Validity of the Clinical Diagnostic Criteria for Vascular Dementia: A Critical Review. Part II
Method of Systematic Literature Review
We conducted a MEDLINE/PubMed search covering the years from 1975 to 2006, using the following key words: “vascular dementia,” “multi-infarct dementia” or “dementia of vascular origin”; and “clinical criteria”; “diagnostic criteria”; and/or “comparison”; and/or “sensitivity and specificity.” Only the articles pertaining to the sensitivity and specificity estimates of the different diagnostic criteria used to detect vascular dementia were selected. However, since different definitions of the cognitive syndrome and vascular causes of vascular dementia may determine different prevalence estimates and identify different subjects, 8 articles comparing prevalence, frequency, and incidence rates of vascular dementia and using different sets of clinical criteria for vascular dementia were also included in the review. Finally, a manual search was performed in the references of the articles previously selected.
The articles were classified into three groups according to their purposes and quality of experimental design. The first group included clinico-neuropathological studies which used the neuropathological diagnosis as the “gold standard” and were specifically designed to assess sensitivity and specificity of different sets of clinical criteria for vascular dementia. Briefly, the sensitivity of a test (or set of criteria) has been defined as the probability of this test to be positive when given to a group of patients with the disease, whereas the specificity is the probability that this test will be negative among patients who do not have the disease. The level of sensitivity and specificity depends only on the qualities of a test (or set of criteria). In order to assess these qualities, it is essential to compare them to a “gold standard.” In the present case, the neuropathological confirmation of the presence of cerebrovascular disease has been considered the ideal gold standard. Another useful approach is to use likelihood ratios (LR) to assess the value of a diagnostic test. 9 The likelihood ratio is the likelihood that a given test result would be expected in a patient with the target disorder (LR + ) compared to the likelihood that the same result would be expected in a patient without the target disorder (LR − ). Because they are ratios, they do not vary in different populations or settings—they are independent of disease prevalence. Therefore, likelihood ratios allow for a quantification of the probability of disease in any individual. These indexes are calculated using formulas involving the sensitivity and specificity values: LR + =sensitivity/(1−specificity) and LR − =(1−sensitivity)/specificity. Since likelihood ratios were not estimated by the authors of the selected studies, the authors computed them when sufficient information was available ( Table 1 ). LR + represents the change in odds favoring disease given a positive test result. LR − expresses the change in odds favoring disease given a negative test result. Generally, a likelihood ratio greater than 1 is considered indicative of a test result associated with the presence of the disease, whereas a likelihood ratio less than 1 would indicate a test result associated with the absence of the disease. Likelihood ratios above 10 (LR + ) and below 0.1 (LR − ) are considered to provide strong evidence to rule in or out diagnoses (respectively) in most circumstances. 10
 |
The second group of studies included clinical studies designed to assess and compare sensitivity and specificity of different sets of criteria for vascular dementia or dementia in general, using the judgment of clinicians as the gold standard for the diagnosis of dementia, and clinico-neuropathological studies not specifically designed to estimate sensitivity and specificity rates of different sets of clinical criteria for vascular dementia. The third and last category of studies included clinical studies designed to assess prevalence, incidence, and/or frequency rates of vascular dementia according to different sets of diagnostic criteria.
RESULTS
General Results
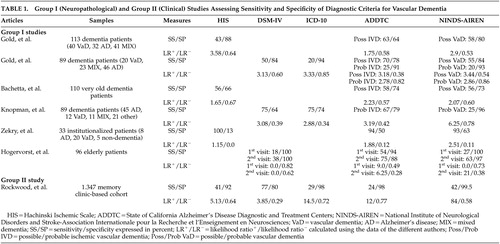
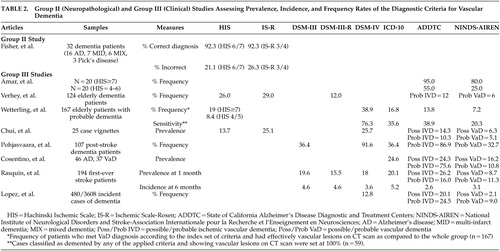
Very few studies aimed specifically at assessing and comparing the sensitivity and specificity rates of the different sets of clinical criteria for the diagnosis of vascular dementia, and few studies used a clinico-pathological design with the neuropathological diagnosis as the gold standard. Only 16 relevant studies were therefore selected and hierarchically classified into the three above-mentioned groups. Six articles pertained to the first group, two to the second, and eight to the third. Table 1 presents group I, neuropathological studies, and group II, clinical studies, that addressed the assessment of sensitivity and specificity of various sets of criteria for vascular dementia. Table 2 presents group II and group III studies that assessed prevalence, incidence, and/or frequency rates of vascular dementia according to the various sets of criteria.
 |
Group I Studies
Six articles pertained to this first group, which specifically assessed the sensitivity and specificity estimates of various sets of criteria for vascular dementia using a neuropathological confirmation of diagnosis ( Table 1 ). The sample size varied from 33 to 113 autopsied cases, and the analyses were generally performed retrospectively. The Ischemic Scale of Rosen, DSM-III, and DSM-III-R criteria have not been investigated and therefore are not illustrated in Table 1 . The principal finding was that the other criteria yield relatively low sensitivity, but high specificity. The main limitation was related to the type of vascular lesions considered by the authors. In the absence of widely accepted markers of vascular dementia (as underlined as a critical issue in Part I of the review), teams of authors developed and used their own neuropathological markers of vascular dementia. These various and different neuropathological definitions prevent accurate comparisons between the reviewed studies and constitute one of the principal limitations of group I studies. Interestingly, the calculated positive likelihood ratios revealed that most diagnostic criteria generate only a minimal to small increase in the likelihood of having vascular dementia (LR + between 0 and 3.58). However, the ADDTC and NINDS-AIREN criteria demonstrated a moderate to large increase in the likelihood of having vascular dementia (LR + values between 6.25 and 21), respectively. According to the LR − values, the capacity of the different sets of criteria to properly identify the true negatives was not very successful, since the LR − values were all higher than 0.1 (except for the Hachinski Ischemic Scale in the study of Zekry et al. 11 ).
The first group I study was performed by Gold et al. in 1997. 12 These authors performed a clinico-neuropathological study comparing the sensitivity and specificity estimates of the ADDTC, the NINDS-AIREN, and the Hachinski Ischemic Scale criteria for the diagnosis of possible vascular dementia on 113 autopsied patients with dementia. They found that the Hachinski Ischemic Scale was the most specific, but the least sensitive, set of criteria, while the ADDTC criteria were the most sensitive. Moreover, the sensitivity/specificity figures for the differential diagnosis between vascular dementia and mixed dementia were 30%/97% for the Hachinski Ischemic Scale, 43%/91% for the NINDS-AIREN, and 58%/88% for the ADDTC criteria. The ADDTC criteria thus reached the best balance between sensitivity and specificity for the differential diagnosis of mixed dementia. The three criteria sets successfully differentiated vascular dementia from Alzheimer’s disease patients since only 3.5% (Hachinski Ischemic Scale), 9.4% (NINDS-AIREN), and 12.7% (ADDTC) of the cases were misclassified as vascular dementia instead of Alzheimer’s disease. Nevertheless, in the absence of neuroimaging data, these findings were limited to the clinical criteria for possible (and not probable) vascular dementia. In a later study, this same group investigated the sensitivity and specificity estimates of the NINDS-AIREN, ADDTC, DSM-IV, and ICD-10 criteria for the diagnosis of possible and probable vascular dementia in 89 dementia patients for whom cerebral imaging data (CT scan or MRI) were available within 6 months of their death. 13 Neuropathological examination was conducted at autopsy. The most sensitive criteria for possible vascular dementia were the ADDTC criteria, followed by the NINDS-AIREN criteria, and DSM-IV. The most specific criteria for probable vascular dementia were the ICD-10, ADDTC, and the NINDS-AIREN criteria. These authors also showed that the proportion of neuropathologically confirmed Alzheimer’s disease cases clinically misclassified as vascular dementia ranged from 0% (ICD-10) to 13% (ADDTC), whereas the proportion of the neuropathologically confirmed cases of mixed dementia clinically misclassified as vascular dementia ranged from 9% (ADDTC probable vascular dementia) to 39% (ADDTC possible vascular dementia). Therefore, the application of the ADDTC criteria resulted in a high rate of misclassification when mixed cases were considered.
