Relationship Between Cognitive Status at Admission and Incident Delirium in Older Medical Inpatients
Risk factors are divided into baseline (mediating) and precipitating (moderating) types. Cognitive impairment is a common baseline risk factor for delirium, especially in older persons. 4 , 5 Cognitive deficit frequency increases in people over 60 years and worsens with advancing age; its severity, but not its etiology, may be quantified using screening cognitive examinations like the Mini-Mental State Examination (MMSE). 6 Cognitive deficit severity (assessed with the MMSE) as a risk factor for delirium has been studied in patients undergoing hip surgery (OR=1.32, 95% CI=1.06–1.64). 7 However, in that study dementia patients unable to give informed consent were excluded, patients with MMSE scores above the cutoff for cognitive impairment were included, and correlations between delirium risk and cognitive impairment were not reported. Further, characteristics of the relationship between these two variables has not been reported in nonsurgical patients. 8
The influence of global cognitive status on the clinical characteristics of delirium is not well studied. Consistently across studies, whether delirium occurs alone or with concurrent dementia, delirium symptoms overshadow dementia symptoms. 9 – 13 However, those reports compared delirium symptom frequencies cross-sectionally between patient groups. To our knowledge, the relationship between admission global cognitive status (as a continuous variable including impairment due to dementia) and incident comorbid delirium severity in hospitalized patients studied longitudinally has not been reported.
This prospective study of inpatients 60 years and over who were newly admitted to internal medicine wards in a tertiary care facility in Colombia had two aims: to evaluate how severity of cognitive impairment at admission modifies the risk for incident delirium, and to assess whether there is a linear relationship between global cognitive status and delirium severity.
METHODS
Design and Subjects
This is a case-control study design, with delirium patients nested in a cohort of patients evaluated daily during their hospitalization. All patients 60 years and older hospitalized in three internal medicine wards with a mean of 13 beds for each one of the Clínica Universitaria Bolivariana (Medellín, Colombia) during a 1-year period were consecutively evaluated. Persons with prevalent delirium at the time of hospitalization and those in coma or stupor were excluded. Patients were initially evaluated during the first 24 hours of hospitalization. Persons who died or were transferred to intensive care or surgery during the follow-up visits before discharge or delirium diagnosis were excluded from the analysis.
This study was approved by the Ethics and Bioethics institute of the Universidad Pontificia Bolivariana. All patients, or their families as proxy for those with cognitive impairment, gave informed consent to participate.
To standardize the raters, who were psychiatrists on the consultation-liaison team, on study tools, training and interrater reliability was accomplished in 15 patients and discussion about differences and difficulties in the scoring were done before the study began. The research psychiatrists who did daily evaluations were different from those who performed the initial interview that included the MMSE.
Procedures
Patients who met the inclusion criteria were evaluated within the first 24 hours of hospitalization using the diagnostic algorithm for delirium, the Confusion Assessment Method—Spanish (CAM-S), 14 , 15 and those who scored “positive” for delirium were excluded from the study (see exclusion criteria above). CAM-S negative patients were then evaluated with the Colombian version of the MMSE 16 to measure global cognitive status. This version is controlled for age, educational level, and visual impairment (defined as visual acuity worse than 20/70 according to the Snellen chart) and has a score range between 0 and 30 points. According to the Colombian MMSE validation study recommendations, 16 we added two points to the score obtained by the patients if there was evidence of visual impairment, one point if they were older than 65, and two points if they were older than 75. Then, according to the same instructions, we used the cutoff value of 21 for those with 5 or fewer years of education, 24 for those with between 6 and 12 years of education, and 26 for those with more than 12 years of education to determine presence of cognitive impairment.
Admission diagnosis and sociodemographic data were collected in this first interview. The use of an indwelling bladder catheter at any time during the study period, the total number of the different medications administered each day and the daily total number of different internal medicine diagnoses were also recorded for cases. These variables were recorded daily through the last evaluation visit day, which was the day when delirium was diagnosed or, for nondelirious comparison subjects, the last day of hospitalization.
