noted for descriptive purposes only.
Five sets of analyses were performed to test the hypotheses described above: (1) VAS ratings before and after active TMS were compared with t tests for paired samples; (2) for the comparison of active TMS versus sham groups, we computed pre/postchange scores for each of the five VAS ratings at each assessment and determined whether the amount of change in the ratings was comparable between the groups using independent sample t tests; (3) likewise, we compared pre/post change scores only within the active TMS group for the TMS responders versus nonresponders with independent sample t tests.
For each of the five mood qualities, repeated measures ANOVAs were used to determine whether the pattern of VAS pre/postchange scores differed across the assessment points, and to evaluate whether these changes were related to receiving active treatment versus sham (4) or response to TMS (5). These ANOVAs used either “response to TMS” or “treatment group versus sham” as the between-group factor, and assessment time (T1, T5, T10 or T15) as the within-group factor. The dependent variables were the five pre/postVAS change ratings. Due to the small sample size and potential violations to the assumptions of these analyses, a Greenhouse-Geisser correction to the degrees of freedom was used.
RESULTS
Hypothesis 1: Acute Effects of Active TMS
The assessments of immediate changes in the five evaluated categories in the active TMS group of 35 patients were as follows: ratings for happiness showed significant improvement at time point T1 (t=–4.67, p<0.0001); at T10, ratings differed at trend level (t=−2.28, p=0.028); sadness ratings significantly decreased at T1 (t=2.77, p=0.009), T5 (t=2.86, p=0.007) and at trend level at T15 (t=2.13, p<0.04); anxiety ratings significantly decreased at T5 (t=3.21, p=0.003) and at trend level at T15 (t=2.16, p=0.038); for tiredness, one trend level decrease was found at T1 (t=2.32, p=0.027); for pain there was one trend level decrease that occurred at T5 (t=2.19, p=0.036).
Hypothesis 2: TMS Versus Sham
The changes in VAS ratings from active treatment versus sham (N=65) were evaluated separately at each time point for each of the five factors ( Table 1 ). Changes from pre- to post on the sadness factor at the four different assessments showed no consistent pattern in favor of TMS and a trend level separation in favor of TMS only at T15 (p=0.046). In pain ratings, the TMS group tended to have higher posttreatment ratings resulting in negative difference scores, but a significant trend toward difference was only found at T5 (p=0.043). In the factors of happiness, anxiety, and tiredness, no significant differences between different scores were found at any assessment. We therefore cannot reject null hypothesis number 2.
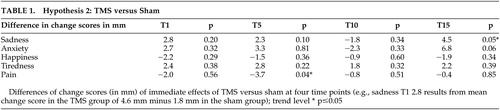 |
Hypothesis 3: TMS Responders Versus Nonresponders
We compared the difference scores between the group of TMS responders and nonresponders for each factor at each assessment. These results revealed only one trend-level significant result for pain/discomfort at T1 (p=0.03). For all other VAS difference scores, no significant differences in the change from pre- to posttreatment were found ( Table 2 ). Thus we cannot reject null hypothesis number 3.
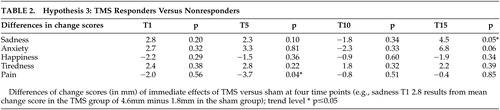 |
Hypothesis 4: TMS Versus Sham Changes Over Time
There were no statistical interactions of assessment period and treatment group versus sham group for any of the five factors: sadness ( F [1, 30]=1.70, not significant [NS]), happiness ( F [1, 30]=0.10 [NS]), anxiety ( F [1, 30]=1.82 [NS]), tiredness ( F [1, 30]=0.35 [NS]) and pain ( F [1, 30]=0.27 [NS]), indicating that over time the pattern of acute changes during the study was not different between the active and sham conditions. In the case of nonsignificant interactions, the main effects can be examined. The main between-group factor of treatment versus sham was not significant for all changes in VAS ratings. Time, the main within-group factor of assessment, was significant for the variable happiness ( F [1, 30]=4.68, p=0.004) only, which means that, collapsing over groups, immediate improvements in happiness ratings grew over time.
Hypothesis 5: TMS Responders Versus Nonresponders Changes Over Time
We could not identify any statistical interactions of assessment period and the two groups for the variables sadness ( F [1, 30]=0.68 [NS]), happiness ( F [1, 30]=0.06 [NS]), anxiety ( F[ (1, 30]=0.25 [NS]), tiredness ( F [1, 30]=1.34 [NS]) or pain ( F [1, 30]=0.08 [NS]), indicating that over time the pattern of acute changes during the study was not different between responder versus nonresponder groups. In the case of these nonsignificant interactions, the main effects were again examined. This further analysis revealed a significant effect between subjects for the variable pain only ( F [1, 30]=4.19, p=0.05). This result was caused by the fact that at T1, the nonresponder group had significantly lower pain ratings before treatment compared with the responders. The posttreatment values for both groups, however, were almost identical. These results mean we cannot reject null hypothesis number 5.
