Efficiency of Venlafaxine in Patients With Psychogenic Nonepileptic Seizures and Anxiety and/or Depressive Disorders
To date, little evidence is available on the most appropriate treatment for these patients. A review of published material from 1966 to 2002 found no randomized controlled trials for the treatment of nonepileptic seizures. 4 In a systematic review two years later, the same team 5 found only one study with a control group, based on confrontation and support psychotherapy, 6 although it was retrospective and used phone call follow-up. This team also found a noncontrolled and nonblind prospective study of 16 patients treated with different types of psychotherapy. 7 A systematic review on nondrug treatments for psychogenic nonepileptic seizures found only three randomized clinical trials: two with hypnosis and one with paradoxical intention; however, the study concludes that, due to poor methodology, no evidence is available to support any specific intervention in patients with psychogenic nonepileptic seizures. 8
Although lacking empirical evidence, it has been suggested that antidepressant, antianxiety, and antipsychotic therapies can treat symptomatic comorbid disorders and indirectly improve nonepileptic seizures. 4 However, in the sole comparative study between benzodiazepines and paradoxical intention therapy, the latter was more effective and resulted in a significant decrease in Hamilton Anxiety Rating Scale (HAM-A) score compared with daily doses of 5–15 mg of diazepam. 9
As for drug treatments, it has been reported that the improvement of anxious and depressive symptoms is often paralleled by the improvement of unexplained medical symptoms, 10 and selective serotonin reuptake inhibitors (SSRIs) have a positive effect on the core symptoms of posttraumatic stress disorder (PTSD), which is often associated with pseudoseizures. 11 Therefore, it has been suggested that treating anxious and depressive comorbid states with SSRIs or their serotoninergic compounds may improve the prognosis of patients with pseudoseizures, 12 , 13 although no information is available on their efficiency or mechanism of action.
The first randomized controlled trial to evaluate the efficacy of SSRI treatment versus cognitive behavior therapy (CBT) versus “treatment as usual” is currently being designed; “treatment as usual” has been defined in a previous study. 14
The number of baseline seizures during 2 weeks has been found to be a prognostic factor for the response to psychopharmacological treatment; in those patients with 10 or more seizures, no response or even worsening has been observed during treatment, compared with those with fewer than 10 seizures during the same period. 15
According to our hypothesis, anxious and/or depressive symptoms present in these patients are associated with the dysfunction of the neurotransmitters serotonin (5HT) and norepinephrine. Given that venlafaxine is a selective 5HT and norepinephrine reuptake inhibitor, 16 it might be useful in the treatment of psychogenic nonepileptic seizures. Our aim is to evaluate the improvement of anxious-depressive symptoms in patients with psychogenic nonepileptic seizures treated with venlafaxine and whether or not this improvement results in a decreased number of episodes.
METHODS
We conducted an open-label prospective study in patients with psychogenic nonepileptic seizures and anxious-depressive symptoms treated with venlafaxine for 5 months.
Patients
Nineteen patients between the ages of 18 and 65 who were diagnosed with psychogenic nonepileptic seizures through continuous video-EEG monitoring over a 1-week period (vEEG/LTM) were included in the study, which took place between March 2005 and July 2007. The inclusion criteria included fulfilling DSM-IV criteria for conversion disorder with seizures or convulsions and major depression, dysthymia, other unipolar mood disorder, and/or generalized anxiety disorder or panic disorder 17 ; an IQ score of at least 80 obtained through neuropsychological assessment during the stay at the Epilepsy Unit; and reading skills to understand the content of the informed consent. All patients gave their written informed consent, and the study protocol was approved by the institutional review board of our hospital. The exclusion criteria included the presence of epilepsy diagnosed with vEEG/LTM or other severe medical illness, schizophrenia, bipolar mood disorder, schizoaffective disorder, or severe obsessive-compulsive disorder; having been treated with psychoactive drugs over the previous month; pending litigation or disability application; or being pregnant.
