Psychopharmacological Neuroprotection in Neurodegenerative Diseases, Part III: Criteria-Based Assessment: A Report of the ANPA Committee on Research
Abstract
Neuroprotective therapies for neurodegenerative diseases (NDDs) have proven elusive. The established psychotropic agents commonly used to treat the neuropsychiatric manifestations of NDDs are potential neuroprotective therapies, and neuropsychiatrists and others may benefit from a knowledge of the neuroprotective properties of these medications. This report identifies FDA-approved, first-line psychotropic drugs affecting intracellular mechanisms and meriting disease-modifying clinical trials in NDDs. The authors evaluated evidence for neuroprotection according to 1) preclinical; and 2) clinical criteria. Despite low-to-moderate preclinical evidence scores and scant clinical evidence, the most promising investigative priorities are 1) lithium and paroxetine in Alzheimer's disease (AD); 2) lithium in tauopathies (frontotemporal lobar degeneration [FTLD], FTDP-17); 3) lithium-plus-valproate in AD and amyotrophic lateral sclerosis; 4) pramipexole and valproate in Parkinson's disease; 5) amantadine and buspirone in multiple system atrophy; and 6) antidepressants in Huntington's disease. Preliminary clinical results signal caution regarding olanzapine use in AD and poor tolerability of lithium in progressive supranuclear palsy and corticobasal degeneration. These preliminary findings can lead to further clinical drug trials on the use of these well-known medications, not only for their psychotropic effects, but also for neuroprotection in NDDs.
Neurodegenerative diseases (NDDs) are common and impose substantial morbidities and costs on patients, caregivers, and society.1 The two most common, Alzheimer's disease (AD) and Parkinson's disease (PD), affect more than 5 million people in the United States and are exponentially increasing with population demographic trends;2 and they result in considerable morbidities and costs.3 Neuropsychiatric disturbances are prominent among these morbidities and can contribute significantly to quality of life in NDDs.1 Clinicians prescribe psychotropic drugs to treat the neuropsychiatric disturbances in NDDs3 without regard to their potential neuroprotective effects. Psychotropics affect the intracellular mechanisms that are common to the pathobiology of a variety of NDDs (i.e., pathological proteins, proteasome, mitochondria, apoptosis), suggesting the possibility that psychotropics may function neuroprotectively across a number of these diseases.1,3 The potential to modify the course of a disease through these effects has substantial implications for both patients and society.1,4 The neuroprotective effects of established psychotropic medications are worth investigating, given the unsatisfying results from clinical trials of newer neuroprotective agents. Also, there is considerable advantage to re-purposing established psychotropic drugs because much is already known about their absorption, metabolism, excretion, toxicity, safety, blood–brain barrier penetration.
For these reasons, we previously reviewed the preclinical neuroprotective literature of psychotropic drugs pertaining to basic common neurodegenerative intracellular mechanisms.1 In that work, we identified particular drugs as especially promising in view of consistent findings supporting putative neuroprotective properties. Subsequently, we considered a wider array of neuroprotective actions of psychotropics by pharmacological class and pharmacodynamic mechanism.3 We further proposed provisional criteria1,3 (Table 1) to assess neuroprotective evidence with a view toward identifying candidate agents most likely to succeed in clinical neuroprotective trials using delayed-start or randomized-withdrawal designs.5
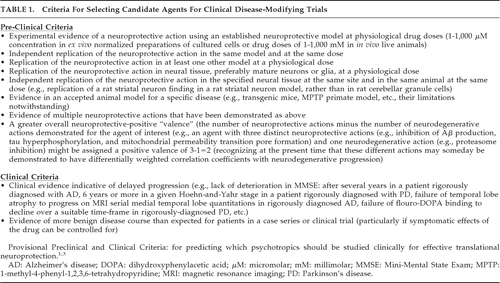 |
This report updates the literature of FDA-approved first-line psychotropics to June 10, 2010. Over the intervening 33 months, there has been an exponential increase in relevant publications. We evaluated these data according to the previously-published provisional criteria,1,3 shown in Table 1. This report focuses on common neurodegenerative intracellular effects in mature neural tissues. Neurons and glia may behave differently from non-neural cells, and immature and mature neurons can behave differently,6 and even, sometimes, oppositely7 with respect to apoptosis. For example, neurons and SK-N-SH neuroblastoma cells are differentially sensitive to antipsychotic-induced apoptosis.6 Since NDDs generally involve mature neurons and glia, we therefore confined the present literature review to these mature cells and excluded studies done in cell-lines related to neuroblastomas (e.g., SK-N-SH, Neuro-2a, N1E-115, etc.), pheochromocytomas (e.g., PC12, KNA, KAT45, etc.), other malignancies, and other immature cell-lines. For this reason, assessment by Table 1 preclinical criteria is limited to findings in mature neural tissues.