The CAM-S was administered daily in nondelirious patients. When it became “positive” for delirium, the patient was administered the Colombian version of the Delirium Rating Scale—Revised-98 (DRS-R98) 17 (score range between 0 and 46, cutoff for delirium diagnosis≥12) to confirm the diagnosis and quantify symptoms. The DRS-R98 is a well-validated, sensitive, specific, and widely used rating scale for delirium with anchored item descriptions 18 that was revalidated in Columbia in its translated version.
Patients with delirium confirmed by the DRS-R98 were considered cases, and study follow-up visits were stopped at that point (study participation concluded) and usual treatment for delirium started. Those who were discharged without becoming delirious were considered comparison subjects and each was followed until discharge.
Statistical Analysis
Data were analyzed using SPSS 15.0 and spreadsheet software. Mean, standard deviation, median, range, and percentages were used for description of sociodemographic variables. The t test and chi-squares were used to evaluate possible differences in sociodemographic variables between cases and comparison subjects, except for the number of days of evaluation, which was compared with the Mann-Whitney U for rank means differences. The individual items of the MMSE in cases and comparison subjects were compared with the Mann-Whitney test; means are presented for these items. Median MMSE scores were compared using the median test.
The means for each patient for daily number of medications and daily number of internal medicine diagnoses were calculated over the assessment period as defined for delirious and comparison patients. Means of those individual patients are reported. The cumulative follow-up period for these two variables, and for the use of an indwelling bladder catheter, was truncated at the follow-up day when about 90% of cases had developed delirium in order to create comparable at-risk periods for cases and comparison subjects. This reduced bias related to the longer expected follow-up period in the comparison group.
A univariate analysis reporting an odds ratio (OR) and its 95% confidence interval (95% CI) was used to evaluate the magnitude of the association of delirium with cognitive impairment as a dichotomous event using the MMSE cutoffs with and without controlling for age. Multivariate stepwise logistic regression analyses (using forward- and backward-stepping algorithms with p values of 0.05 for entry and 0.1 for removal variables from the model) included age (dichotomous), educational level, visual impairment, the four most frequent admission diagnoses, use of indwelling bladder catheter, mean daily number of medications, and mean daily number of internal medicine diagnoses in the whole sample (cases and comparison subjects).
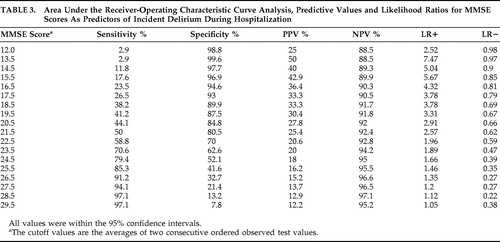
Sensitivity and specificity of MMSE scores for prediction of incident delirium were assessed using receiver-operating characteristic curve analysis. Positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio and negative likelihood ratio are reported.
Logistic regression was also applied to the group with admission cognitive impairment to analyze the way the MMSE score, as a continuous variable, was associated with the odds of having delirium as the dependent (dichotomous) variable. Literature recommendations were taken into account for logistic regressions, including evaluation of the goodness of fit with the logistic model (Hosmer and Lemeshow test for goodness of fit), Wald test for analysis of the statistical significance of each variable coefficient (β) in the model, and agreement of the risk with linearity 19 , 20 (i.e., verify whether worsening of MMSE scores increases the risk for delirium in a linear manner). As recommended by Hosmer and Lemeshow, 21 the MMSE scores of persons with cognitive impairment were divided into three approximately equal categories, and three logistic models were run in order to obtain model coefficients. Then those coefficients were graphed versus the mathematical midpoint of the corresponding ranks to evaluate the agreement of the risk with the linear gradient.
Linear regression was used in order to evaluate the relationship between global cognitive status at admission with MMSE as a continuous variable and delirium severity on the first day of delirium measured with the DRS-R98 in those with delirium.
