Anxiety, Depression, and Quality of Life in Parkinson’s Disease
Several studies have found that clinically relevant depression in Parkinson’s disease is underdiagnosed and that it is associated with increased disability and reduced quality of life. The mean incidence of depression in Parkinson’s disease is about 40%, 4 – 7 with reported rates varying between 4% and 70% in different studies, according to different methodological and diagnostic criteria. Anxiety disorders are also common, with higher incidence than in other chronic medical conditions (38% versus 11%). 8 A study by Nuti et al. 9 suggested the existence of a wide spectrum of psychiatric disorders in Parkinson’s disease, ranging from pure depressive disorders, to pure anxiety, to comorbid depression and anxiety disorders.
Previous studies were not conclusive about the etiology of both depression and anxiety disorders in Parkinson’s disease patients. 9 Results suggest a multifactorial process including a reactive state to a chronic disabling disease and/or an underlying neuropathological process involving abnormalities of central monoaminergic neurotransmitter systems. 10 In addition, the descriptive and clinical characteristics of depression and anxiety in Parkinson’s disease have also not been carefully investigated. 11 , 12
This study has the main purpose of evaluating the impact of Parkinson’s disease severity, depression, and anxiety on the health-related quality of life of patients.
METHODS
We performed a cross-sectional study including all idiopathic Parkinson’s disease patients observed in a specialized movement disorders consultation of a general hospital during a 6-month period. An informed consent was obtained from each patient. Idiopathic Parkinson’s disease was diagnosed according to the clinical criteria of the United Kingdom Parkinson’s Disease Society Brain Bank (UK-PDS-BB). 13 The Mini-Mental State Examination (MMSE) was used to exclude dementia, according to cutoff score levels of 15 for illiterate patients, 22 for patients who had from 1 to 11 years of schooling, and 27 for patients with more than 11 years. 14 , 15 Patients with additional functional impairment due to medical comorbidity were also excluded from this study.
The assessment instruments were the Short Form-36 Health Survey questionnaire, the Hospital Anxiety and Depression Scale, and the Hoehn and Yahr degree of disability scale. The first two were administrated in a face-to-face interview, in order to minimize missing values and evaluate helpfulness of collateral information. In addition, patients were questioned about psychiatric relevant history and current psychotropic medication.
Assessment Instruments
Short Form-36 Health Survey Questionnaire
The Short Form-36 Health Survey 16 is the most widely used quality-of-life measurement not specific to age, disease, or treatment. It generates a profile of scores for eight health dimensions: physical functioning, role limitations due to physical problems, bodily pain, general health perceptions, social functioning, role limitations due to emotional problems, mental health, and vitality. These eight dimensions may be grouped in two summary domains of physical and mental health: physical summary score includes physical functioning, physical role functioning, bodily pain, general health, and vitality; mental summary score includes mental health, emotional role functioning, social functioning, vitality, and general health dimensions.
This questionnaire has good psychometric qualities, and in Portuguese validation 17 , 18 only the social functioning dimension demonstrated a reliability coefficient lower than 0.80. On the other hand, some of Short Form-36 Health Survey items are not relevant to elderly patients, resulting in high levels of missing data; to minimize this we used the face-to-face administration of the questionnaire, which has been reported as equally consistent with self-administration scores. 19
Hospital Anxiety and Depression Scale
The Hospital Anxiety and Depression Scale 20 contains 14 items and consists of anxiety and depression subscales. Each item is rated on a four-point scale of 0–3, giving maximum scores of 21 for anxiety and depression.
The depression subscale is weighted toward the emotional aspects of depression (emphasizing anhedonia rather than sadness) and does not include physical and cognitive symptoms or suicidal ideation. Only the item regarding feeling “slowed down” overlaps with core Parkinson’s disease symptoms.
The authors of this scale suggested the cutoff value of 8 for both subscales. Its use as a severity measure is controversial, but they mentioned the classification of mild (8–10), moderate (11–15), and severe anxiety or depression (16–21) scores. The total Hospital Anxiety and Depression Scale may be used as a measure of global mood disorder. The Portuguese version 21 offers good psychometric properties similar to those in international studies.