This report particularly emphasizes the extent of replication of these findings (Table 1: Preclinical Criteria 2–5). Also, we consider findings in disease-specific animal models (Table 1: Preclinical Criterion 6). In some cases (e.g., antidepressants), the disease-specific animal model literature also evaluates psychotropic effects on neuronal stem cells and glial progenitors in the mature brain (e.g., hippocampal neural stem cells in AD models, subventricular zone neurogenesis in Huntington's disease [HD] models), which are intrinsic to a drug's overall neuroprotective properties (via neuro-restoration), and so we selectively consider these findings in these models, provided they derive from or pertain to a disease-related brain structure of interest in mature adult animals. Multiple neuroprotective actions (Table 1: Preclinical Criterion 7) constituting neuroprotective valences (Table 1: Preclinical Criterion 8) are considered for each drug. Finally, this report reviews and assesses the clinical-trial literature (Table 1: Clinical Criteria).
Pending criteria predictive validity, the findings below represent an advance and refinement in selecting currently-prescribed psychotropics as candidates for disease-modifying clinical trials in NDDs.
METHOD
Preclinical findings in mature neural tissue and clinical findings in NDDs treated with psychotropics were evaluated by Table 1 criteria. Relevant studies were identified through a literature search (PubMed search terms, February 1, 2010: (alpha-synuclein OR beta-amyloid OR tau OR TDP-43 OR ubiquitin OR proteasome OR mitochondrial viability OR mitochondria OR mitochondrial transition pore OR cytochrome c release OR endosome OR lysosome OR autophagy OR endoplasmic reticulum OR leukocyte viability OR apoptosis) AND (pramipexole OR ropinirole OR amantadine OR haloperidol OR fluphenazine OR trifluoperazine OR thiothixene OR chlorpromazine OR thioridazine OR risperidone OR olanzapine OR quetiapine OR ziprasidone OR aripiprazole OR clozapine OR paliperidone OR iloperidone OR asenapine OR tetrabenazine OR pimavanserin OR lithium OR carbamazepine OR oxcarbazepine OR valproate OR amitriptyline OR imipramine OR nortriptyline OR desipramine OR clomipramine OR trimipramine OR doxepin OR protriptyline OR maprotiline OR bupropion OR fluoxetine OR sertraline OR fluvoxamine OR paroxetine OR citalopram OR s-citalopram OR trazodone OR nefazodone OR venlafaxine OR duloxetine OR mirtazapine OR buspirone OR diazepam OR chlordiazepoxide OR flurazepam OR temazepam OR chlorazepate OR clonazepam OR lorazepam OR oxazepam OR alprazolam OR zaleplon OR zolpidem OR zopiclone OR s-zopiclone OR cyproheptadine OR hydroxyzine OR benztropine OR trihexyphenidyl OR modafinil OR melatonin OR ramelteon) AND (neuron OR neuronal OR neurons OR glia OR glial OR neuroglia)).