DISCUSSION
There has been very little data published on the immediate effects of TMS on mood in depressed patients. Despite well-documented positive effects with multisession (5 to 20) high frequency TMS, 6 , 23 immediate effects in depressed patients have received little attention. Our data present the largest sample of patients so far assessed during a major depressive episode. The results document significant immediate effects from TMS on five patient-rated VAS assessing sadness, anxiety, happiness, tiredness, and pain at various time points. However, these changes did not differ significantly from the outcome of sham treatment. These findings are in contrast with one prior sham-controlled study with a smaller sample (N=14) and a similar high frequency TMS procedure over the left DLPFC. 21 The previous study showed significant positive effects in depression, anxiety, and anger measured before and after the treatments compared to sham. However, the clinical significance of these results is diminished by the small number of subjects and the fact that two subjects received active and sham treatments on an alternating schedule, which may have compromised the blinding due to different scalp sensations. Also, despite significant changes on the POMS, a 6-item Hamilton Depression Rating Scale applied immediately before and after TMS detected no significant changes. Results from the assessment of immediate mood changes may be dependent on the assessment instrument. There is little literature on the optimization or standardization of the assessment of acute mood changes, but the use of uniform measures in future studies would improve the comparability of results.
Most studies assessing acute mood changes of TMS with VAS have been performed with small groups of healthy volunteers. Statistically but not clinically significant negative mood changes from stimulation over the left DLPFC have been reported by some, 15 , 16 , 18 but not others. 19 , 20
As opposed to studies with nondepressed subjects, there are several methodological considerations regarding our sample. The patients were not only acutely depressed but were also considered medication-resistant, which may result in an effect of TMS different from medication naïve patients or healthy subjects. Changes in prefrontal metabolism and neurochemistry in depressed patients may interfere with their acute response to TMS. This may be especially relevant in patients who are considered treatment-resistant and those receiving concomitant psychopharmacological treatment. TMS in depressed patients might not cause immediate positive mood effects, and a full course of TMS over several weeks may be required for more profound changes in cortico-limbic circuits which, in turn, produce sustained mood improvement. 24
In addition, we attempted to identify whether the immediate response to TMS in early sessions could help predict ultimate response to the treatment. Therefore we analyzed the interaction of responder versus nonresponder groups and time. As opposed to other studies on predictors of response which investigated demographic and clinical characteristics in depressed patients 25 , 26 there have been no prior reports on how early mood changes could be a useful predictor of outcome of TMS. By using VAS ratings, we could not identify early acute effects of TMS which predicted overall clinical response. In addition, there were no significant differences in the difference scores between the two groups over time.
CONCLUSIONS
Our study found that, although immediate mood changes were associated with TMS, those changes were no greater than in the sham condition. In addition, immediate changes with TMS were not associated with ultimate clinical response to the TMS. The available data on immediate mood effects of high frequency TMS in depressed patients are still very few and results remain controversial. Differences in patient characteristics, assessment tools, and treatment protocols may account for these different study results.