Previous studies indicate the validity of using the Hospital Anxiety and Depression Scale to screen for depressive and anxious symptoms in Parkinson’s disease. 11 , 12 Until now there was no consensus about optimal cutoff scores for Parkinson’s disease patients. 22 , 23
Hoehn and Yahr Degree of Disability Scale
The Hoehn and Yahr scale 24 is a standard clinical measure of Parkinson’s disease progression. It globally measures signs and symptoms of functional impairment, including postural instability, rigidity, tremor, and bradykinesia. The stages 1 to 3 represent low to moderate incapacity, and stages 4 to 5 indicate serious incapacity. The descriptive analysis of this study uses a modified version 25 that includes intermediate stages.
Scoring and Statistical Analysis
Summary scores as well as the subdimensions of the Short Form-36 Health Survey were calculated according to the scoring algorithm. The difference in proportions was assessed by chi-square or Fisher’s exact test. Mean values were compared by the t test or Aspin-Welch unequal variance test. When normality conditions were not guaranteed, we compared difference in medians by Mann-Whitney test. Spearman rank correlation coefficients were calculated to assess the direction and magnitude of association between variables. Multiple regression analysis was used to determine the factors that best accounted for variance in quality-of-life scores.
PASS 2008 (Kaysville, Utah, version 08.05) was used to calculate sample size and statistical power. Statistical analysis was performed with NCSS 2007 (Kaysville, Utah, version 07.1.5). Statistical significance was only accepted at a significance level of 0.05, on a bilateral hypothesis test.
RESULTS
Descriptive Analysis
Forty-five consecutive patients were evaluated. Two were excluded from statistical analysis by outlier and missing values, leaving 43 for the study sample. There were 24 women (56%), and patients had a mean age of 72 years old (range=55 to 86 years).
The age of Parkinson’s disease onset varied between 49 and 82 years old, with a mean of 65 years and disease duration of 1 to 23 years. The severity of Parkinson’s disease according to the modified Hoehn and Yahr scale was classified as stage 1 in three patients (7%), stage 2 in 26 patients (60%), stage 2.5 in seven patients (16%), stage 3 in five patients (12%), and stage 4 in two patients (5%).
Three patients were taking antidepressant medication with infratherapeutic dose; none of the patients had relevant psychiatric history.
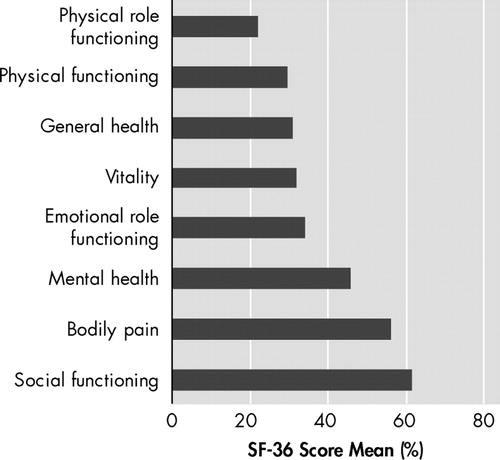
The means of the eight dimensions of Short Form-36 Health Survey showed that the most impaired aspects in Parkinson’s disease were those included in physical summary score, with the exception of bodily pain score ( Figure 1 ). The lowest mean score in the “role limitations due to physical problems” dimension is compatible with the Parkinson’s disease compromise of fine movements. There were no significant differences between sex and age.
Anxiety and depression (cutoff value of 8 in the Hospital Anxiety and Depression Scale subscales) were present in 58% of patients (n=25) in both cases; 44% of these patients presented a moderate to severe degree of anxiety (n=11), and 60% presented a moderate to severe degree of depression (n=15).
Correlational Analysis in Patients With Original Hoehn and Yahr Stage 2 Parkinson’s Disease
The original Hoehn and Yahr scale, without intermediate stages, persists as the preferred Hoehn and Yahr version for investigational purposes. To control confounding by illness severity, we restricted subsequent correlational analysis to patients with original Hoehn and Yahr stage 2 Parkinson’s disease (n=33). This left us with a subsample of 18 women and 15 men; mean and limits of age and years since diagnosis were similar to the total sample.