Citations were reviewed to exclude findings in non-neural tissue and immature or malignancy-related cell lines. The review was limited to mature neural tissues (except in disease-specific animal models, where stem cells in mature brain were also considered) for reasons detailed above. We considered studies of any methodology but excluded models distinct from NDD (e.g., stroke, tardive dyskinesia, etc.). Cell culture conditions not typical of NDD (e.g., hyperosmotic stress, oxygen deprivation, etc.) were also excluded. The sole exception involved mature cerebellar granule cells (CGNs) that require hyperkalemic conditions for survival and reliably undergo apoptosis after withdrawal of serum or potassium.8
We focused on intracellular processes common across NDDs specified in the search terms (proteins, ubiquitin–proteasomal system, mitochondria, and apoptosis), and did not consider studies of intracellular calcium influx or other disease mechanisms unless those studies also considered the intracellular processes of interest. DNA fragmentation and condensation was required to ascertain apoptosis, whereas other indices (apoptotic mediator concentration, cell viability) were considered insufficient. The single exception to this involved CGN studies (as noted, CGNs are well documented to undergo apoptosis with serum/potassium deprivation). A single reviewer evaluated each abstract to determine whether it might potentially provide information on relevant intracellular processes after meeting the above methodological inclusions, even if not clearly stated, in which case the article itself was then reviewed.
In assessing Preclinical Criterion 6 (disease-specific animal models), outcomes consistent with putative neuroprotection were considered even if the study did not specifically address the intracellular targets required for cell and tissue studies, provided that outcomes were relevant to disease-specific clinical outcomes. In interpreting the results and applying the findings in specific NDDs, findings from mature neural tissues in a disease-specific animal model were considered to be relevant to only that disease, whereas data pertaining to neural stem-cells in mature animals were considered to be potentially applicable to any disease, regardless of model.
Two studies considered combined treatment of lithium with valproate. The results of this combined treatment were considered separately and not included in the neuroprotective scores for either drug unless findings pertain to either drug given by itself.
Clinical findings evaluated by Table 1 Clinical Criteria were identified through a literature search (PubMed search terms, June 10, 2010: (pramipexole OR ropinirole OR amantadine OR haloperidol OR fluphenazine OR trifluoperazine OR thiothixene OR chlorpromazine OR thioridazine OR risperidone OR olanzapine OR quetiapine OR ziprasidone OR aripiprazole OR clozapine OR paliperidone OR iloperidone OR asenapine OR tetrabenazine OR pimavanserin OR lithium OR carbamazepine OR oxcarbazepine OR valproate OR amitriptyline OR imipramine OR nortriptyline OR desipramine OR clomipramine OR trimipramine OR doxepin OR protriptyline OR maprotiline OR bupropion OR fluoxetine OR sertraline OR fluvoxamine OR paroxetine OR citalopram OR s-citalopram OR trazodone OR nefazodone OR venlafaxine OR duloxetine OR mirtazapine OR buspirone OR diazepam OR chlordiazepoxide OR flurazepam OR temazepam OR chlorazepate OR clonazepam OR lorazepam OR oxazepam OR alprazolam OR zaleplon OR zolpidem OR zopiclone OR s-zopiclone OR cyproheptadine OR hydroxyzine OR benztropine OR trihexyphenidyl OR modafinil OR ramelteon) AND (clinical trial OR randomized controlled trial) AND (neuroprotection OR neuroprotective OR disease-modifying OR disease modifying OR disease modification OR progression OR disease progression OR biomarker OR beta-amyloid OR tau protein OR alpha-synuclein OR huntingtin OR TAR DNA binding protein 43 OR TAR DNA-binding protein 43 OR TDP-43 OR TDP 43 cerebrospinal fluid OR imaging OR magnetic resonance imaging OR single photon emission computed tomography OR positron emission tomography) AND (neurodegenerative disease OR Alzheimer's disease OR Parkinson's disease OR Huntington's disease OR frontotemporal lobar degeneration OR FTLD OR FTD OR frontotemporal dementia OR ALS OR amyotrophic lateral sclerosis OR progressive supranuclear palsy OR corticobasal degeneration OR Pick's disease OR MSA OR multiple system atrophy)). Methods specific to the quantitative evaluation of Table 1 criteria are described in respective Results sections.