1 . Burt T, Lisanby SH, Sackeim HA: Neuropsychiatric applications of transcranial magnetic stimulation: a meta analysis. Int J Neuropsychopharmacol 2002; 5:73–103Google Scholar
2 . Holtzheimer PE, 3rd, Russo J, Avery DH: A meta-analysis of repetitive transcranial magnetic stimulation in the treatment of depression. Psychopharmacol Bull 2001; 35:149–169Google Scholar
3 . Holtzheimer PE, Avery D, Schlaepfer TE: Antidepressant effects of repetitive transcranial magnetic stimulation. Br J Psychiatry, 2004184, 541-a-542Google Scholar
4 . Kozel FA, George MS: Meta-analysis of left prefrontal repetitive transcranial magnetic stimulation (rTMS) to treat depression. J Psychiatr Pract 2002; 8:270–275Google Scholar
5 . Martin JLR, Barbanoj MJ, Schlaepfer TE et al: Repetitive transcranial magnetic stimulation for the treatment of depression: systematic review and meta-analysis, Br J Psychiatry 2003; 182:480–491Google Scholar
6 . Gershon AA, Dannon PN, Grunhaus L: Transcranial magnetic stimulation in the treatment of depression. Am J Psychiatry 2003; 160:835–845Google Scholar
7 . Baxter LR, Jr, Schwartz JM, Phelps ME et al: Reduction of prefrontal cortex glucose metabolism common to three types of depression. Arch Gen Psychiatry 1989; 46: 243–250Google Scholar
8 . Soares JC, Mann JJ: The anatomy of mood disorders–review of structural neuroimaging studies, Biol Psychiatry 1997; 41:86–106Google Scholar
9 . Goodwin GM: Neuropsychological and neuroimaging evidence for the involvement of the frontal lobes in depression. J Psychopharmacol 1997; 11:115–122Google Scholar
10 . Mayberg HS: Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. Br Med Bull 2003; 65:193–207Google Scholar
11 . Fox P, Ingham R, George MS, et al: Imaging human intra-cerebral connectivity by PET during TMS. Neuroreport 1997; 8:2787–2791Google Scholar
12 . Chouinard PA, Van Der Werf YD, Leonard G et al: Modulating neural networks with transcranial magnetic stimulation applied over the dorsal premotor and primary motor cortices. J Neurophysiol 2003; 90:1071–1083Google Scholar
13 . Milak MS, Parsey RV, Keilp J, et al: Neuroanatomic correlates of psychopathologic components of major depressive disorder. Arch Gen Psychiatry 2005; 62:397–408Google Scholar
14 . Barrett J, Della-Maggiore V, Chouinard PA, et al: Mechanisms of action underlying the effect of repetitive transcranial magnetic stimulation on mood: behavioral and brain imaging studies. Neuropsychopharmacology 2004; 29:1172–1189Google Scholar
15 . George MS, Wassermann EM, Williams WA, et al: Changes in mood and hormone levels after rapid-rate transcranial magnetic stimulation (rTMS) of the prefrontal cortex. J Neuropsychiatry Clin Neurosci 1996; 8:172–180Google Scholar
16 . Pascual-Leone A, Catala MD, Pascual-Leone Pascual A: Lateralized effect of rapid-rate transcranial magnetic stimulation of the prefrontal cortex on mood. Neurology 1996; 46:499–502Google Scholar
17 . Dearing J, George MS, Greenberg BD, et al: Mood effects of prefrontal repetitive high frequency transcranial magnetic stimulation (rTMS) in healthy volunteers. CNS Spectrums 1997; 2:53–68Google Scholar
18 . Padberg F, Juckel G, Prassl A, et al: Prefrontal cortex modulation of mood and emotionally induced facial expressions: a transcranial magnetic stimulation study. J Neuropsychiatry Clin Neurosci 2001; 13:206–212Google Scholar
19 . Cohrs S, Tergau F, Riech S, et al: High-frequency repetitive transcranial magnetic stimulation delays rapid eye movement sleep. Neuroreport 1998; 9:3439–3443Google Scholar
20 . Mosimann UP, Rihs TA, Engeler J, et al: Mood effects of repetitive transcranial magnetic stimulation of left prefrontal cortex in healthy volunteers. Psychiatry Res 2000; 94:251–256Google Scholar
21 . Szuba MP, O’Reardon JP, Rai AS, et al: Acute mood and thyroid stimulating hormone effects of transcranial magnetic stimulation in major depression. Biol Psychiatry 2001; 50:22–27Google Scholar
22 . Sher L, Matthews JR, Turner EH, et al: Early response to light therapy partially predicts long-term antidepressant effects in patients with seasonal affective disorder. J Psychiatry Neurosci 2001; 26:336–338Google Scholar
23 . Avery DH, Holtzheimer III PE, Fawaz W, et al: A controlled study of repetitive transcranial magnetic stimulation in medication-resistant major depression. Biol Psychiatry 2006; 59:187–194Google Scholar
24 . Catafau AM, Perez V, Gironell A, et al: SPECT mapping of cerebral activity changes induced by repetitive transcranial magnetic stimulation in depressed patients: a pilot study. Psychiatry Res Neuroimaging 2001; 106:151–160Google Scholar
25 . Eschweiler GW, Plewnia C, Bartels M: Which patients with major depression benefit from prefrontal repetitive magnetic stimulation. Fortschr Neurol Psychiatr 2001; 69:402–409Google Scholar
26 . Brakemeier E-L, Luborzewski A, Danker-Hopfe H, et al: Positive predictors for antidepressive response to prefrontal repetitive transcranial magnetic stimulation (rTMS). J Psychiatr Res 2007; 41:395–403Google Scholar