More recently, the same team of researchers provided the first neuropathological validation of the criteria for vascular dementia in a hospital-based cohort composed of the oldest-old patients. 14 The authors analyzed 110 autopsied cases of dementia over 90 years of age and reported comparably low sensitivity rates between the Hachinski Ischemic Scale, the ADDTC, and NINDS-AIREN criteria for possible ischemic vascular dementia/vascular dementia (from 56% to 58%). More than 40% of the neuropathologically confirmed vascular dementia cases were not identified by any of the clinical criteria studied. In regards to specificity, the Hachinski Ischemic Scale was the least specific. Both the NINDS-AIREN and ADDTC criteria performed very well at excluding Alzheimer’s disease. However, 30% of the mixed dementia cases were misdiagnosed by the ADDTC and NINDS-AIREN criteria, whereas up to 45.9% of mixed dementia cases were erroneously diagnosed as having pure vascular dementia by the Hachinski Ischemic Scale. Some limitations should nevertheless be mentioned concerning these three studies. 12 – 14 First, they were conducted on hospital-based cohorts, and hence were not representative of the full spectrum of patients with vascular dementia. The studies did not clearly specify what kind of cognitive assessment was performed to define dementia. The studies only mentioned a mental state examination—insufficient to properly characterize the cognitive syndrome. Furthermore, in the absence of widely accepted quantitative neuropathological criteria for vascular dementia and to guarantee that the dementia in this context was related to a predominant vascular pathology, the studies applied a restricted definition of significant vascular lesions. This was based on the presence of a lacunar state in the basal ganglia or on both macroscopic and microscopic cortical infarcts involving at least three cortical association areas (excluding the primary and secondary visual cortex). Vascular lesions confined to subcortical structures other than the basal ganglia were not considered for a diagnosis of vascular dementia. In this respect, the findings of these studies concerned the validity of diagnostic criteria for the detection of multi-ischemic dementia, but not other forms of vascular dementia, such as Binswanger’s subcortical encephalopathy, cerebral amyloid angiopathy, dementia linked to hypoperfusion, and hemorrhagic dementia.
Knopman et al. 15 retrospectively identified incident cases of dementia from the Rochester Epidemiology Project in order to investigate clinico-pathological correlations of vascular dementia. Of 482 incident cases of dementia, 419 were deceased at the time of their study, and neuropathological diagnoses were available in 89 cases. The best sensitivity rates were obtained with the ICD-10 and DSM-IV criteria, whereas the worst rates were achieved with the NINDS-AIREN criteria for probable vascular dementia. Conversely, the NINDS-AIREN were the most specific, and the DSM-IV the least specific criteria. The lack of sensitivity of the diagnostic criteria for pure vascular dementia was due to five patients (of 12 cases) with pure vascular dementia at neuropathological examination who lacked a temporal relationship between clinical stroke and onset of their dementia. Only half of the subjects with radiological evidence of critical ischemic lesions in fact had a history of clinical stroke or focal motor signs. This study was principally limited by its retrospective collection of data, which may have led to high rates of dementia diagnoses; the lack of details about the cognitive tests they used to determine the presence of dementia; and by the lack of independence between the clinical and pathological diagnosis. Moreover, patients with a temporal relationship between dementia and stroke tended to undergo autopsy more frequently than did others, and autopsied patients had two or more strokes more often than did the nonautopsied patients. This situation may not be representative of all cases of vascular diseases associated with dementia. Since only a few patients of the total cohort underwent routine autopsy, their estimates of sensitivity and specificity might have been biased. However, this limitation is quite common in any autopsy study.
Zekry et al. 11 carried out a prospective clinico-neuropathological study of 33 institutionalized patients age 75 years and over. Although all sets of criteria were highly sensitive, the Hachinski Ischemic Scale was the most sensitive set of criteria for vascular dementia (100%) but, conversely, the least specific compared to the NINDS-AIREN and ADDTC clinical criteria for vascular dementia/ischemic vascular dementia. In addition, the most sensitive criteria for diagnosing pure versus mixed cases were still the Hachinski Ischemic Scale (86%), and the most specific were the NINDS-AIREN (63%). The best diagnostic agreement between clinical criteria and the neuropathological examination was observed when all cases of mixed dementia were excluded (88%), demonstrating that this diagnosis was clinically underestimated. Thus, Zekry et al. 11 concluded that all sets of criteria distinguished pure Alzheimer’s disease from vascular dementia with a high accuracy whereas mixed dementia remained problematic. However, this study was performed on a small sample of patients (N=33), which was not representative of the general population because of a selection bias. This bias might have explained why the sensitivity rates were so high and so different from the other studies reviewed herein. A psychometric battery and scales were used for the diagnosis of dementia and therefore to describe the cognitive syndrome, but the authors did not clearly specify them. In addition, they did not take into account subcortical vascular lesions in their neuropathological diagnosis; only bilateral infarcts and unilateral infarcts involving the territories of either the posterior or anterior cerebral artery were felt to be consistent with the diagnosis of vascular dementia.
The last clinico-pathological study was conducted by Hogervorst et al. 16 on 96 elderly dementia patients and control subjects as part of the Oxford, England, Project to Investigate Memory and Ageing (OPTIMA) longitudinal study. The definition of dementia was based on a brief scale (i.e., Mini-Mental State Examination) and global scales (i.e., Clinical Dementia Rating and the Cambridge Examination for Mental Disorders of the Elderly). Although the primary goal was to investigate the validity and reliability of the clinical criteria for Alzheimer’s disease and vascular dementia using a computerized “dementia diagnosis system,” the study also reported sensitivity and specificity results for various sets of clinical criteria. In particular, they found that none of the criteria for vascular dementia (using the DSM-IV, ADDTC, and NINDS-AIREN criteria) at the first clinical visit had very good sensitivity for predicting autopsy findings, despite excellent specificity. However, with clinical data from follow-up visits, reasonable sensitivity and specificity were obtained with the ADDTC criteria compared with the NINDS-AIREN and DSM-IV criteria. This study included only a small sample of patients with cerebrovascular disease and vascular dementia (N=11). Moreover, for postmortem confirmation of cerebrovascular disease or vascular dementia, the authors only included dementia patients with moderate to severe cerebrovascular disease with or without additional (non-Alzheimer’s disease) pathology at autopsy. In these cases, cerebrovascular disease was substantial when consisting of either multiple infarcts and/or cribriform (or lacunar) state accompanied by surrounding tissue rarefaction and gliosis or white matter myelin pallor. In these cases, vascular disease was considered to have contributed to the dementia to varying extent.