RESULTS
The preclinical search returned 623 citations. Review of abstracts resulted in 103 potentially relevant articles that, after review, yielded 96 studies with potentially contributory results. The findings are presented and summarized for each Table 1 preclinical criterion, quantitatively for the composite preclinical criteria, and by disease for the clinical criteria.
Pre-Clinical Criterion 1
The search was limited to neurons and glia. The review was limited to established preclinical models using physiological doses in mature neurons and glia.
Pre-Clinical Criteria 2–5
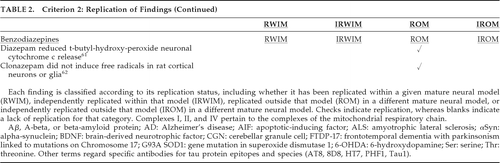
Replicated neuroprotection was classified as replication within a given model, independent replication within the model, replication in at least one other model, and independent replication in at least one other model. The degree to which replication was achieved for findings is indicated in Table 2 (unreplicated findings are not included in this table). As seen in Table 2, findings that have been the best established in terms of independent replications within and across different models include apoptosis of cortical neurons induced by haloperidol, reduced tau phosphorylation (Thr181, Tau1, AT8, Thr231) and apoptosis by lithium, and neuroprotective increases in alpha-synuclein (αSyn) by valproate. Table 3 displays the replication of findings across various antidepressants, irrespective of the specific antidepressant with which the finding was associated (Table 3 findings were not included in the quantitative analysis).
 |
 |
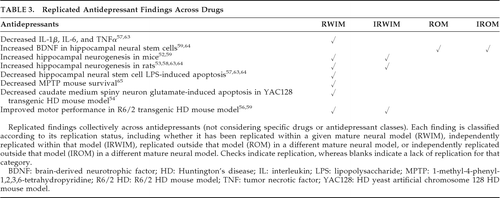 |
Within animal models, multiple benefits in the amyotrophic lateral sclerosis (ALS) G93A transgenic mouse (Table 2) of lithium, hippocampal neurogenesis in rodents with antidepressants (Table 3), and improved motor performance in the R6/2 HD transgenic mice with nortriptyline and sertraline (Table 3) have each been independently replicated. Across models, Complex I inhibition by haloperidol and other neuroleptics, decreased Aβ42, tau phosphorylation (Ser199, Ser202, Ser396, Ser404, PHF1), and cytochrome c release with lithium, apoptosis with carbamazepine, and decreased apoptosis with valproate have each been independently replicated (Table 2). Independently replicated findings for antidepressants across models include hippocampal neurogenesis with imipramine, decreased neuronal apoptosis with desipramine and nortriptyline, hippocampal neural stem-cell stimulation with fluoxetine (Table 2), and upregulated hippocampal neural stem-cell brain-derived neurotrophic factor (BDNF) in rats with imipramine and mice with sertraline (Table 3). Other findings have not been independently replicated.
Findings for Criteria 1–5 (Table 2 and Table 3) suggest the potential danger of haloperidol, especially in cortical dementias, and the potential benefit of lithium, especially in AD and tauopathies, and valproate in PD and synucleinopathies. Less well-replicated findings suggest the potential danger of haloperidol, neuroleptics, and carbamazepine in NDDs (especially PD with neuroleptics) and the potential benefit of lithium in ALS, AD, and tauopathies, valproate in various NDDs, and various antidepressants in AD, hippocampal sclerosis, and HD.