RESULTS
Delirium and Nondelirium Group Characteristics
All patients who were CAM-S positive for delirium also met the delirium cutoff using the DRS-R98. Table 1 describes sociodemographic variables for 34 incident delirium cases (11.7%) and 257 nondelirious comparison subjects (88.3%). The mean age of the entire sample of 291 patients was 74.4 years old (SD=8.79, range=60–99 years). There was a significant difference of 4.47 years between delirium and nondelirium groups but no difference on any other sociodemographic variable.
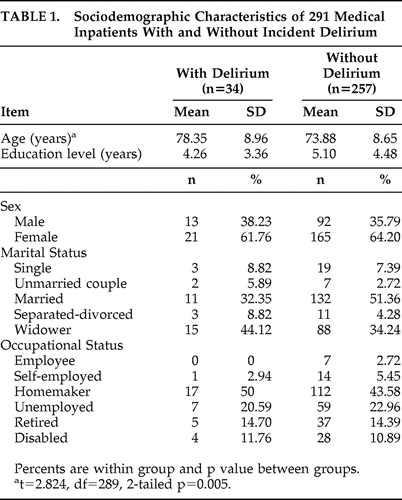 |
The four most frequent admission medical diagnoses were: pneumonia in 66 case (22.7%), urinary infection in 51 cases (17.5%), acute renal failure in 37 cases (12.7%), and complications related to cancer in 21 cases (7.2%). The remaining 116 patients (39.9%) had a variety of other admission diagnoses. At admission, eight patients in the delirium group (23.5%) had pneumonia, versus 58 patients in the comparison group (22.6%); 10 in the delirium group (29.4%) had urinary tract infection, versus 41 in the comparison group (15.9%); six in the delirium group (17.6%) had acute renal failure, versus 31 in the comparison group (12.1%); two in the delirium group (5.9%) had complications related to cancer, versus 19 in the comparison group (7.4%); and 23 in the delirium group (67.6%) had visual impairment, versus 146 in the comparison group (56.8%). Only the presence of urinary tract infection distinguished the groups (χ 2 =3.763, df=1, p=0.05).
The overall group’s mean MMSE score at admission was 23.86 (SD=4.33). In the nondelirium group, mean MMSE score was 24.23 (SD=4.01), which was significantly higher than the delirium group at 20.65 (SD=4.65) (t=4.276, df=289, two-tailed p<0.001). Box plots ( Figure 1 ) reveal overlapping MMSE score distributions between delirium and nondelirium groups, though median scores were significantly higher in the nondelirium group (χ 2 for median test=11.969, df=1, p=0.001). MMSE items compared between groups revealed significant differences for orientation, verbal recall, visuoconstructional ability, writing, and comprehension but no difference for attention ( Table 2 ). Eight of 15 in the delirium group (53.3%) who did not meet the calculated cognitive impairment criteria had MMSE scores between 21 and 24, versus 53 of 194 (27.3%) in the nondelirium group.
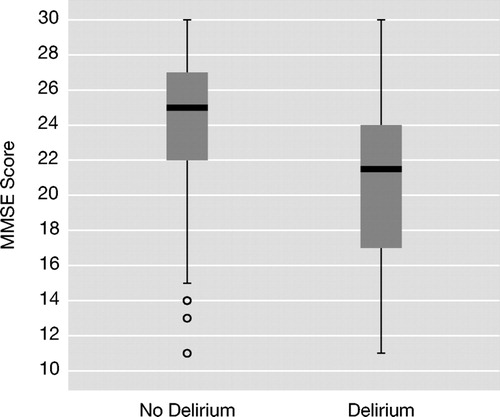
Median scores are denoted by the horizontal lines within the 50th percentile boxes; tails denote the 25th percentiles and circles are outliers. Medians were significantly different between groups (p=0.001).