The scores of the Hospital Anxiety and Depression Scale (cutoff value of 8 in each subscale) showed that anxiety was present in 55% of patients (n=18) and depression in 58% of patients (n=19). There was a moderate to severe degree of anxiety (cutoff value of 11) in 33% of patients (n=6) and of depression in 84% of patients (n=16) meeting Hoehn and Yahr stage 2 criteria. Using the Mann-Whitney test, we found that women had significantly higher anxiety and depression scores than males (z=−2.49 and −2.41, respectively; p<0.05).
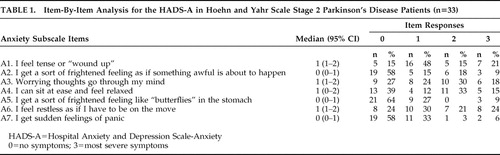
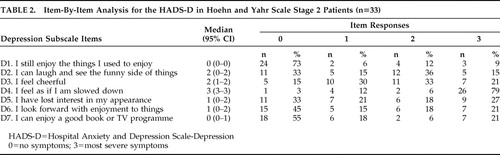
In order to identify the specific components of anxiety and depression in Parkinson’s disease, we undertook an item-by-item analysis of the patients’ scores on the Hospital Anxiety and Depression Scale-A and Hospital Anxiety and Depression Scale-D. We calculated the percentage of patients obtaining scores of 0–3 for each item separately. This analysis suggested that feeling tense, experiencing excessive worrying thoughts, an inability to relax, and restlessness were the main characteristics of anxiety ( Table 1 ). Inability to laugh, feeling unhappy, feeling slowed down, losing interest in personal appearance, and losing interest in formerly enjoyable activities were the main characteristics of depression for these patients ( Table 2 ).
 |
 |
The presence of anxiety or depression led to lower quality of life (z=3.33 and 2.52, respectively; p<0.05), including a significant impact in the physical summary score (z=2.28 and 2.48, respectively; p<0.05).
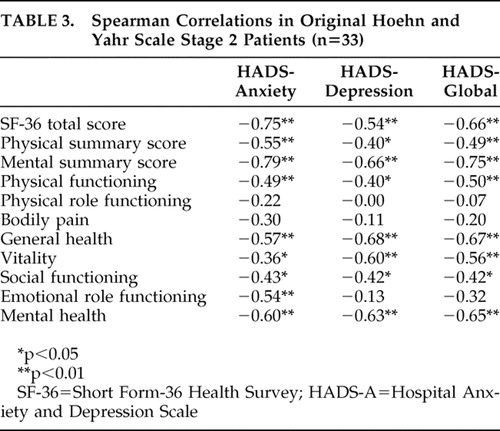
The numerical Hospital Anxiety and Depression Scale gives us more statistical power. The Spearman coefficients ( Table 3 ) showed a significant negative correlation between Hospital Anxiety and Depression Scale and global quality-of-life scores, higher in anxiety (r=−0.75, p<0.01) than depression (r=−0.54, p<0.01). Both anxiety and depression scores were significantly correlated with physical summary quality-of-life score, also with a stronger magnitude for anxious (r=−0.55, p<0.01) than depressive symptoms (r=−0.40, p<0.05). In addition, anxiety score was significantly correlated to the majority of Short Form-36 Health Survey dimensions. There was no significant correlation between the Hospital Anxiety and Depression Scale or Short Form-36 Health Survey scores and age, age at Parkinson’s disease onset, or disease duration.
 |
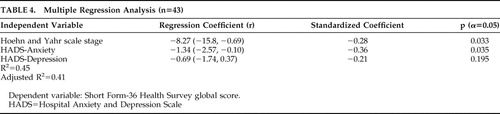
Multiple Regression Analysis
To determine which factor contributed most to quality-of-life score, we performed a multiple regression analysis including illness severity and anxious/depressive symptoms (the independent variables were operated as if they were interval ones).
