Neurometabolic Correlations of Donepezil and Rivastigmine in Dementia Patients: A Different Neuroprotective Effect
To the Editor: Alzheimer’s disease is the most frequently encountered type of primary degenerative dementia, which is the primary cause of morbidity and mortality in the elderly population. Recent studies have shown that functionality is preserved much longer in patients who received therapy in the early phases of dementia. 1 This increased the importance of rapid initiation of choline esterase inhibitors widely used in the treatment of Alzheimer’s disease.
The evidence for the neuroprotective role of acetylcholine esterase inhibitors is rapidly replicating. 2 , 3 Rivastigmine is an effective cholinesterase inhibitor of acetyl and butyrylcholine esterase. Studies examining its superiority to donepezil revealed similar efficiency in the improvement of cognitive functions.
However, the existing clinical data were not combined with magnetic resonance spectroscopy (MRS) in any of these studies. MRS can provide quantitative analysis, unlike a subjective radiological evaluation limited with MRI and CT. In light of these findings, we compared the neurometabolic and the clinical correlations of rivastigmine and donepezil simultaneously.
Each group included 10 patients (five men and five women for the rivastigmine group; four men and six women for the donepezil group). The average age for rivastigmine and donepezil groups was 68.9±8.6 and 63.5±6.4 years old, respectively, and the target dose was determined as 12 mg/day and 10 mg/day for rivastigmine tartrate and donepezil hydrochloride, respectively. The Mini-Mental State Examination (MMSE), and MRS assays were repeated after 12 weeks of therapy.
Discussion
We found similar significant posttreatment improvements in cognitive function scores ( Figure 1 ) in both of the treatment groups as well as strong correlations with the cerebral metabolite values. However, although rivastigmine and donepezil caused similar significant increases in N -acetylaspartate/choline level (rivastigmine: pretreatment 0.70±36, posttreatment 1.06±0.33; donepezil: pretreatment 0.86±0.40, posttreatment 1.17±0.27) (Wilcoxon rank sum test, p<0.05) ( Figure 2 ), there was relatively more increasing effect of rivastigmine on İ 270 N -acetylaspartate levels than donepezil (rivastigmine: pretreatment 15.1±7.7, posttreatment 19.9±7.7; donepezil: pretreatment 20.0±7.9, posttreatment 22.6±4).
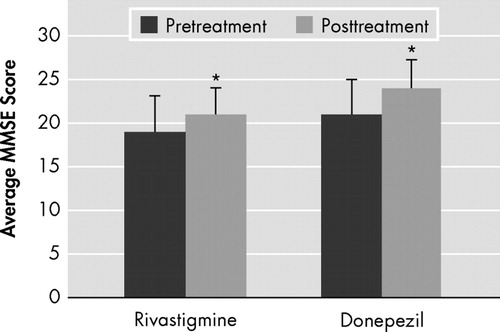
The posttreatment Mini-Mental State Examination (MMSE) scores were statistically significantly higher in the donepezil and rivastigmine groups (paired t test, p=0.029 and p=0.001, respectively).
*Significantly different (p≤0.05).
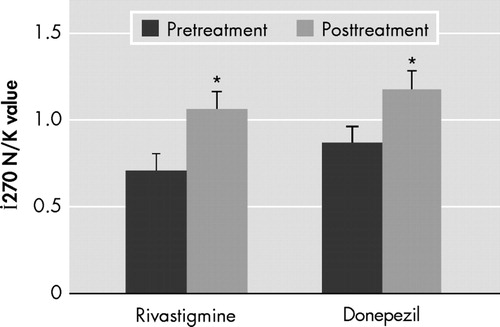
In both treatment groups, the posttreatment increase in İ 270 N -acetylaspartate levels was found to be statistically significant.
*Significantly different (p≤0.05).
In light of recent developments suggesting N -acetylaspartate as a marker for neuronal loss and rapidly replicating evidences about the neuroprotective role of acetylcholine esterase inhibitors, 3 , 4 we can hypothesize that this difference in N -acetylaspartate levels can be attributed to the dual effect of rivastigmine on cholinergic neurotransmission (acetyl and butyrylcholine esterase inhibition), which can contribute to its possible neuroprotective effect.
This is suggested by preclinical studies which indicated the enhancing effect of cholinergic activity in neuroprotection as well as the reversal of such effect under cholinergic blockage in different models of traumatic brain injury. 5
From this point of view, we think that this is an interesting study that compares not only the neuropsychological effects of two different groups of acetylcholine esterase inhibitors but also their effect on the quantitative neurometabolic assay that can reflect their possible underlying antiapoptotic and/or antinecrotic effect. This was also suggested by recent MRI hippocampal volumetric studies with donepezil. 3
Further experiments to evaluate the underlying neuroprotective mechanisms as well as long- and short-term clinical reflections of such neuroprotective effects using MRS and positron emission tomography studies would be the logical future steps in the field of psychiatry research.
1. Doody RS, Stevens JC, Beck C, et al: Practice parameter: management of dementia (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2001; 56:1154–1166Google Scholar
2. Akaike A: Preclinical evidence of neuroprotection by cholinesterase inhibitors. Alzheimer Dis Assoc Disord 2006; 20:8–11Google Scholar
3. Mori E, Hashimoto M, Krishnan KR, et al: What constitutes clinical evidence for neuroprotection in Alzheimer’s disease: support for the cholinesterase inhibitors? Alzheimer Dis Assoc Disord 2006; 20:S19–26Google Scholar
4. Demougeot C, Gamier P, Mossiat C, et al: N-acetylaspartate: a marker of both cellular dysfunction and neuronal loss: its relevance to studies of acute brain injury. J Neurochemistry 2001; 77:408–415Google Scholar
5. Jonnala RR, Graham JH 3rd, Terry AV, et al: Relative levels of cytoprotection produced by analogs of choline and the role of alpha7-nicotinic acetylcholine receptors. Synapse 2003; 47:262–269Google Scholar