To summarize, these data revealed that the ADDTC criteria for possible ischemic vascular dementia achieved the best balance of sensitivity and specificity. Nevertheless, there was a marked age-related decrease in the sensitivity rates of the ADDTC criteria for possible ischemic vascular dementia. 14 The sensitivity rates of the NINDS-AIREN criteria for possible vascular dementia and the Hachinski Ischemic Scale were higher in the oldest-old cohort or comparable to those reported in the old population by previous studies. 12 – 14 The NINDS-AIREN criteria were consistently found to be the most specific criteria. However, the results of Bacchetta et al. 14 compared to those of other researchers also revealed a decrease in the specificity rates of all three sets of criteria compared to those reported in the younger elderly cohort. 12 , 13 In addition, the data of Hogervorst et al. 16 suggested that a 6-month follow-up visit may be useful to increase both sensitivity and specificity rates of the vascular dementia criteria. In regards to the likelihood ratios, most studies demonstrated that the diagnostic criteria did not reach the threshold values for acceptance of a diagnostic test (LR + >10 and LR − <0.1). 9 , 17 In fact, only the NINDS-AIREN criteria showed, in two different studies, 15 , 16 a moderate to large increase in the likelihood of having vascular dementia (LR + values between 6.25 and 21), whereas the ADDTC criteria showed a moderate increase in the likelihood of having vascular dementia (LR + values between 6.25 and 9) in only one study. 16
Group II Studies
Two studies (one clinical and one clinico-neuropathological) were included in this group. The DSM-III and DSM-III-R criteria were again not investigated. The clinical study by Rockwood et al. 18 yielded rather different results compared to those found in the group I studies. The neuropathological study by Fischer et al. 19 was limited to the ischemic scales, and thus could not be comprehensively compared to the group I studies. Overall, the DSM-IV criteria were found to be the most sensitive, and the NINDS-AIREN criteria were found to be the most specific. The ischemic scales gave high rates of misclassification (vascular dementia instead of Alzheimer’s disease). The only group II study which addressed the question of sensitivity and specificity of clinical criteria was performed by Rockwood et al. 18 in a multicenter prospective cohort study design ( Table 1 ). Compared with the clinical judgment of geriatricians and neurologists (the “gold standard” used in this study), who diagnosed 101 out of 1347 participants with vascular dementia, the NINDS-AIREN criteria were found to be the most specific. The DSM-IV criteria were the most sensitive, while, in contrast to the above studies, the ADDTC criteria were the least sensitive. Thus, the DSM-IV identified the greatest numbers of patients as having vascular dementia. However, lower proportions of these individuals had vascular risk factors and focal neurological signs compared with those identified by other criteria. The analysis of the cerebral imaging data revealed that greater proportions of ADDTC classified patients showed a multi-infarct profile, whereas white matter changes were more common among those diagnosed by the DSM-IV. In general, neuroimaging was felt to change the final diagnosis in 10.8% of patients. Rockwood et al. 18 concluded that consensus-based criteria for vascular dementia omitted patients who did not meet dementia criteria modeled on Alzheimer’s disease. Even for patients who did meet these criteria, the proportion identified with vascular dementia varied widely. Interestingly, the clinical judgment of clinicians was also compared with the various diagnostic criteria sets in 324 out of 1347 participants diagnosed with vascular cognitive impairment. They found the following sensitivity and specificity rates: 14%/99% (NINDS-AIREN); 14%/99% (ADDTC); 13%/99% (ICD-10); 53%/85% (DSM-IV); and 29%/96% (Hachinski Ischemic Scale). Once again, the DSM-IV criteria were the most sensitive and the NINDS-AIREN and ADDTC criteria were the most specific. However, values above the threshold for the positive likelihood ratio were obtained only by the NINDS-AIREN, the ICD-10, and ADDTC criteria, respectively ( Table 1 ), indicating better diagnostic capacity of these criteria over the DSM-IV. One major limitation of this study was a selection bias, since the patient sample came from a memory clinic and thus was not representative of the entire population. This cohort may have included a higher number of cases with mixed dementia, because, according to the authors, mixed dementia is more likely to be diagnosed as vascular cognitive impairment in memory clinics. Moreover, they only relied on the Mini-Mental State Examination (MMSE) and some other functional scales to determine the presence or absence of dementia. The conclusions of this study were further limited because clinicians used their clinical judgment as the gold standard to compare the diagnosis of vascular dementia with the consensus diagnostic criteria. Nevertheless, their prevalence results were similar to previous clinical work (see group III studies).
The next group II study was a prospective clinico-neuropathological study that did not specifically address the question of sensitivity and specificity ( Table 2 ). It aimed instead at validating the Hachinski ischemic scales in a consecutive series of 32 elderly demented patients, as determined by a score lower than 24 on the MMSE, who had a neuropathological confirmation of their diagnosis. 19 The authors found that the Hachinski ischemic scales were able to diagnose mixed dementia (coexistence of Alzheimer’s disease and multi-ischemic dementia) and multi-ischemic dementia correctly in 92.3% of cases, independent of the cutoff and scoring system used. However, there was a high rate of false positive cases in individuals who had been clinically diagnosed as having mixed dementia or multi-ischemic dementia, but were found to have Alzheimer’s disease at autopsy. The results of this study should be interpreted with caution given the very small sample size.
Group III Studies
Eight clinical studies pertained to this group ( Table 2 ). The studies generally aimed at determining prevalence/incidence or frequency rates of vascular dementia according to different criteria. The sample sizes varied from 25 to 480 cases according to the type of experimental design (case vignette analysis versus longitudinal multicenter study). Overall, these studies suggested that the ADDTC criteria led to the highest proportion of vascular dementia diagnosis whereas the NINDS-AIREN criteria led to the lowest proportion. However, false positive diagnoses of vascular dementia were most frequent when the ADDTC criteria were used.
Amar et al. 20 retrospectively applied the NINDS-AIREN and the ADDTC criteria to two groups of patients who underwent a relatively detailed neuropsychological examination and who were thought to be suffering from vascular dementia per their initial scores on the Hachinski Ischemic Scale. The first group (N=20) had a high Hachinski Ischemic Scale score of ≥7, and the second group (N=20) had a score between 4 and 6. The authors found the ADDTC to be the most sensitive criteria as they yielded the highest proportion of vascular dementia diagnosis. However, this study was limited by the small sample size and the lack of distinction between diagnoses of possible or probable vascular dementia.
Verhey et al. 21 studied 124 dementia patients from a memory clinic and found that frequency values of vascular dementia were the highest with the ischemic scales: the Ischemic Scale of Rosen resulted in nearly five times as many patients with vascular dementia as when the NINDS-AIREN criteria were applied. Only eight patients fulfilled all criteria sets. The authors concluded that the criteria sets could not be interchanged. However, they noticed that in clear-cut patients, as defined by clear evidence of multiple strokes by history, clinical examination, and CT scans, different criteria led to similar diagnoses. The criteria diverged when information from one category did not confirm the other (e.g., when there was evidence of stroke on CT scan without focal neurological symptoms or vice versa). This study also showed that if a temporal connection and/or neuroimaging data were taken into account, the diagnostic outcome was considerably different. Unfortunately, the authors did not mention what kind of cognitive assessment they performed in order to reach the diagnosis of vascular dementia.