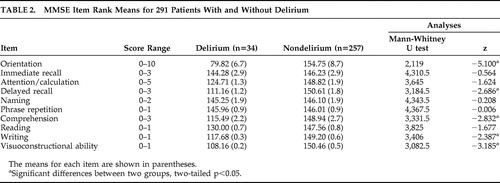 |
The median number of days from the first evaluation to the development of delirium was 3, with a range from 2 to 18 days and mean rank of 91.6, while the median number of days from the first evaluation to discharge in comparison subjects was 5, with a range from 2 to 29 days and mean rank of 153.2 (Mann-Whitney U for the mean ranks differences=2521.0, two-tailed p<0.001). Thirty-one patients in the delirious group (91.2%) had incident delirium by the end of day 7, while 194 comparison subjects (75.5%) had been discharged by the end of that day.
The mean daily number of medical diagnoses by day 7 of hospitalization in the delirium group was 5.0 (SD=1.5), which was not significantly different from that using the same ceiling for follow-up in the comparison group, 4.5 (SD=1.8) (t=1.596, df=289, 2-tailed p=0.112). The mean daily number of medications by day 7 of follow-up in the delirium group was 8.3 (SD=2.3), which was not different from that using the same ceiling for follow-up in the comparison group, 8.1 (SD=2.6; t=0.345, df=189, 2-tailed p=0.73). Five patients from the delirium group (14.7%) and 23 from the comparison group (8.9%) had required an indwelling bladder catheter by day 7 (χ 2 =1.144, df=1, p=0.285). In addition, a post hoc analysis using day 3 (median for delirium development) and day 5 (median for comparison subjects discharge) as the ceiling for follow-up revealed no differences between cases and comparison subjects for these variables (data not shown).
Using the dichotomous cognitive impairment cutoff score, 82 patients (28.2%) had cognitive impairment at admission. Mean MMSE score was significantly worse in the group with cognitive impairment at 18.71 (SD=3.40) with a range of 11 to 24, versus 25.81 (SD=2.5) in the 209 patients without deficits (71.8%).
Prediction of Delirium in the Whole Study Group
There was a significantly higher frequency and almost four times the odds of having cognitive impairment at admission in the delirium group as compared to the nondelirium group (χ 2 =14.6, df=1, p<0.001, OR=3.901, 95% CI=1.872–8.128). Besides cognitive impairment, age was a modest risk factor for delirium (logistic regression) (χ 2 =5.706, df=8, p=0.680 for Hosmer and Lemeshow test for goodness of fit; Wald test=7.49, df=1, p=0.006, OR=1.059, 95% CI=1.017–1.104), but it was not a linear effect. In order to control for effects of age we dichotomized age using the whole sample median (74) as a cutoff, and though its odds ratio increased to 2.425 (Wald test=5.229, df=1, p<0.022, 95% CI=1.135–5.181), its effect was not significant when combined in a logistic model with cognitive impairment. Cognitive impairment remained a significant risk factor in that combined model (χ 2 =0.396, df=2, and p=0.82 for Hosmer and Lemeshow test for goodness of fit; Wald test=11.241, df=1, p=0.001, OR=3.565, 95% CI=1.696–7.495).
Using multivariate analysis, age (dichotomized), educational level, visual impairment, each of the four most frequent admission diagnoses, use of an indwelling bladder catheter, mean daily number of internal medicine diagnoses and mean daily number of medications were excluded in the stepwise logistic model but cognitive impairment remained as a unique risk factor for delirium (data not shown).
The area under the curve from receiver-operating characteristic curve analysis of MMSE scores as a predictor of incident delirium in the whole group was 0.723 (SE=0.47, 95% CI=0.632–0.814). Table 3 displays sensitivity, specificity, predictive values, and likelihood ratios. The cutoff of 24.5 correctly classified 79.4% (95% CI=63.2%–89.7%) of patients who developed delirium during hospitalization. A post hoc analysis of the area under the curve in those older than 74 showed a small reduction in accuracy (area under the curve=0.682, SE=0.064) but sensitivity, specificity, predictive values and likelihood ratios were similar for those reported for the whole sample (data available from the corresponding author).