The analysis about global quality of life revealed that the most important predictive factor was the Hospital Anxiety and Depression Scale score (r=−0.51, p=0.0002), followed by Hoehn and Yahr stage (r=−0.30, p=0.0198). Together they accounted for 42% of the variance of global Short Form-36 Health Survey score. With regard to the overlap of contents in Hospital Anxiety and Depression Scale global and its subscales’ scores, we performed a separate analysis including these last scores. This model accounted for 41% of the variance of the quality-of-life scores, with the largest standardized regression coefficient related to anxiety score (r=−0,36, p=0.03), and a nonsignificant one to depression score ( Table 4 ).
 |
This analysis watched for normality assumptions and potential sources of error like collinearity. Power analysis revealed that a sample size of 43 achieves 80% power to detect an R 2 of 0.22 attributed to three independent variables using a significance level of 0.05.
DISCUSSION
Previous studies demonstrated that Parkinson’s disease affects not only motor functioning, but also all measured aspects of quality of life, including emotional well-being and social functioning. 4 – 7 Severity of motor features of Parkinson’s disease is a major factor in how patients subjectively experience the impact of the disease, but previous multivariate analyses reported depression as the main factor correlated with worse quality of life in Parkinson’s disease, accounting for up to 60% of quality-of-life impairment. 4 – 7
In this study, with the exception of significantly higher anxiety and depression scores in females, we did not find any significant association between anxiety or depression and demographic characteristics (sex, age) or clinical features of Parkinson’s disease (age at onset, disease duration, Hoehn and Yahr rating). This suggests that anxiety and depression in Parkinson’s disease are not closely correlated with the severity of motor symptoms or degree of disability. In addition, the multiple regression analysis revealed that the strongest predictor of quality of life in Parkinson’s disease was the Hospital Anxiety and Depression Scale score, which, together with Hoehn and Yahr score, accounted for 42% of the variance of global quality-of-life score.
The results showed that anxious and depressive symptoms had a negative correlation with global quality of life and that a cutoff value of 8 in both Hospital Anxiety and Depression Scale subscales was sensitive to it. Anxious symptoms had the strongest impact in the quality of life of Parkinson’s disease patients, even in a sample with a clearly lower proportion of moderate-severe anxiety cases compared with depressive ones. Anxiety was also strongly correlated with physical summary score, suggesting two hypotheses: anxiety gives a subjective perception of higher motor compromise, and/or anxiety is the somatic translation of a central biochemical disturbance that accompanies Parkinson’s disease.
The homogeneity in clinical and psychiatric approach and the absence of missing values in our database were advantages compared with a previous study. 26 The sample size guaranteed statistical power concerning the regression analysis conclusion of this study—anxiety had the strongest predictive value in quality of life of Parkinson’s disease patients. However, the sample heterogeneity about Parkinson’s disease severity and underrepresentation of most Hoehn and Yahr stages limit generalization of results. In this context, we emphasize the interpretation of these results mainly in patients with original Hoehn and Yahr stage 2 Parkinson’s disease.
The use of a generic measure for quality-of-life assessment (Short Form-36 Health Survey questionnaire) excluded specific aspects of Parkinson’s disease management, like iatrogenic symptoms. 2 – 4 In fact, this study did not comprise a detailed assessment of clinical Parkinson’s disease characteristics, but previous studies noted that the Hoehn and Yahr scale had stronger correlation with quality-of-life scores than other more detailed clinical assessment scales (e.g., the Unified Parkinson's Disease Rating Scale). 4
The complexity of the concept of quality of life is certainly the main limitation of this study, because many variables that potentially contribute to quality of life, such as social support, individual coping strategies, and cultural context, were not directly measured.
While anxiety or depression etiology in Parkinson’s disease is unclear (biochemical changes, psychosocial factors, and situational stressors have all been implicated), their adverse effect on the quality of patients’ lives becomes reinforced. In addition, it is recognized that severe anxiety and/or depression can, in turn, make the motor symptoms of Parkinson’s disease worse.