Wetterling et al. 22 studied 167 patients admitted for probable dementia. The authors found that 109 patients met at least one of four definitions for dementia (either ICD-10, DSM-IV, ADDTC, or NINDS-AIREN criteria). However, only 59 of these patients (54.1%) showed vascular lesions on a CT scan. Among them, 74.6% had bilateral vascular lesions and 42.2% had pure white matter lesions. Small-vessel disease was the most frequent type of lesion seen on CT (48.6%), while large-vessel infarcts were the second most frequent type of lesion (11.9%). Approximately 5.5% of patients presented with small and large vessel lesions. Of the 65 cases meeting the DSM-IV criteria for vascular dementia, only 69% had vascular lesions on a CT scan. Of the 28 cases meeting the ICD-10 criteria for vascular dementia, 75% had vascular lesions on a CT scan. In order to evaluate the sensitivity values of the four diagnostic criteria sets, the diagnoses of dementia according to any of these clinical criteria were contrasted with the vascular lesions on a CT scan (considered as the “gold standard”). The DSM-IV criteria were the most sensitive criteria for vascular dementia (the NINDS-AIREN was the least sensitive), but their specificity rate was the lowest as CT scans revealed no vascular lesions in 30.8% of cases. In fact, a high proportion of patients also fulfilled the National Institute of Neurological and Communicative Diseases and Stroke/Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) criteria for Alzheimer’s disease (or other sets of criteria for Alzheimer’s disease).
With regards to concordance, Wetterling et al. 22 found that 86.4% of their patients were diagnosed with vascular dementia by at least one of the four diagnostic criteria, but only five cases met the criteria for vascular dementia according to all the diagnostic criteria. These five subjects were characterized by large-vessel infarcts involving cortical areas, three or more focal neurological signs, and a stepwise deterioration. Finally, the findings occurring in less than one third of the sample included evidence of two or more strokes, evidence of two or more infarcts on CT scan, a history of multiple transient ischemic attack, and focal neurological symptoms. It should be mentioned that the patients in this study were only administered very short scales (i.e., MMSE) and a structured interview to determine the presence of dementia.
Chui et al. 23 aimed at assessing concordance in classification, as well as the interrater reliability, for different clinical criteria of vascular dementia using 25 case vignettes representing a spectrum of cognitive impairment (21 out of 25 case vignettes had information regarding cognitive tests) and subtypes of dementia. They found that the DSM-IV and the Ischemic Scale of Rosen were the most liberal criteria. The ADDTC criteria, as well as the Hachinski Ischemic Scale, produced an intermediate frequency of vascular dementia, while the NINDS-AIREN criteria were the most conservative. The interrater reliability was the highest for the Hachinski Ischemic Scale (κ=0.61), and was the lowest for the ADDTC for possible vascular dementia (κ=0.15). Interrater reliability for the NINDS-AIREN for probable or possible vascular dementia was intermediate (κ=0.42).
Pohjasvaara et al. 24 studied 107 dementia patients who underwent a very comprehensive neuropsychological battery following an ischemic stroke and demonstrated that the number of cases classified as vascular dementia according to the different criteria varied considerably. Consistent with previous studies, the DSM-IV criteria were found to be the most sensitive, while the NINDS-AIREN criteria for probable vascular dementia were the least sensitive. Only 31 patients (29%) fulfilled all criteria sets for vascular dementia, and only 40 patients (37.4%) showed focal neurological signs on neurological examination 3 months after their stroke. Patients with small-vessel subcortical vascular dementia frequently did not show clear-cut focal neurological signs. However, this study included only poststroke patients and excluded other types of vascular diseases.
More recently, Cosentino et al. 25 retrospectively analyzed a series of ambulatory patients who also underwent an extensive neuropsychological assessment. Thirty-seven patients were diagnosed with vascular dementia (per a multidisciplinary team diagnosis) and 46 patients with Alzheimer’s disease (per a multidisciplinary team diagnosis and the NINCDS-ADRDA criteria). Most of the patients met the ADDTC criteria for probable ischemic vascular dementia in contrast to the NINDS-AIREN; very few patients met the latter criteria. However, the ADDTC criteria for probable ischemic vascular dementia were the only criteria resulting in false positive diagnosis of vascular dementia; 15.2% of Alzheimer’s disease patients also met the criteria for probable ischemic vascular dementia. Moreover, only 45.9% of cases with vascular dementia were diagnosed by more than one diagnostic scheme. Regardless of the diagnostic scheme that was used, Cosentino et al. 25 demonstrated that the most common clinical characteristics associated with vascular dementia were hypertension; neuroradiological evidence of extensive periventricular and deep white matter alterations; and differential impairment on neuropsychological tests assessing visuoconstruction and the ability to establish and maintain mental set, with relatively higher scores on tests of memory using the delayed recognition paradigm. In fact, there was a double dissociation as the Alzheimer’s disease patients presented the inverse neuropsychological profile. The authors thus suggested that these features could perhaps be utilized as indicators of vascular rather than Alzheimer’s disease pathology in the future.
Rasquin et al.’s study 26 aimed at determining, in a sample of 194 first-time stroke patients, the influence of different diagnostic criteria on the prevalence and cumulative incidence of poststroke dementia using a longitudinal design as clinically evaluated at 1, 6, 12, and 24 months after stroke. The authors used an extensive cognitive battery in patients whose MMSE score was higher than 15. The highest prevalence rate of dementia at 1 month was obtained using the ADDTC criteria (for possible ischemic vascular dementia) and the ICD-10 criteria, and the lowest prevalence rate was obtained using the NINDS-AIREN criteria (for both possible and probable vascular dementia). The incidence rates were highest at 6 months, ranging from 2.6% (ADDTC) to 5.2% (ICD-10).
Finally, Lopez et al. 27 classified 480 incident cases of vascular dementia from the multicenter Cardiovascular Health Study–Cognition Study. In this study, patients were administered an extensive neuropsychological battery as well as a brain MRI during their follow-up. The authors aimed at comparing and contrasting the diagnosis of vascular dementia based solely on history of strokes or severe vascular disease versus that aided by neuroimaging. The pre-MRI classification showed that of 480 incident cases, 52 participants (10.8%) had vascular dementia and 76 (15.8%) had both Alzheimer’s disease and vascular dementia. The post-MRI classification showed that the highest proportion of vascular dementia cases was detected using the ADDTC criteria. The DSM-IV criteria ranked second, while the lowest proportion of patients was diagnosed using the NINDS-AIREN criteria. The diagnosis of vascular dementia by the DSM-IV and NINDS-AIREN criteria identified a group of participants with severe vascular disease. The ADDTC criteria identified participants in the border zone between Alzheimer’s disease and vascular dementia or patients with no history of strokes but with severe MRI-identified vascular disease. Of the 132 subjects with a pre-MRI diagnosis of vascular dementia (alone or mixed), only 38 (29%) were classified as vascular dementia by all three diagnostic criteria. None of the vascular dementia criteria were able to successfully identify all of the MRI-confirmed cases of vascular dementia. Interestingly, based on the composite scores of neuropsychological data available in 243 patients, it was found that patients with probable vascular dementia had poorer visuospatial and fine motor control performance than did those with a diagnosis of probable vascular dementia with Alzheimer’s disease, possible vascular dementia with probable Alzheimer’s disease and possible vascular dementia with possible Alzheimer’s disease. No other statistical differences among groups were noted on tasks of memory, language, or executive functioning. The strengths of this study included a standardized MRI protocol for the 480 subjects and extensive premorbid longitudinal clinical data that allowed the researchers to examine the relationship between dementia and vascular disease.
DISCUSSION
The present paper is, to our knowledge, the first review aimed at assessing the diagnostic properties of the Hachinski Ischemic Scale and Ischemic Scale of Rosen, and the ICD-10, DSM-III, DSM-III-R, DSM-IV, ADDTC, and NINDS-AIREN criteria for the diagnosis of vascular dementia.