Materials
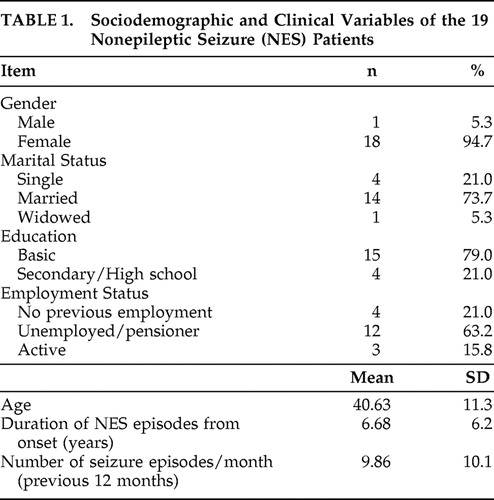
Epidemiologic and clinical data of the patients were recorded in a form designed for this purpose ( Table 1 ). Psychiatric diagnoses were established using the Structured Clinical Interview for DSM-IV (SCID-I). 18 The severity of depressive and anxious symptoms was evaluated with the hetero-administered Hamilton Depression Rating Scale (HAM-D) 19 and Hamilton Anxiety Rating Scale (HAM-A), 20 respectively. The Spanish version of the self-administered Hospital Anxiety and Depression Scale (HADS) 21 was also used.
 |
The number of seizure episodes was based on a self-recording form designed for this purpose, which was filled out by the patient under the supervision of a family member.
Procedure
Patients were evaluated with vEEG/LTM at the Epilepsy Unit of the Department of Neurology of our hospital in order to rule out epilepsy. Diagnosis of nonepileptic seizures was disclosed according to the standardized protocol of Shen et al. 22 Subsequently, patients were referred to the Department of Psychiatry for follow-up and treatment. The screening visit took place within 30 to 60 days after discharge from the Epilepsy Unit. A 15-day washout period was included if the patient had received antidepressant treatment.
The psychiatrist responsible for the clinical assessment, inclusion, and treatment (LP) was blind to the results of scales and questionnaires which were administered by a team psychologist (EB).
At the first (screening) visit, we administered the SCID-IV and filled in the epidemiologic and clinical data form. We administered HAM-D, HAM-A, and HADS, and the patient and his or her family member filled in the self-recording form for seizure episodes during the previous 15 days.
Visit 2 was scheduled 15 days after inclusion. Following the clinical assessment, drug treatment with venlafaxine was prescribed at 75 mg/day. HAM-D, HAM-A, and HADS were also administered. We calculated baseline mean number of seizures by the addition of the retrospective information obtained at the screening visit (previous 15 days) and the number of episodes recorded during the 15-day period from the screening visit to visit 2. We calculated baseline scale scores as the mean of scores obtained at screening visit and visit number 2.
Monthly visits were held over 5 months. Doses of venlafaxine were adjusted in the range of 75 mg/day to 300 mg/day according to the clinical criterion of the psychiatrist (LP) and based on the clinical response of the patient.
In each case, the minimum efficient dose was established to improve anxious and/or depressive symptoms. At each follow-up visit, we administered HAM-D, HAM-A, and HADS, and we collected the self-recording form for the number of seizure episodes during the past month.
In case the patient did not attend the scheduled visit, he or she was reminded by phone of the date of the next visit, which prevented the definitive exclusion of various patients during the follow-up (see the Results section). In case no therapeutic response was obtained after having reached the maximum dose allowed in the study, or there was worsening of the symptoms or nontolerated side effects, the patient was excluded and other proper therapeutic options were offered.
Statistical Analysis
Descriptive statistics were used for relevant sociodemographic and clinical variables. Means obtained at each of the five follow-up visits were compared with baseline values (repeated measurements) with the Wilcoxon nonparametric test. Differences between the number of seizures during the 15-day period previous to the inclusion visit and the number of seizures during the 15-day period after the inclusion visit were analyzed through the Wilcoxon nonparametric mean comparison test for paired data.
The Mann-Whitney nonparametric test (nonrepeated measurement) was used to compare the means of sociodemographic or clinical variables, including the number of seizures during follow-up, of those patients with different number of baseline seizure episodes (cutoff point of either 10 or five baseline seizure episodes) during the period from the day after the inclusion visit until visit 2 (first day of treatment).