This study emphasizes the impact of anxiety on quality of life of these patients. Anxiety may be high shortly after diagnosis but declines as patients increasingly cope with the experience of living with the illness. Nevertheless, several factors may predispose its persistence during illness course: Parkinson’s disease patients can become embarrassed about their motor impairment, particularly the tremor, stooped posture, and shuffling gait, and may avoid social situations because of the fear of negative evaluation. In addition, the unpredictability of function associated with on-off fluctuations, the disturbance of balance and risk of falls when walking are among the symptoms more likely to contribute to anxiety.
Physicians tend to ignore anxiety and depression in older patients, concentrating instead on physical complaints. Additionally, many older patients are reluctant to talk about their feelings or deny feeling sad or depressed. In this context, clinicians must be aware of other relevant signs, including worries about being a burden, feelings of worthlessness or self-loathing, losing interest in pleasurable pastimes, social withdrawal, and isolation (reluctance to be with friends, engage in activities, or leave home). All these symptoms make dealing with Parkinson’s disease more difficult and create a vicious cycle that impairs quality of life.
In this study, item analysis report concerning Hospital Anxiety and Depression Scale reemphasizes the importance of assessing these psychosocial issues. The main characteristics of anxiety and depression included feeling tense, experience of excessive worrying thoughts, feeling unhappy, and losing interest in personal appearance, which have no relation with somatic symptoms of Parkinson’s disease and that reflect a lower ability to get over the personal and social challenges that Parkinson’s disease patients experience.
In this context, clinicians should ensure the diagnosis and treatment optimization of depressive or anxious symptoms, including a first psychopharmacological intervention (mainly antidepressants) followed by a psychiatrist consultation if symptoms persist. In fact, some patients may benefit from psychotherapy or cognitive behavior therapy, in order to help them work through stressful life changes, change negative thinking patterns, and develop better coping skills. In addition, support groups can be a great help to connect people with others who are going through the same challenges.
The Hospital Anxiety and Depression Scale has the advantage of assessing anxiety and depressive symptoms, with a low overlap with somatic symptoms of Parkinson’s disease is a self-rating possibility and takes little time to administer. In this context, we think it can ameliorate the detection of reversible depressive and anxious symptoms, especially when used by nonpsychiatric clinicians.
CONCLUSION
This study supports the notion that depressive or anxiety disorders do not have a simple linear relationship with Parkinson’s disease severity or duration. Additionally, it highlights their impact on quality of life, reemphasizing the need for psychopathological assessment in Parkinson’s disease multidisciplinary care. 26
The results showed that anxiety had a prevailing impact on quality of life and reinforced the different meanings of anxious and depressive symptoms in Parkinson’s disease patients. Difficulties in coping with disease, a comorbid depressive syndrome, and the underlying neuropathological process are all possible etiologies that call for different therapeutic strategies. We think a therapeutic approach must be individualized, including an association between antiparkinson drugs and psychopharmacological treatment and/or psychotherapeutic interventions whenever indicated.
Further investigation is needed to clarify the pathophysiology of anxiety and depression in Parkinson’s disease, in order to guide pharmacological intervention (e.g., antidepressive drug selection) and to optimize multidisciplinary care of these patients. The Hospital Anxiety and Depression Scale has the advantage of screening both anxious and depressive states, allowing for better therapeutic management of psychopathological symptoms in Parkinson’s disease patients.