 |
Prediction of Delirium in Those With Cognitive Impairment
In the 82 subjects with cognitive impairment at admission, the logistic model using MMSE score as the independent variable and delirium as the dichotomous outcome found a linear relationship where lower MMSE scores were associated with higher delirium risk (χ 2 =6.107, df=8, p=0.635 for Hosmer and Lemeshow test for goodness of fit; Wald test=4.151, df=1, p=0.042, OR=1.164, 95% CI=1.006–1.347). Linear gradient concordance for the risk (odds ratio) is shown in Figure 2 . For every point the MMSE decreases in the cognitive impairment group, the odds for becoming delirious increases by 0.164. Age (dichotomous), educational level, visual impairment, each of the four most frequent admission diagnoses, use of an indwelling bladder catheter, mean daily number of internal medicine diagnoses and mean daily number of medications were excluded in stepwise analysis and were not related with the risk of developing delirium in these subjects (data not shown).
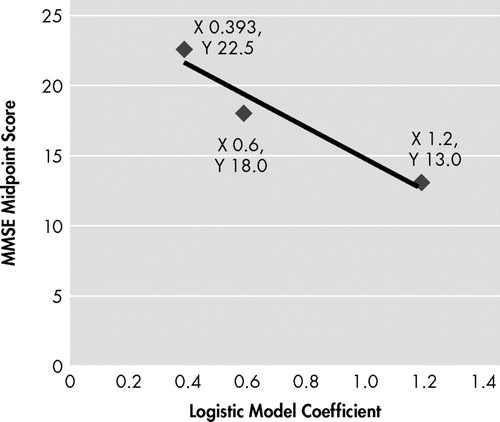
Linear gradient between the mathematical midpoints of three MMSE score categories (ranges) applied to 82 persons with cognitive impairment and the constant for each one of the 3 corresponding logistic models.
Prediction of DRS-R98 Scores in Those With Delirium
In the 34 patients with delirium, linear regression using the DRS-R98 score as the dependent variable and MMSE score as the independent variable showed a linear relationship where 12% of the variance of the DRS-R98 score (corrected R 2 ) was explained by the admission MMSE score (SE=4.9). Further, the correlation between severity of cognitive impairment and delirium severity was significant (F=5.39, df=1, p=0.027), where for each unit that the MMSE worsened, the DRS-R98 score increased by 0.4. Both variables exhibited a normal distribution in the delirium subjects (Kolmogorov-Smirnov test: Z=0.605, two-tailed p=0.858; and Z=0.687, two-tailed p=0.732, for DRS-R98 and MMSE, respectively). The other continuous variables age (years), educational level, mean daily number of internal medicine diagnoses, and mean daily number of medications, did not correlate with the DRS-R98 score in delirious subjects. Further, mean DRS-R98 score was independent from visual impairment, each of the four most frequent admission diagnoses, and use of an indwelling bladder catheter (data not shown).
DISCUSSION
We report a prospective longitudinal study of the relationship between admission cognitive impairment and incident delirium in a cohort of medically ill geriatric inpatients. Patients were evaluated daily for delirium onset, and delirium severity was assessed using widely used, validated tools. We found that cognitive impairment on admission as measured by the MMSE was linearly associated with both an increased risk of incident delirium and increased delirium severity as measured on the DRS-R98. Further, in delirious patients, 12% of the variance on the DRS-R98 was explained by this admission level of cognitive impairment. Using CAM-S for detection and the DRS-R98 for diagnosis validation our finding of an 11.7% delirium incidence was essentially the same as that found by Villalpando-Berumen et al. 22 over a 6-month period (12%) in a medical ward in Mexico.
Cognitive deficits constitute an important risk factor for delirium and when comorbid with delirium may result in a worse postdelirium follow-up prognosis. Fick et al., 8 in a systematic review, found that delirium in persons with dementia (defined in diverse ways), either as a unique variable or in multivariate models, is associated with more hospital readmissions and higher rates of institutionalization and mortality. They reported a prevalence of delirium in dementia patients to be between 22% and 89%.