RESULTS
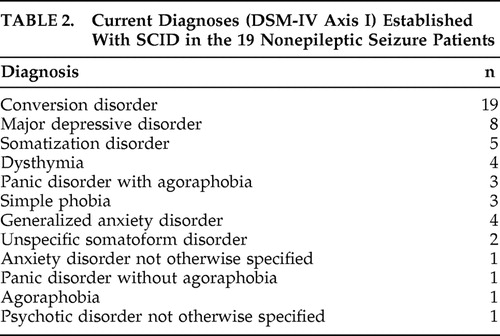
At the psychiatric assessment with SCID-IV, one patient (5.2%) had five different DSM-IV axis I diagnoses, two patients (10.5%) had four diagnoses, seven patients (36.8%) had three diagnoses, and nine (47.3%) had two diagnoses (i.e., conversion disorder with nonepileptic seizures and another anxiety or depressive disorder). The mean number of diagnoses per patient was 2.68. Table 2 shows the types and total number of diagnoses.
 |
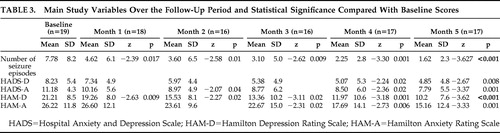
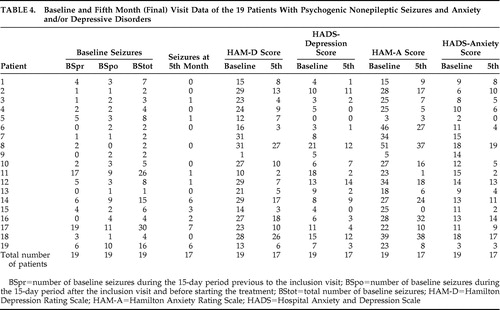
Mean values of the main variables (number of seizure episodes, HAM-D and HAM-A scores, and scores for the depression and anxiety subscales of HADS) used for the monthly clinical assessment of the patients over the 5-month follow-up are shown in Table 3 , which also shows statistically significant decreases in these main variables. The number of patients evaluated at each scheduled visit is also shown. Table 4 shows the results of the main variables obtained at baseline assessment and at month 5 in the 19 patients. Of the 19 patients, 13 attended all the control visits (baseline through month 5), 17 completed the follow-up, and six missed some of the visits. As shown in Table 3 , a total of 11 visits were not performed during the study (missed control visits). Patient 2 missed visits of months 3 and 4; after having been contacted twice by telephone, the patient came to the fifth visit and reported having had difficulties coming to previous control visits. However the patient reported a slight improvement. Patient 7 missed the final visit and, during a subsequent phone call, reported improvement and the decision not to come to the visit. Patient 8 reported having forgotten to come to the first two follow-up visits. Patient 9 was lost to follow-up after the month 1 visit and could not be located by telephone; the reason for the patient abandoning the study remains unknown. Patient 12 forgot to come to the month 2 visit. Patient 14 missed the month 3 visit due to nausea and dizziness and therefore stopped the treatment during several days but resumed it later.
 |
 |
The average doses of venlafaxine over the course of follow-up were: first month, 157.9 mg/day (SD=34.4); second month, 167.65 mg/day (SD=49.8); third month, 176.47 mg/day (SD=58.9); fourth month, 182.81 mg/day (SD=61.0); fifth month, 189.71 mg/day (SD=59.9).
No significant differences were observed in sociodemographic or clinical variables, including the number of seizures during follow-up (at a cutoff point of either 10 or five baseline seizure episodes) during the period from the day after the inclusion visit until the second visit (first day of treatment).
No significant differences were found between the number of seizures during the 15-day period previous to the inclusion visit and the number of seizures during the 15-day period after the inclusion visit (z=−0.288, p=0.77).
At the end of follow-up, the number of seizures decreased by over 50% in 15 patients (88.2%), HAM-D score decreased by over 50% in 11 patients (64.7%), and HAM-A score decreased by over 50% in seven patients (41.2%). Improvement for these three variables (HAM-D, HAM-A, and number of seizures) was considered as a ≥50% decrease between baseline and final scores. Of those two patients who did not decrease the number of seizures, one improved anxiety and depression scores, but the other improved neither anxiety nor depression scores. Of the 15 patients who decreased the number of seizures, five did not improve anxiety or depression scores, six improved both anxiety and depression scores, and four improved depression but not anxiety scores.