Pre-Clinical Criterion 6
Evidence in accepted disease-specific animal models is presented in Table 4. Findings for each drug in specific animal models are presented with corresponding neuroprotective action scores (NPASs). NPASs indicate the number of times neuroprotective (+), absence of a significant difference (±), and pro-degenerative (–) findings were obtained over the total number of experiments detailed in the papers assessing the property. For example, an NPAS of 13+/7±/4– indicates that of a total of 24 experiments detailed in articles addressing the property, 13 found neuroprotection, 7 found no effect (neither neuroprotective nor neurodegenerative), and 4 found a prodegenerative effect.
 |
 |
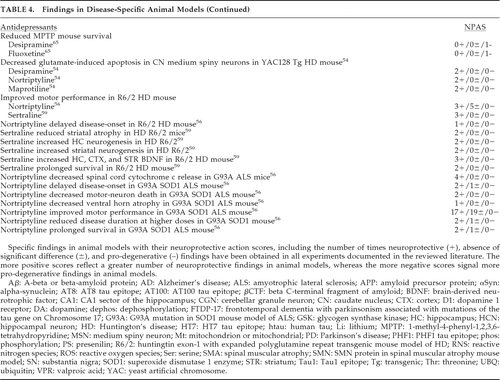 |
In Table 4, drugs showing benefit in AD animal models include quetiapine, lithium, valproate, and paroxetine. In tauopathic diseases, including FTDP-17, lithium has potential. In PD models, pramipexole, lithium, and valproate have had positive results. In HD, trifluoperazine, tetrabenazine, lithium, desipramine, nortriptyline, maprotiline, and sertraline are all promising. ALS models suggest low-dose clozapine, lithium, valproate, lithium-plus-valproate, and nortriptyline as possible treatments. For spinal muscular atrophy (SMA), valproate has had benefit. Deterioration occurred with desipramine and fluoxetine in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of PD, but this might be unique to the MPTP model. Deterioration in the ALS mouse was noted with standard doses of clozapine, whereas a lower dose, similar to the dose used in PD patients, actually extended survival.
Pre-Clinical Criterion 7
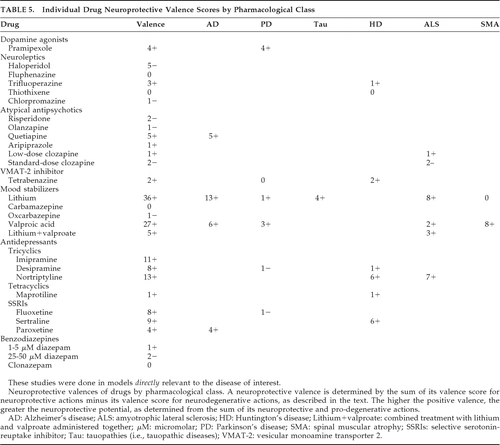
Evidence of multiple neuroprotective actions was derived from assessment of NPASs. NPASs were transformed into neuroprotective valences. The neuroprotective valence for a drug is the net sum of its neuroprotective and neurodegenerative pre-valence scores. If the sum of the three components (i.e., +, ±, and – scores) of an NPAS for an individual action was a positive value, the resulting pre-valence score for the action was 1+. Likewise, if the NPAS sum was a negative value, then the pre-valence score for that action was 1–, indicating an overall prodegenerative finding across studies for the action. To obtain the valence for the drug, the positive and negative pre-valence for the individual actions were summed. Valences for a drug >1+ indicate multiple neuroprotective actions. Higher valences indicate a greater number of documented neuroprotective actions. All findings, including those in animal models, are included in these scores. Drug valence scores correct for countervailing pro-degenerative actions by their subtraction from the total score. Consequently, the higher the positive valence score, the greater the number of neuroprotective actions outweighing any coexisting pro-degenerative actions that the drug might have. (Similarly, the greater the negative valence score, the more likely a drug is to promote or accelerate NDD.) Valences are presented in Table 5 and are also stated in terms of findings in specific disease models. (It is worth mentioning that although a single substantive clinically-proven pro-degenerative action would likely be sufficient to preclude further drug development, the clinical relative risks of preclinical pro-degenerative actions relative to preclinical neuroprotective actions are not yet clear. For example, it is not currently clear whether anti-mitochondrial effects will outweigh salutary pathogenic protein effects or direct antiapoptotic effects. Thus, the existence of preclinical deleterious actions should not necessarily exclude clinical consideration of a drug if noteworthy preclinical beneficial actions are apparent.)