Our study analyzed the relationship between delirium and global cognitive status in multiple ways and consistently found a significant relationship between cognitive deficits and an increased risk for an incident episode of delirium during the hospitalization. That risk was independent of the effects of age. Further, we found that in patients with cognitive impairment, the odds for delirium increased by 0.164 for each point of decline on the MMSE, which means that a person with an MMSE score of 10 will have almost 2.5 times more risk of developing delirium than a person with an MMSE score of 25. This relationship between cognitive deficit severity and delirium risk may imply that those with more cognitive impairment are predisposed to develop delirium with relatively mild medical illness stressors, as suggested by Inouye and Charpentier’s model for vulnerability and precipitating factors. 23 However, we found no difference between groups regarding other risk factors or etiologies for explaining delirium risk including age, education, medications, medical diagnoses, use of an indwelling catheter, or visual impairment during the hospitalization; rather, only admission MMSE scores predicted delirium risk. This suggests that impairment of the brain itself, through functional, microstructural and/or structural dysfunction is sufficient to predispose to incident delirium in the elderly.
Receiver-operating characteristic curve analysis found an MMSE cutoff score of 24.5 as the optimal predictor for delirium with (approximately) a sensitivity of 80%, specificity of 52%, positive predictive ability of 18% and negative predictive ability of 95%, which indicates that patients whose MMSE score is equal to or above the cutoff will probably not develop delirium. Based on associated likelihood ratios, patients who develop delirium have a 1.66 times increased probability of having an MMSE score equal to or below the MMSE cutoff score of 24.5 as compared with those who do not develop delirium. The positive likelihood ratio gradually increases with the MMSE cutoff score reduction, and those who develop delirium have a 7.47 times increased probability of having an MMSE score equal to or below the cutoff of 13.5.
Our findings with the bedside global cognitive test are consistent with findings of increased delirium risk reported by others using more focused neuropsychological testing. Lowery et al. 24 reported that nondemented elderly had a 4–5 times increased risk of delirium if their reaction time or fluctuation of attention was greater than one standard deviation above the mean. Using neuropsychological testing, Katz et al. 25 and Rudolph et al. 26 found that executive function impairment predicted delirium. Impairment of executive function was associated with a 2.8 times increased risk of delirium, after adjustment for age, education, sex, and medical morbidity. 26 It is quite possible that some of our nondemented elderly patients who developed delirium had mild cognitive impairment which includes executive dysfunction. 27
We may have missed cognitive impairment at the extremes. The MMSE is a screening test for global cognitive impairment with ceiling effects, so subtler deficits may have been missed, especially when applying the Colombian MMSE score adjustments. 16 We excluded prevalent delirium and did not have cases with MMSE scores below 11. Because we found that severity of cognitive impairment predicted delirium severity, this might also apply to more severely impaired patients even though we did not assess them.
We did not find that visual impairment was a risk factor for delirium, whereas Inouye and Charpentier’s predictive model for geriatric delirium based on admission characteristics included MMSE score <24 and visual impairment. 5 We did not measure medical status severity at admission, though there were no differences between groups for mean number of diagnoses or medications during the hospitalization. Nonetheless, this is a limitation for interpretation of our data because medical severity may in part explain the delirium risk and severity. In Inouye and Charpentier’s model, patients with a history of severe dementia were excluded, while we included any patient who was not delirious with moderate to severe dementia, as suggested by an MMSE score of 11. Our findings regarding cognitive impairment as the sole predictor are quite consistent with those of Edlund et al., 28 who prospectively studied delirium superimposed on dementia in older inpatients admitted with femoral neck fracture and found that cognitive impairment (dementia), but not age or other medical variables, was the only predictive factor (OR=3.53) for delirium in a multivariate logistical analysis.