1. Marinus J, Ramaker C, van Hilten JJ, et al: Health related quality of life in Parkinson’s disease: a systematic review of disease specific instruments. J Neurol Neurosurg Psychiatry 2002; 72:241–248Google Scholar
2. Meyers AR, Gage H, Hendricks A: Health-related quality of life in neurology. Arch Neurol 2000; 57:1224–1227Google Scholar
3. Hobson P, Holden A, Meara J: Measuring the impact of Parkinson’s disease with the Parkinson’s Disease Quality of Life Questionnaire. Age Ageing 1999; 28:341–346Google Scholar
4. Schrag A, Jahanshahi M, Quinn N: What contributes to quality of life in patients with Parkinson’s disease? J Neurol Neurosurg Psychiatry 2000; 69:308–312Google Scholar
5. Schrag A, Jahanshahi M, Quinn N: How does Parkinson’s disease affect quality of life? A comparison with quality of life in the general population. Mov Disord 2000; 15:1112–1118Google Scholar
6. Schrag A: Quality of life and depression in Parkinson’s disease. J Neurol Sci 2006; 248:151–157Google Scholar
7. Ravina B, Camicioli R, Como PG, et al: The impact of depressive symptoms in early Parkinson’s disease. Neurology 2007; 69:342–347Google Scholar
8. Pincus JH, Tucker GJ: Behavioral Neurology, 4th ed. New York, Oxford University Press, 2002, pp 177–195Google Scholar
9. Nuti A, Ceravolo R, Piccinni A, et al: Psychiatric comorbidity in a population of Parkinson’s disease patients. Eur J Neurol 2004, 11:315–320Google Scholar
10. Mayeux R, Stern Y, Williams JB: Clinical and biochemical features of depression in Parkinson’s disease. Am J Psychiatry 1986; 143:756–759Google Scholar
11. Mondolo F, Jahanshahi M, Granà A, et al: Evaluation of anxiety in Parkinson’s disease with some commonly used rating scales. Neurol Sci 2007; 28:270–275.Google Scholar
12. Mondolo F, Jahanshahi M, Granà A, et al: The validity of the Hospital Anxiety and Depression Scale and the Geriatric Depression Scale in Parkinson’s disease. Behav Neurol 2006; 17:109–115Google Scholar
13. Hughes AJ, Daniel SE, Kilford L, et al: Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992; 55:181–184Google Scholar
14. Folstein MF, Folstein SE, McHugh PR: “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinicians. J Psychiatr Res 1975; 12:189–198Google Scholar
15. Guerreiro MS, Botelho MA, Leitão O, et al: Adaptação à população portuguesa da tradução do minimental state examination (MMSE). Rev Port Neurol 1994; 3(suppl 1):9–10Google Scholar
16. Ware JE, Snow KK, Kosinski M, et al: SF-36 Health Survey: Manual and Interpretation Guide. Boston, The Health Institute, New England Medical Center, 1993Google Scholar
17. Ferreira PL: Criação da versão portuguesa do MOS SF-36: parte I—adaptação cultural e linguística. Acta Med Port 2000; 13:55–66Google Scholar
18. Ferreira PL: Criação da versão portuguesa do MOS SF-36: parte II—testes de validação. Acta Med Port 2000; 13:119–127Google Scholar
19. Weinberger M, Oddone EZ, Samsa GP, et al: Are health-related quality-of-life measures affected by the mode of administration? J Clin Epidemiol 1996; 49:135–140Google Scholar
20. Zigmond AS, Snaith RP: The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand 1983; 67:361–370Google Scholar
21. Pais-Ribeiro J, Silva I, Ferreira T, et al: Validation study of a Portuguese version of the Hospital Anxiety and Depression Scale. Psychol Health Med 2007; 12:225–237Google Scholar
22. Schrag A, Barone P, Brown RG, et al: Depression rating scales in Parkinson’s disease: critique and recommendations. Mov Disord 2007; 22:1077–1092Google Scholar
23. Miyasaki JM, Shannon K, Voon V, et al: Practice parameter: evaluation and treatment of depression, psychosis, and dementia in Parkinson’s disease (an evidence-based review): report of the quality standards subcommittee of the American Academy of Neurology. Neurology 2006; 66:996–1002Google Scholar
24. Hoehn M, Yahr MD: Parkinsonism: onset, progression, and mortality. Neurology 1967; 17:427–442Google Scholar
25. Shenkman ML, Clark K, Xie T, et al: Spinal movement and performance of standing reach task in participants with and without Parkinson’s disease. Phys Ther 2001; 81:1400–1411Google Scholar
26. Rochow SB, Blackwell AD, Brown VJ: Quality of life in Parkinson’s disease: movement disorders clinic versus general medical clinic: a comparative study. Scott Med J 2005; 50:18–20Google Scholar