Assessment of Sensitivity and Specificity
Following the analyses of the six clinico-neuropathological studies, this review revealed that the ADDTC criteria for possible (or possible with probable) ischemic vascular dementia were the most sensitive diagnostic criteria in four out of six (66.67%) clinico-pathological studies. 12 – 14 , 16 Knopman et al. 15 and Zekry et al. 11 were the only clinico-pathological studies in which the ADDTC criteria were not found to be the most sensitive; they were surpassed by the ICD-10 and DSM-IV, 15 and by the Hachinski Ischemic Scale. 11 In these two studies, the ADDTC were the second most sensitive criteria. The NINDS-AIREN criteria for possible (and possible with probable) vascular dementia were the second most sensitive criteria in four out of six clinico-pathological studies (66.67%). The ADDTC criteria for possible vascular dementia appear to be the best criteria to use for case detection in clinical settings, at least for younger cohorts of older patients (≤ 90 years old). 14
Regarding specificity, the NINDS-AIREN criteria for possible or probable vascular dementia were found to be the most specific in two out of six (33.33%) of the clinico-pathological studies, and the second most specific in four out of six (66.67%) of them. The best performance in terms of specificity has been occupied differently depending on the study, either by the Hachinski Ischemic Scale, ICD-10, ADDTC possible, or DSM-IV criteria. Because of their very high specificity estimates, the NINDS-AIREN criteria may be most indicated for clinical research, as they would prevent researchers from including false positive cases of vascular dementia. By contrast, the only clinical study that assessed sensitivity and specificity found the ADDTC criteria to be the least sensitive criteria for the diagnosis of vascular dementia. 18 In fact, in this particular study, the DSM-IV criteria were the most sensitive and the least specific, whereas the NINDS-AIREN criteria were the second most sensitive and the most specific. However, as seen in the first part of our qualitative review, 7 the DSM and ICD-10 criteria and the Hachinski ischemic scales did not exclude systemic disorders or other brain disease such as Alzheimer’s disease and have not provided clear instructions in regards to the mandatory vascular lesions required for a diagnosis of vascular dementia. Consequently, these criteria may perhaps misdiagnose the cases of mixed dementia in clinical settings more frequently than the NINDS-AIREN or ADDTC criteria. All sets of clinical criteria appear to distinguish, with relative success, pure Alzheimer’s disease from vascular dementia. 11 – 14 , 19 However, they are much less effective in detecting mixed cases of dementia. 11 – 14 , 19 The NINDS-AIREN and ADDTC criteria were generally the most sensitive, and the Hachinski Ischemic Scale was the least sensitive, for the diagnosis of mixed dementia in clinico-pathological studies.
Comparison of the Prevalence, Incidence, and/or Frequency Rates
The ADDTC criteria for possible or probable ischemic vascular dementia yielded the highest prevalence and/or frequency rates in half of the group III studies, 20 , 25 – 27 whereas the DSM-IV yielded the highest proportion of vascular dementia diagnosis in three out of eight (37.5%) studies. 22 – 24 However, most of the studies that found the highest proportion of vascular dementia diagnosis using the ADDTC criteria did not include the DSM-IV criteria in their analyses. Should they have done so, the results might have been different in that regard. Nevertheless, it should be underlined that some studies have found a high rate of false positives with the ADDTC criteria. These criteria misdiagnosed Alzheimer’s disease patients 25 and mixed dementia patients 12 as having vascular dementia. Thus if the exclusion of mixed cases is important, the DSM-IV 13 or the NINDS-AIREN criteria for vascular dementia 11 might be preferred. On the other hand, the NINDS-AIREN criteria were consistently found to be the most conservative for vascular dementia, yielding the lowest prevalence and/or frequency rates of vascular dementia diagnosis in all group III studies. These results are in accordance with the low sensitivity rates found in the class I studies.
Possible Causes for the Lack of Validity and Comparability
The lack of sensitivity and comparability of the diagnostic criteria can be attributed, in part, to several factors that relate to the issues presented and discussed in Part I of our qualitative review. 7 Indeed, differences in the definition of the cognitive syndrome and its distribution (i.e., the requirement of patchy or unequal distribution of impairments in higher cognitive functions per the DSM and ICD-10) made the detection of vascular dementia difficult and created difficulty in comparing the various diagnostic criteria. 22 , 23 , 25 – 27 Prominent memory deficits in the current definition of vascular dementia given by the DSM and NINDS-AIREN criteria, a concept which evolved from our current understanding of Alzheimer’s disease, might not be appropriate. Episodic memory impairment represents a core aspect of Alzheimer’s disease, but several recent findings have clearly indicated that executive dysfunction, inattention, and impairment of psychomotor speed are more characteristic of vascular dementia, vascular lesions, and vascular cognitive impairment. 28 – 34 A patchy and unequal neuropsychological profile is also not specific to vascular dementia.
Furthermore, the requirement of focal neurological signs and symptoms by the DSM-III, DSM-III-R, ICD-10, and NINDS-AIREN criteria limits the accurate diagnosis of vascular dementia as shown in several studies. 13 , 21 , 23 , 24 In fact, both Wetterling et al. 22 and Pohjasvaara et al. 24 found that only one third of their samples presented with focal neurological symptoms, and these symptoms were relatively rare in subcortical vascular dementia patients. 24 The unique combination in the NINDS-AIREN criteria of the requirement of focal neurological signs and the exclusion of diseases that might cause dementia might explain the low sensitivity and prevalence rates of vascular dementia diagnoses according to this classification. 23 The DSM-IV criteria have been found to be the most liberal criteria in the present review, probably because they do not require focal neurological signs and symptoms to be present and do not clearly specify brain imaging lesions. The ADDTC criteria have been found to be the second most liberal criteria. They require only focal neurological signs or evidence of cerebrovascular disease.
Moreover, the difficulty in establishing a temporal relationship between dementia and cerebrovascular disease in terms of type of onset and evolution makes it difficult to properly identify patients with vascular dementia. 13 , 15 , 21 , 23 , 27 It has also been shown that some types of vascular dementia begin and develop in an insidious fashion without any clinically apparent stroke 15 and that an abrupt onset or stepwise decline is not always present even in the pure cerebrovascular disease cases. 35
Finally, some authors found that in “clear-cut” patients with multiple strokes, current vascular dementia criteria are rather reliable. 21 , 22 However, small-vessel diseases rather than stroke appear to be the most frequent type of vascular diseases, 22 , 25 which make periventricular and deep white matter changes the most frequent types of vascular lesions. 25 In this context, the absence of a requirement for neuroimaging evidence of cerebrovascular disease and the lack of consideration of subtypes of vascular dementia according to CT or MRI findings by several diagnostic criteria (DSM-III, DSM-III-R, DSM-IV, and ICD-10 criteria, the Hachinski Ischemic Scale and Ischemic Scale of Rosen) certainly have had an impact on both the sensitivity and specificity estimates of these criteria. Overall, these problems related to the diagnosis of vascular dementia are consistent with Paul et al.’s five clinical “myths” 36 that complicate the diagnostic process for clinical neuropsychologists: a stepwise versus an insidious decline, a patchy neuropsychological profile, the prominent memory deficit, the specificity of neuroimaging findings, and the distinctions between vascular dementia and Alzheimer’s disease.