MMSE scores on admission may reasonably reflect a true baseline in our patients. We ruled out delirium at admission and the MMSE attention/calculation item was not different between those who did and did not develop delirium. Because inattention is the cardinal symptom of delirium, this supports that admission MMSE impairments were related to other preexisting causes including dementia or mild cognitive impairment and not prevalent delirium. In a longitudinal study of the trajectory of cognitive function in older persons living in nursing homes or high-rise congregate apartment complexes, Katz et al. 25 found that MMSE scores between prehospitalization baseline and day of hospitalization were not statistically different.
Delirium neuropathophysiology involves a relative reduction in cholinergic function. A possible explanation for the linear increment in delirium risk and severity in older persons with cognitive deficits due to a variety of causes including aging, vascular lesions or degenerative brain processes where cholinergic neurotransmission hypofunction may underlie reduced cognitive reserve as a delirium risk factor. 29 , 30
In summary, we confirmed the prime importance of cognitive impairment as a risk factor for incident delirium in hospitalized elderly. Additionally, we established a linear relationship between admission cognitive impairment and delirium severity using the DRS-R98 as a sensitive and specific measure. We neither limited nor excluded dementia as some other studies have done. Our data suggest that older patients should be screened for cognitive impairment upon admission to the hospital and those with cognitive impairment, even if they are not yet delirious, should be recognized for their increased risk for delirium during the hospitalization. The MMSE is a widely used tool that appears adequate to assess this risk. This assessment could be coupled with extra nursing attention for detecting subsyndromal symptoms of delirium, including sleep-wake cycle disruption, attentional deficits, motor activity changes, thought process impairments, and other cognitive deficits. 30
1. Franco JG, Trzepacz PT, Mejia M, et al: Factor analysis of the Colombian translation of the Delirium Rating Scale (DRS), Revised-98. Psychosomatics 2009; 50:255–262Google Scholar
2. Bucht G, Gustafson Y, Sandberg O: Epidemiology of delirium. Dement Geriatr Cogn Disord 1999; 10:315–318Google Scholar
3. Curyto KJ, Johnson J, TenHave T, et al: Survival of hospitalized elderly patients with delirium: a prospective study. Am J Geriatr Psychiatry 2001; 9:141–147Google Scholar
4. Trzepacz PT, Teague GB, Lipowski ZJ: Delirium and other organic mental disorders in a general hospital. Gen Hosp Psychiatry 1985; 7:101–106Google Scholar
5. Inouye SK, Viscoli CM, Horwitz RI, et al: A predictive model for delirium in hospitalized elderly medical patients based on admission characteristics. Ann Intern Med 1993; 119:474–481Google Scholar
6. Folstein MF, Folstein SE, McHugh PR: “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189–198Google Scholar
7. Galanakis P, Bickel H, Gradinger R, et al: Acute confusional state in the elderly following hip surgery: incidence, risk factors, and complications. Int J Geriatr Psychiatry 2001; 16:349–355Google Scholar
8. Fick DM, Agostini JV, Inouye SK: Delirium superimposed on dementia: a systematic review. J Am Geriatr Soc 2002; 50:1723–1732Google Scholar
9. Liptzin B, Levkoff SE, Gottlieb GL, et al: Delirium: background papers for DSM-IV. J Neuropsychiatry Clin Neurosci 1993; 5:154–160Google Scholar
10. Trzepacz PT, Mulsant BH, Dew MA, et al: Is delirium different when it occurs in dementia? A study using the Delirium Rating Scale. J Neuropsychiatry Clin Neurosci 1998; 10:199–204Google Scholar
11. Laurila JV, Pitkala KH, Strandberg TE, et al: Delirium among patients with and without dementia: does the diagnosis according to the DSM-IV differ from the previous classifications? Int J Geriatr Psychiatry 2004; 19:271–277Google Scholar