DISCUSSION
In this study, venlafaxine seemed to be useful in treating patients with psychogenic nonepileptic seizures and anxiety and/or depressive symptoms that fulfilled DSM-IV diagnostic criteria for anxiety and/or depressive disorders. Comorbid anxiety or depression disorders had already been found to be a positive prognostic factor for the outcome of conversion symptoms; 23 however, this has not been confirmed in a homogeneous group of chronic patients showing a specific conversion disorder such as psychogenic nonepileptic seizures and using a methodology focused on a diagnosis based upon clinical and physiological aspects as would be advisable from a neuropsychiatric perspective. 24
When anxiety and depression symptoms are measured with standardized and validated scales, we observe an evident improvement of these symptoms and a decreased number of nonepileptic seizure episodes.
The reliability of our study seems to be improved due to both the self-administered HADS and physician-administered HAM-D and HAM-A showing a decrease in anxiety and depression symptoms over the 5-month follow-up period. Nevertheless, the psychiatrist’s assessment reveals more severe symptoms compared with the perception of the patient. The difficulty in evaluating or the low level of awareness of symptoms has already been observed in social introversion as measured by the Minnesota Multiphasic Personality Inventory (MMPI) scale, which was within a normal range in patients with pseudoseizures and severely deteriorated interpersonal relationships. 25 Biological studies also attempted to identify specific neural correlates associated with hysterical conversion symptoms; these studies seem to point to a modulation of sensorimotor representations by primary affective or stress-related factors, perhaps involving primitive reflexive mechanisms of protection and alertness that are partly independent of conscious control. 26 The mechanism that relates the improvement of anxiety and/or depression symptoms and a decreased number of nonepileptic seizures is still not known; some authors who attempt to theoretically conceptualize nonepileptic seizures consider that depressive states are closely related to somatizations so that somatic components of depression are emphasized by the patient to the exclusion of affective and cognitive aspects. 27 Moreover, functional MRI in patients with conversion disorder revealed the activation of different brain areas in these patients relative to control subjects simulating similar neurological problems, namely left insula, left inferior frontal gyrus, putamen, and lingual gyri bilaterally, and the deactivation of right middle frontal and orbitofrontal cortices 28 —all of these areas being related to affective and anxiety disorders.
According to the prognostic indicators for nonepileptic seizure cessation reported by LaFrance et al., 4 the patients in our sample are severely ill and fulfill the following criteria: long duration of nonepileptic seizures (>2 years), extensive history of psychiatric illnesses, and age older than 18; children or adolescents have a better prognosis. The mean number of psychiatric diagnoses per patient is 2.68, which is in line with previous studies in nonepileptic seizure patients. 3
We did not observe significant differences in the change of the number of seizures in those patients with greater than 10 episodes in 15 days compared with those with fewer than 10 episodes during the same period; this contrasts with the findings of the pilot study of LaFrance et al. 15 This might be due to the fact that only two patients in our sample had ≥10 seizure episodes in 15 days, so we cannot state that the clinical characteristics revealed different illness severity between the samples of the two studies.
In our study, the Hawthorne effect (i.e., “the positive effect observed in patients from the attention received in a study” 29 ) was taken into account; therefore, baseline number of episodes was calculated as the sum of the episodes during the 15 days previous to inclusion and the 15 days after inclusion.
In contrast with LaFrance et al., 15 who started their study more than a year after diagnosis confirmation, the time span between diagnosis confirmation and start of treatment in our study was 1 or 2 months. However, this difference does not seem relevant as the mean duration of seizure episodes was 6 years in our patients (i.e., they can be considered as chronic nonepileptic seizure patients).
In line with the previously mentioned study, 15 we were cautious not to include young patients in our sample, as they usually show greater improvement, and to exclude patients showing both epilepsy and nonepileptic seizures simultaneously. We also withdrew all previous antiepileptic or antidepressant treatments at the beginning of the study, as they might have biased the findings. These treatments are used in higher doses and for longer periods in nonepileptic seizure patients compared with epilepsy patients 30 with no evident therapeutic benefits. We also prevented other possible bias suggested in previous studies 31 such as including heterogeneous cohorts or different conversion subtypes and mixing acute and chronic patients.