Suggestions for Improvements
First of all, the definition of the cognitive syndrome associated with vascular dementia should be revised. As currently conceptualized, the presence of executive dysfunction is not a necessary criterion for the diagnosis of vascular dementia, while memory impairment generally is. The fact that executive deficits represent a common and important aspect of vascular dementia, and may help distinguish it from Alzheimer’s disease, raises the question of whether greater importance should be assigned to this feature. Subcortical pathology due to vascular factors leading to a dementia syndrome has been recognized for over a century, 37 but whether all vascular dementia patients have significant subcortical pathology and executive dysfunction needs further examination. Cognitive effects of cerebrovascular disease are highly variable. 35 Therefore, executive dysfunctions may be added as a supportive deficit rather than a required deficit in future criteria. On the other hand, an impairment of episodic memory should not be a mandatory deficit anymore in future criteria; it could instead be a supportive deficit. Moreover, a patchy distribution of cognitive deficits is not specific to vascular dementia; many forms of dementia differentially impact regions of the brain at a given level of disease severity. This criterion should thus be excluded from future criteria sets. Pohjasvaara et al. 38 underlined the importance of further refining categories of impairment in the diagnosis of vascular dementia as well as the method of assessment of dementia. These authors demonstrated that the prevalence estimates for vascular dementia according to different criteria varied greatly depending on the type of cognitive assessment used—a short, clinical assessment such as the MMSE 39 and the Modified MMSE 40 versus an exhaustive neuropsychological evaluation. The prevalence rates of vascular dementia were 14.1% (DSM-III), 9.7% (DSM-III-R), and 8.4% (NINDS-AIREN criteria) using a short clinical assessment of the cognitive syndrome, whereas these rates were 27.3% (DSM-III), 4.0% (DSM-III-R), and 25.6% (NINDS-AIREN criteria) when a complete and exhaustive neuropsychological assessment of the cognitive syndrome was administered. Regardless of the assessment method used and consistent with previous studies, the NINDS-AIREN criteria led to the lowest proportion of vascular dementia diagnosis whereas the DSM-III criteria led to the highest. As shown here, cognitive functions in the reviewed studies were assessed either by very short examination (i.e., MMSE), global battery (i.e., Cambridge Examination for Mental Disorders of the Elderly), or by extensive neuropsychological testing. This may add to the heterogeneity in vascular dementia case detection. In future studies of vascular dementia, an exhaustive neuropsychological test battery should be administered in order to better define the cognitive syndrome. Shorter test batteries, which might find a clinical role in the diagnosis of vascular dementia, could be included in these studies, so as to assess the diagnostic accuracy of these tests in comparison to the neuropsychological battery.
In regards to the vascular causes, various mechanisms and types of vascular lesions might be involved, not only strokes with related abrupt onset of dementia. 15 , 25 , 41 , 42 Further studies, aimed at differentiating subtypes of vascular dementia according to the various underlying vascular mechanisms, may greatly improve both detection and treatment of vascular-related cognitive impairment. For instance, to improve sensitivity of the diagnostic criteria for the insidious subtype of vascular dementia, better tools for distinguishing nonspecific white matter lesions from severe ischemic leukoencephalopathy are required. However, reports of volume and white matter abnormalities on MRI and other cerebral imaging techniques are common among healthy elderly 43 , 44 and Alzheimer’s disease patients. 45 , 46 As a result, it is difficult to determine whether evidence of cerebrovascular disease on cerebral imaging is clinically meaningful or simply represents “background noise.” To overcome these limitations, it has been suggested that white matter changes/lesions should exceed 10 cm 2 in size to provoke cognitive abnormalities 47 and involve at least 25% of the total white matter in order to meet criteria for vascular dementia, 6 , 48 although the exact threshold remains unknown. Unfortunately, in clinical settings radiological reports rarely interpret the severity of subcortical ischemic disease in terms of percentages. Perhaps clinicians should consider this possibility to improve the differential diagnosis of vascular dementia versus Alzheimer’s disease and mixed dementia. In the future, differentiation between ischemic lesions using structural and functional MRI versus in vivo Alzheimer’s disease pathological changes in the hippocampus, eventually using fluorine-18 (FDDNP) in conjunction with positron emission tomography, 49 , 50 may improve recognition of vascular dementia versus Alzheimer’s disease.
In addition, because vascular dementia and Alzheimer’s disease may share more pathological, 45 , 46 neurochemical, 51 , 52 and clinical features than was previously believed, it might be appropriate to include affective and behavioral symptoms in the diagnostic criteria. These symptoms, especially anxiety, apathy, and depression, are relatively common in patients with cerebrovascular risk factors, 53 CNS diseases, 54 white matter hyperintensities, 55 stroke, 56 , 57 and vascular dementia. 58 – 60 Psychosis has been found to be as frequent in vascular dementia as in Alzheimer’s disease in patients with moderate to severe dementia. 58 , 59 However, depression was significantly more frequent in patients with moderate to severe vascular dementia than in Alzheimer’s disease patients. 58 , 60 Among patients with vascular dementia and Alzheimer’s disease, those with severe vascular dementia were the most likely to be anxious. 58 Another study showed that depression significantly contributed to frontal cognitive dysfunction in 67 patients with CNS disease, including 46 patients with cerebrovascular disease. 54 Furthermore, Flint et al. 61 showed that in the acute stroke stage, delirium was found in 13% of the patients compared to 2% in acute coronary patients. Although these findings require replication, they nevertheless suggest that depression in moderate to severe vascular dementia could be added as a supportive criterion, whereas the presence of psychosis and delirium might not be considered as exclusion criteria in patients with moderate to severe dementia suspected of vascular dementia. Should these three additions be made in future criteria, one would expect an increase in the sensitivity rates.
The suggestions for improvement are consistent with those recommended by Garrett et al. 62 in future clinical and research work on vascular dementia. They include: (a) a description of the nature of vascular changes in order to facilitate predictions regarding the neuropsychological profile of subtypes of vascular dementia; (b) the measurement of cognitive constructs in addition to test scores to describe the neuropsychological profiles of types of vascular dementia; (c) the integration of direct MRI observations of the brain and other collateral data in the diagnostic process; and (d) the consideration of the term “vascular cognitive impairment-no dementia” for suspected prodromal vascular dementia. Indeed, a continuum of cerebrovascular-related cognitive impairment has been developed under the term vascular cognitive impairment 63 , 64 or vascular cognitive disorders. 65 This vascular cognitive impairment continuum extends from the brain-at-risk stage for cerebrovascular disease, to clinical signs of cognitive impairment without evidence of significant loss of function, and later to vascular dementia. 63 , 64 , 66 Nonetheless, the earliest stages (i.e., brain-at-risk and vascular cognitive impairment-no dementia) have received very little attention compared to vascular dementia or even mild cognitive impairment, a surprising situation as it is widely accepted that vascular dementia can be prevented. Individuals may control risk factors for stroke and small vessel ischemic disease. 60 , 67 – 69
Limitations of the Literature Reviewed
A major limitation of the reviewed studies is the absence of clinico-pathological validation of the clinical criteria for vascular dementia using prospective longitudinal experimental designs. Sample size varied considerably across studies, ranging from 25 case reports 23 and 32 dementia patients 19 to 1,347 patients. 18 This difference was principally due to the experimental design (cross-sectional versus longitudinal design; one setting versus multicenter cohort analysis) as well as to the type of study (clinico-pathological versus clinical study). Since all the participants included in these studies came from various recruitment sites, such as memory-clinic, stroke-clinic, and institution-, hospital-, or community-based cohort, the samples were also quite different with regard to age (patients in the clinico-pathological studies were typically older than patients in clinical studies) and degree of cognitive impairment. Some patients presented dementia according to the DSM-III-R or DSM-IV, whereas other patients presented with only mild cognitive impairment or were not impaired. Despite these limitations, however, it is clear that the currently available literature has provided a considerable amount of useful information related to the properties of the various diagnostic criteria for vascular dementia, as well as provided useful suggestions to improve future criteria.
CONCLUSION
Several sets of clinical criteria have been proposed to diagnose vascular dementia. However, this review has evidenced marked variability among reported sensitivities and specificities, incidence, and prevalence rates as well as substantial differences in the clinical classification of cases of dementia. This situation has a direct impact on recognition and treatment of this important neurological condition. None of the criteria sets distinguished mixed dementia from vascular dementia (although the criteria were better at excluding pure Alzheimer’s disease), nor did they recognize early vascular cognitive changes. None of these criteria have been satisfactorily validated by longitudinal prospective studies. Therefore, the current clinical criteria for vascular dementia have limited validity for the whole vascular cognitive impairment continuum, and they are not interchangeable.