12. Voyer P, Cole MG, McCusker J, et al: Prevalence and symptoms of delirium superimposed on dementia. Clin Nurs Res 2006; 15:46–66Google Scholar
13. Edlund A, Lundström M, Sandberg O, et al: Symptom profile of delirium in older people with and without dementia. J Geriatr Psychiatry Neurol 2007; 20:166–171Google Scholar
14. Inouye SK, van Dyck CH, Alessi CA, et al: Clarifying confusion: the confusion assessment method: a new method for detection of delirium. Ann Intern Med 1990; 113:941–948Google Scholar
15. Gonzalez M, de Pablo J, Fuente E, et al: Instrument for detection of delirium in general hospitals: adaptation of the confusion assessment method. Psychosomatics 2004; 45:426–431Google Scholar
16. Rosselli D, Ardila A, Pradilla G, et al: The Mini-Mental State Examination as a selected diagnostic test for dementia: a Colombian population study. Rev Neurol 2000; 30:428–432Google Scholar
17. Franco JG, Mejía MA, Ochoa SB, et al: Delirium Rating Scale—Revised-98 (DRS-R-98): Colombian adaptation of the Spanish version. Actas Esp Psiquiatr 2007; 35:170–175Google Scholar
18. Trzepacz PT, Mittal D, Torres R, et al: Validation of the Delirium Rating Scale—Revised-98: comparison with the delirium rating scale and the cognitive test for delirium. J Neuropsychiatry Clin Neurosci 2001; 13:229–242Google Scholar
19. Bagley SC, White H, Golomb BA: Logistic regression in the medical literature: standards for use and reporting, with particular attention to one medical domain. J Clin Epidemiol 2001; 54:979–985Google Scholar
20. Franco JG, Gaviria AM, Torres Y, et al: Regresión logística en la literatura psiquiátrica: evaluación de los artículos publicados entre 2002 y 2005 en una prominente revista. Rev Bras Epidemiol 2007; 10:370–379Google Scholar
21. Hosmer DW, Lemeshow S: Model building strategies and methods for logistic regression, in Applied Logistic Regression. New York, John Wiley & Sons-Interscience, 1989, pp 82–134Google Scholar
22. Villalpando-Berumen JM, Pineda-Colorado AM, Palacios P, et al: Incidence of delirium, risk factors, and long-term survival of elderly patients hospitalized in a medical specialty teaching hospital in Mexico City. Int Psychogeriatr 2003; 15:325–336Google Scholar
23. Inouye SK, Charpentier PA: Precipitating factors for delirium in hospitalized persons: predictive model and interrelationship with baseline vulnerability. JAMA 1996; 275:852–857Google Scholar
24. Lowery DP, Wesnes K, Ballard CG: Subtle attentional deficits in the absence of dementia are associated with an increased risk of post-operative delirium. Dement Geriatr Cogn Disord 2007; 23:390–394Google Scholar
25. Katz IR, Curyto KJ, TenHave T, et al: Validating the diagnosis of delirium and evaluating its association with deterioration over a one-year period. Am J Geriatr Psychiatry 2001; 9:148–159Google Scholar
26. Rudolph JL, Jones RN, Grande LJ, et al: Impaired executive function is associated with delirium after coronary artery bypass graft surgery. J Am Geriatr Soc 2006; 54:937–941Google Scholar
27. Tschanz JT, Welsh-Bohmer KA, Lyketsos CG, et al: Conversion to dementia from mild cognitive disorder: the Cache County Study. Neurology 2006; 67:229–234Google Scholar
28. Edlund A, Lundström M, Brännström B, et al: Delirium before and after operation for femoral neck fracture. J Am Geriatr Soc 2001; 49:1335–1340Google Scholar
29. Trzepacz PT: Is there a final common neural pathway in delirium? Focus on acetylcholine and dopamine. Semin Clin Neuropsychiatry 2000; 5:132–148Google Scholar
30. Trzepacz PT, Meagher DJ: Neuropsychiatric aspects of delirium, in American Psychiatric Publishing’s Textbook of Neuropsychiatry, 5th ed. Edited by Yudofsky S, Hales RE. Washington, DC, American Psychiatric Publishing, 2008, pp 445–517Google Scholar