There are a wide range of treatments developed for nonepileptic seizure patients, and these treatments should be tailored to individual needs. 13 Treatments include anecdotal improvement with neuroleptics 32 and neurofeedback 33 and more widely confirmed improvement with psychoeducation, relaxation, coping skills training with a CBT approach, cognitive restructuring that attempts to transform somatic interpretations into verbal expression, psychodynamic interventions, and group therapy. Our work supports the idea that pharmacotherapeutic interventions may be useful in improving clinical symptoms in these patients. A previous study observed that 40% of conversion disorder patients experienced treatment failure with the initial SSRI antidepressant but responded to venlafaxine between 37.5 and 300 mg per day as needed. 31 Considering that nonepileptic seizures are the result of complex interactions between psychiatric disorders, psychosocial stressors, dysfunctional coping styles, and CNS vulnerability, 34 and these factors have been associated with altered 5-HT and norepinephrine neurotransmission, it seems proper to suggest that treatment with dual antidepressants may be useful in improving conversion disorders.
The findings of our study have some limitations such as lack of a comparison group, no double-blind design, not being a controlled trial treatment, small sample size, relatively short follow-up period with no information on long-term outcomes, lack of direct efficiency outcomes of venlafaxine on conversion symptoms in absence of affective or anxiety symptoms, and no standardized measurement of side effects of venlafaxine. The most important limitation is the absence of a placebo-controlled group, as a review of 75 clinical trials on placebo response in major depression 35 found an improvement rate of 29.7% for placebo response compared with an improvement rate of 50.1% for antidepressant treatment (improvement was defined as a >50% decrease of the HAM-D score). This finding indicates the need to control for placebo effect in drug trials.
Although our findings support the idea that pharmacotherapeutic treatments with dual antidepressants may be useful in improving clinical symptoms in patients with psychogenic nonepileptic seizures, the usefulness of such treatments should be confirmed by further double-blind comparative controlled efficiency studies.
1. Lesser RP: Psychogenic seizures. Neurology 1996; 46:1499–1507Google Scholar
2. Pintor L, Bailles E: Patients with psychogenic non-epileptic seizures: what happens to them? in Focus on Posttraumatic Stress Disorder Research. Edited by Corales TA. New York, Nova Science Publishers, 2005, pp 225–244Google Scholar
3. Bowman ES, Markand ON: Psychodynamics and psychiatric diagnoses of pseudoseizure subjects. Am J Psychiatry 1996; 153:57–63Google Scholar
4. LaFrance WC Jr, Devinsky O: Treatment of nonepileptic seizures. Epilepsy Behav 2002; 3(suppl 5):19–23Google Scholar
5. LaFrance WC Jr, Devinsky O: The treatment of nonepileptic seizures: historical perspectives and future directions. Epilepsia 2004; 45(suppl 2):15–21Google Scholar
6. Aboukasm A, Mahr G, Gahry BR, et al: Retrospective analysis of the effects of psychotherapeutic interventions on outcomes of psychogenic nonepileptic seizures. Epilepsia 1998; 39:470–473Google Scholar
7. McDade G, Brown SW: Non-epileptic seizures: management and predictive factors of outcome. Seizure 1992; 1:7–10Google Scholar
8. Brooks JL, Goodfellow L, Bodde NM: Nondrug treatments for psychogenic nonepileptic seizures: what’s the evidence? Epilepsy Behav 2007; 11:367–377Google Scholar
9. Ataoglu A, Ozcetin A, Icmeli C, et al: Paradoxical therapy in conversion reaction. J Korean Med Sci 2003; 18:581–584Google Scholar
10. O′Malley PG, Jackson JL, Santoro J, et al: Antidepressant therapy for unexplained symptoms and symptom syndromes. J Fam Pract 1999; 48:980–990Google Scholar