All of these criteria sets have very different specificities and sensitivities. Although the ADDTC criteria might be most useful in clinical settings, and the NINDS-AIREN criteria the most useful in research settings, the lack of comparability between diagnostic criteria is a barrier for both of these criteria. They share similar principles and flaws, in that they recognize cases too late and use a cognitive diagnostic paradigm inappropriately based on that of Alzheimer’s disease. 63 , 64
Vascular cases presenting with cognitive impairment should ideally be recognized before dementia onsets, as vascular dementia may be prevented. 60 These shortcomings in the diagnosis of vascular dementia have led to the development of the concept of vascular cognitive impairment, a broader term that is intended to detect cognitive loss before dementia evolves beyond effective treatment. 63 There is now a vital need for an international agreement on clinical criteria not only for the vascular dementia diagnosis and its subtypes, but also for the full spectrum of cognitive impairments associated with vascular diseases and vascular risk factors. Such agreement would clarify the selection and domain measurements related to the vascular cognitive impairment construct, including cognitive functions, behavioral/affective/psychotic symptoms, and social functioning.
1 . Jellinger KA: Vascular-ischemic dementia: an update. J Neural Transm Suppl 2002; 62:1–23Google Scholar
2 . Yanagihara T: Vascular dementia in Japan. Ann N Y Acad Sci 2002; 977:24–28Google Scholar
3 . Hachinski VC, Iliff LD, Zilhka E, et al: Cerebral blood flow in dementia. Arch Neurol 1975; 32:632–637Google Scholar
4 . Rosen WG, Terry RD, Fuld PA, et al: Pathological verification of ischemic score in differentiation of dementias. Ann Neurol 1980; 7:486–488Google Scholar
5 . Chui HC, Victoroff JI, Margolin D, et al: Criteria for the diagnosis of ischemic vascular dementia proposed by the State of California Alzheimer’s Disease Diagnostic and Treatment Centers. Neurology 1992; 42:473–480Google Scholar
6 . Román GC, Tatemichi TK, Erkinjuntti T, et al: Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology 1993; 43:250–260Google Scholar
7 . Wiederkehr S, Simard M, Fortin C, et al: Comparability of the clinical diagnostic criteria for vascular dementia: a critical review. Part 1. J Neuropsychiatry Clin Neurosci 2008; 20:150–161Google Scholar
8 . Erkinjuntti T, Ostbye T, Steenhuis R, et al: The effect of different diagnostic criteria on the prevalence of dementia. N Engl J Med 1997; 337:1667–1674Google Scholar
9 . Warner J: Clinicians’ guide to evaluating diagnostic and screening tests in psychiatry. Advances in Psychiatric Treatment 2004; 10:446–454Google Scholar
10 . Jaeschke R, Guyatt G, Lijmer J: Diagnostic tests, in User’s Guides to the Medical Literature. Edited by Guyatt G, Rennie D. Chicago, American Medical Association Press, 2002, pp 121–140Google Scholar
11 . Zekry D, Duyckaerts C, Belmin J, et al: Alzheimer’s disease and brain infarcts in the elderly. J Neurol 2002; 249:1529–1534Google Scholar
12 . Gold G, Giannakopoulos P, Montes-Paixao S, et al: Sensitivity and specificity of newly proposed clinical criteria for possible vascular dementia. Neurology 1997; 49:690–694Google Scholar
13 . Gold G, Bouras C, Canuto A, et al: Clinicopathological validation study of four sets of clinical criteria for vascular dementia. Am J Psychiatry 2002; 159:82–87Google Scholar
14 . Bacchetta J-P, Kovari E, Merlo M, et al: Validation of clinical criteria for possible vascular dementia in the oldest-old. Neurobiol Aging 2007; 28:579–585Google Scholar
15 . Knopman DS, Parisi JE, Boeve BF, et al: Vascular dementia in a population-based autopsy study. Arch Neurol 2003; 60:569–575Google Scholar
16 . Hogervorst E, Bandelow S, Combrinck M, et al: The validity and reliability of 6 sets of clinical criteria to classify Alzheimer disease and vascular dementia in cases confirmed postmortem: added value of a decision tree approach. Dement Geriatr Cogn Disord 2003; 16:170–180Google Scholar
17 . Jaeschke R, Guyatt GH, Sackett DL: Users’ guides to the medical literature. III. How to use an article about a diagnostic test. B. What are the results and will they help me in caring for my patients? The Evidence-Based Medicine Working Group. JAMA 1994; 271:703–707Google Scholar
18 . Rockwood K, Davis H, MacKnight C, et al: The consortium to investigate vascular impairment of cognition: methods and first findings. Can J Neurol Sci 2003; 30:237–243Google Scholar
19 . Fischer P, Jellinger K, Gatterer G, et al: Prospective neuropathological validation of Hachinski’s Ischemic Score in dementia. J Neurol Neurosurg Psychiatry 1991; 54:580–583Google Scholar
20 . Amar K, Wilcock GK, Scott M: The diagnosis of vascular dementia in the light of the new criteria. Age Ageing 1996; 25:51–55Google Scholar
21 . Verhey FRJ, Lodder J, Rozendaal N, et al: Comparison of seven sets of criteria used for the diagnosis of vascular dementia. Neuroepidemiology 1996; 15:166–172Google Scholar
22 . Wetterling T, Kanitz RD, Borgis KJ: Comparison of different diagnostic criteria for vascular dementia (ADDTC, DSM-IV, ICD-10, NINDS-AIREN). Stroke 1996; 27:30–36Google Scholar
23 . Chui HC, Mack W, Jackson JE, et al: Clinical criteria for the diagnosis of vascular dementia: a multicenter study of comparability and interrater reliability. Arch Neurol 2000; 57:191–196Google Scholar
24 . Pohjasvaara T, Mantyla R, Ylikoski R, et al: Comparison of different clinical criteria (DSM-III, ADDTC, ICD-10, NINDS-AIREN, DSM-IV) for the diagnosis of vascular dementia. Stroke 2000; 31:2952–2957Google Scholar
25 . Cosentino SA, Jefferson AL, Carey M, et al: The clinical diagnosis of vascular dementia: a comparison among four classification systems and a proposal for a new paradigm. Clin Neuropsychol 2004; 18:6–21Google Scholar
26 . Rasquin SMC, Lodder J, Verhey FRJ: The effect of different diagnostic criteria on the prevalence and incidence of poststroke. Neuroepidemiology 2005; 24:189–195Google Scholar
27 . Lopez OL, Kuller LH, Becker JT, et al: Classification of vascular dementia in the Cardiovascular Health Study Cognition Study. Neurology 2005; 64:1539–1547Google Scholar
28 . Charlton RA, Morris RG, Nitkunan A, et al: The cognitive profiles of CADASIL and sporadic small vessel disease. Neurology 2006; 66:1523–1526Google Scholar
29 . Chen CF, Lan SH, Khor GT, et al: Cognitive dysfunction after acute lacunar infarct. Kaohsiung J Med Sci 2005; 21:267–271Google Scholar
30 . Garrett KD, Browndyke JN, Whelihan W, et al: The neuropsychological profile of vascular cognitive impairment-no dementia: comparisons to patients at risk for cerebrovascular disease and vascular dementia. Arch Clin Neuropsychol 2004; 19:745–757Google Scholar
31 . Looi JCL, Sachdev PS: Differentiation of vascular dementia from Alzheimer’s disease on neuropsychological tests. Neurology 1999; 53:670–678Google Scholar
32 . Nys GM, Van Zandvoort MJ, De Kort PL, et al: Domain-specific recovery after first-ever stroke: a follow-up study of 111 cases. J Int Neuropsychol Soc 2005; 11:795–806Google Scholar
33 . Nys GM, Van Zandvoort MJ, De Kort PL, et al: The prognostic value of domain-specific cognitive abilities in acute first-ever stroke. Neurology 2005; 64:821–827Google Scholar
34 . Poore QE, Rapport LJ, Fuerst DR, et al: Word list generation performance in Alzheimer’s disease and vascular dementia. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 2006; 13:86–94Google Scholar
35 . Reed BR, Mungas DM, Kramer JH, et al: Clinical and neuropsychological features in autopsy-defined vascular dementia. Clin Neuropsychol 2004; 18:63–74Google Scholar
36 . Paul R, Garrett K, Cohen R: Vascular dementia: a diagnostic conundrum for the clinical neuropsychologist. Appl Neuropsychol 2003; 10:129–136Google Scholar
37 . Bingswanger O: Die Abrenzung der allegmeinen progresiven Paralyze. Berliner Kilnische Wochenschrift 1894; 13:1137–1139Google Scholar
38 . Pohjasvaara T, Ylikoski R, Leskelä M, et al: Evaluation of various methods of assessing symptoms of cognitive impairment and dementia. Alzheimer Dis Assoc Disord 2001; 15:184–193Google Scholar
39 . Folstein MF, Folstein SE, McHugh PR: Minimental state: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189–198Google Scholar
40 . Teng EL, Chui HC: The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry 1987; 48:314–318Google Scholar
41 . Esiri MM, Wilcock GK, Morris JH: Neuropathological assessment of the lesions of significance in vascular dementia. J Neurol Neurosurg Psychiatry 1997; 63:749–753Google Scholar
42 . Fein G, Di Sclafani V, Tanabe J, et al: Hippocampal and cortical atrophy predict dementia in subcortical ischemic vascular disease. Neurology 2000; 55:1626–1635Google Scholar
43 . Henry Feugeas MC, De Marco G, Peretti II, et al: Age-related cerebral white matter changes and pulse-wave encephalopathy: observations with three-dimensional MRI. Magn Reson Imaging 2005; 23:929–937Google Scholar
44 . Maclullich AM, Wardlaw JM, Ferguson KJ, et al: Enlarged perivascular spaces are associated with cognitive function in healthy elderly men. J Neurol Neurosurg Psychiatry 2004; 75:1519–1523Google Scholar
45 . Swartz RH, Black SE: Anterior-medial thalamic lesions in dementia: frequent, and volume dependently associated with sudden cognitive decline. J Neurol Neurosurg Psychiatry 2006; 77:1307–1312Google Scholar
46 . Xie S, Xiao JX, Gong GL, et al: Voxel-based detection of white matter abnormalities in mild Alzheimer disease. Neurology 2006; 66:1845–1849Google Scholar
47 . Boone KB, Miller BL, Lesser IM, et al: Neuropsychological correlates of white-matter lesions in healthy elderly subjects: a threshold effect. Arch Neurol 1992; 49:549–554Google Scholar
48 . Erkinjuntti T, Haltia M, Palo J, et al: Accuracy of the clinical diagnosis of vascular dementia: a prospective clinical and postmortem neuropathological study. J Neurol Neurosurg Psychiatry 1988; 51:1037–1044Google Scholar
49 . Agdeppa ED, Kepe V, Liu J, et al: 2-Dialkylamino-6-acylmalononitrile substituted naphthalenes (DDNP analogs): novel diagnostic and therapeutic tools in Alzheimer disease. Mol Imaging Biol 2003; 5:404–417Google Scholar
50 . Shoghi-Jadid K, Small GW, Agdeppa ED, et al: Localization of neurofibrillary tangles and beta-amyloid plaques in the brains of living patients with Alzheimer’s disease. Am J Geriatr Psychiatry 2002; 10:24–35Google Scholar
51 . Gottfries CG, Blennow K, Karlsson I, et al: The neurochemistry of vascular dementia. Dementia 1994; 5:163–167Google Scholar
52 . Jia JP, Jia JM, Zhou WD, et al: Differential acetylcholine and choline concentrations in the CSF of patients with Alzheimer’s disease and vascular dementia. Chinese Medicine Journal 2004; 117:1161–1164Google Scholar
53 . Sheline YI, Barch DM, Garcia K, et al: Cognitive function in late life depression: relationships to depression severity, cerebrovascular risk factors and processing speed. Biol Psychiatry 2006; 60:58–65Google Scholar
54 . van Reekum R, Simard M, Clarke D, et al: The role of depression severity in the cognitive functioning of elderly subjects with CNS disease. J Psychiatry Neurosci 2000; 25:262–268Google Scholar
55 . O’Brien JT, Firbank MJ, Krishnan MS, et al: White matter hyperintensities rather than lacunar infarcts are associated with depressive symptoms in older people: the LADIS study. Am J Geriatr Psychiatry 2006; 14:834–841Google Scholar
56 . Brodaty H, Sachdev PS, Withall A, et al: Frequency and clinical, neuropsychological and neuroimaging correlates of apathy following stroke: the Sydney, Australia, Stroke Study. Psychol Med 2005; 35:1707–1716Google Scholar
57 . Nys GM, van Zandvoort MJ, van der Worp HB, et al: Early depressive symptoms after stroke: neuropsychological correlates and lesion characteristics. J Neurol Sci 2005; 228:27–33Google Scholar
58 . Ballard C, McKeith I, O’Brien J, et al: Neuropathological substrates of dementia and depression in vascular dementia, with a particular focus on cases with small infarct volumes. Dement Geriatr Cogn Disord 2000; 11:59–65Google Scholar
59 . Leroi I, Voulgari A, Breitner JC, et al: The epidemiology of psychosis in dementia. Am J Geriatr Psychiatry 2003; 11:83–91Google Scholar
60 . O’Brien JT, Erkinjuntti T, Reisberg B, et al: Vascular cognitive impairment. Lancet Neurol 2003; 2:89–98Google Scholar
61 . Flint AC, Loh JP, Brust JC: Vivid visual hallucinations from occipital lobe infarction. Neurology 2005; 65:756Google Scholar
62 . Garrett KD, Paul RH, Libon DJ, et al: Defining the diagnosis of vascular dementia. Applied Neuropsychology 2004; 11:202–207Google Scholar
63 . Hachinski V: Vascular dementia: a radical redefinition. Dement Geriatr Cogn Disord 1994; 5:130–132Google Scholar
64 . Hachinski VC, Bowler JV: Vascular dementia: diagnostic criteria for research studies. Neurology 1993; 43:2159–2160Google Scholar
65 . Sachdev P: Vascular cognitive disorder. Int J Geriatr Psychiatry 1999; 14:402–403Google Scholar
66 . Devasenapathy A, Hachinski VC: Vascular cognitive impairment. Curr Treat Options Neurol 2000; 2:61–71Google Scholar
67 . Goldstein LB, Adams R, Alberts MJ, et al: Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council: cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation 2006; 113:873–923Google Scholar
68 . Hanon O, Forette F: Prevention of dementia: lessons from SYST-EUR and PROGRESS. J Neurol Sci 2004; 226:71–74Google Scholar
69 . Tzourio C, Anderson C, Chapman N, et al: Effects of blood pressure lowering with perindopril and indapamide therapy on dementia and cognitive decline in patients with cerebrovascular disease. Arch Intern Med 2003; 163:1069–1075Google Scholar