11. Stein DJ, Seedat S, van der Linden GJ, et al: Selective serotonin reuptake inhibitors in the treatment of post-traumatic stress disorder: a meta-analysis of randomized controlled trials. Int Clin Psychopharmacol 2000; 15:31–39Google Scholar
12. Reuber M, House A: Treating patients with psychogenic non-epileptic seizures. Curr Opin Neurol 2002; 15:207–211Google Scholar
13. LaFrance WC Jr, Barry J: Update on treatments of psychological nonepileptic seizures. Epilepsy Behav 2005; 7:364–374Google Scholar
14. LaFrance WC Jr, Rusch MD, Machan JT: What is “treatment as usual” for nonepileptic seizures? Epilepsy Behav 2008; 12:388–394Google Scholar
15. LaFrance WC Jr, Blum AS, Miller IW, et al: Methodological issues in conducting treatment trials for psychological nonepileptic seizures. J Neuropsychiatry Clin Neurosci 2007; 19:391–398Google Scholar
16. Rudolph RL, Entsuah R, Chitra R: A meta-analysis of the effects of venlafaxine on anxiety associated with depression. J Clin Psychopharmacol 1995; 18:136–144Google Scholar
17. American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Washington, DC, American Psychiatric Association, 1994Google Scholar
18. First MB, Spitzer RL, Gibbon M, et al: Structured Clinical Interview for DSM-IV Axis I Disorders—Clinician Version (SCID-CV). Washington, DC, American Psychiatric Press, 1997 (Versión Española, Masson SA, 1999)Google Scholar
19. Hamilton M: A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56–62Google Scholar
20. Hamilton M: The assessment of anxiety states by rating. Br J Med Psychol 1959; 32:50–55Google Scholar
21. Herrero MJ, Torres X, Blanch J, et al: A validation study of the Hospital Anxiety and Depression Scale (HADS) in a Spanish population. Gen Hosp Psychiatry 2003; 25:277–283Google Scholar
22. Shen W, Bowman E, Markand ON: Presenting the diagnosis of pseudoseizure. Neurology 1990; 40:756–759Google Scholar
23. Crimlisk HL, Bhatia K, Cope H, et al: Slater revisited: 6 year follow up study of patients with medically unexplained motor symptoms. BMJ 1998; 316:582–586Google Scholar
24. Krem MM: Motor conversion disorders reviewed from a neuropsychiatric perspective. J Clin Psychiatry 2004; 65:783–790Google Scholar
25. Pintor L, Pérez G, Torres X, et al: Trastornos psiquiátricos, personalidad y experiencias traumáticas en pacientes con crisis no epilépticas conversivas. Actas Españolas de Psiquiatría 2002; 30:233–239Google Scholar
26. Vuilleumier P: Hysterical conversion and brain function. Prog Brain Res 2005; 150:309–329Google Scholar
27. Katon W: Depression: somatic symptoms and medical disorders in primary care. Compr Psychiatry 1982; 23:274–287Google Scholar
28. Stone J, Zeman A, Simonotto E, et al: fMRI in patients with motor conversion symptoms and controls with simulated weakness. Psychosom Med 2007; 69:961–969Google Scholar
29. West ED, Jackson A, Physentides A, et al: Randomized comparative trial of a ward discussion group. Br J Psychiatry 1982; 141:76–80Google Scholar
30. Hantke NC, Doherty MJ, Haltiner AM: Medication use profiles in patients with psychogenic nonepileptic seizures. Epilepsy Behav 2007; 10:333–335Google Scholar
31. Voon V, Lang AE: Antidepressant treatment outcomes of psychogenic movement disorder. J Clin Psychiatry 2005; 66:1529–1534Google Scholar
32. Rampello L, Raffaele R, Nicoletti G, et al: Hysterical neurosis of the conversion type: therapeutic activity of neuroleptics with different hyperprolactinemic potency. Neuropsychobiology 1996; 33:186–188Google Scholar
33. Swingle PG: Neurofeedback treatment of pseudoseizure disorder. Biol Psychiatry 1998; 44:1196–1199Google Scholar
34. Mökleby K, Blomhoff S, Malt UF, et al: Psychiatric comorbidity and hostility in patients with psychogenic nonepileptic seizures compared with somatoform disorders and healthy controls. Epilepsia 2002; 43:193–198Google Scholar
35. Walsh BT, Seidman SN, Sysko R, et al: Placebo response in studies of major depression: variable, substantial, and growing. JAMA 2002; 287:1840–1847Google Scholar