 |
Pre-Clinical Criterion 8
Neuroprotective valence was determined as for Criterion 7, and, in Table 6, drugs are listed in rank order according to their valences. Although Criterion 8 seems redundant with the preceding one for the drugs at hand, it is actually distinct because it is possible to have a large number of neuroprotective actions yet still have a low or even negative valence because of an even greater number of pro-degenerative actions. Fortunately, the drugs considered here had minimal countervailing effects, so valences parallel NPAS and pre-valence scores.
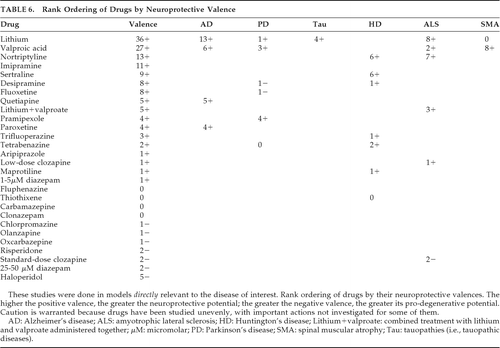 |
On the basis of these valences, the glycogen synthase kinase 3-β (GSK-3β)-inhibitor lithium is the most promising neuroprotective agent, followed by the GSK-3β and histone deacetylase (HDAC) inhibitor valproate, the tricyclic antidepressants nortriptyline and imipramine, and the atypical antipsychotic quetiapine. Less-robust agents are the antidepressants sertraline, desipramine, and fluoxetine. Drugs warranting caution in NDDs include oxcarbazepine, diazepam, and the antipsychotics, particularly haloperidol, clozapine, and olanzapine. Valences can covary with the intensity with which a drug has been studied, and some effective agents may have low scores due to minimal investigation. Furthermore, there is a difference in how various drugs have been studied. For example, investigations of neuroleptics have focused primarily upon their mitochondrial respiratory chain effects, fostering more negative valences. Also, whereas lithium, valproate, and quetiapine have exerted neuroprotective actions, antidepressant valences primarily reflect neuro-restorative effects on neural stem-cell proliferation in animal models because of the orientation of the investigations. Thus, caution is warranted in applying valence findings because drugs have been studied unevenly, with important domains not investigated for some of them.
Quantitative Evaluation Of Psychotropics According To The Provisional Pre-Clinical Criteria
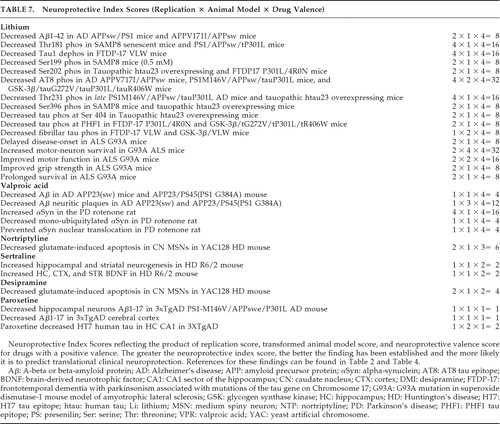
Drugs were quantitatively assigned a neuroprotective index score for the degree to which they met the Table 1 provisional preclinical neuroprotective criteria. To provide a measure of the quality and strength of the available preclinical evidence regarding a given drug's effect on neurodegenerative processes, we devised an equation to consider three factors represented in the provisional preclinical criteria, resulting in a neuroprotective index score for each drug. The neuroprotective index score is the multiplied product of three factors, specifically: 1) replication; 2) NPAS; and 3) valence scores, reflecting degree of replication, experimental weight, and multiplicity of actions, respectively. Thus, the neuroprotective index score can be affected by how often a finding has been replicated across various models, how consistently it has been observed in animal models, and the number of different beneficial actions relative to detrimental actions for the drug associated with the finding. Criterion 1 was not scored, since all drugs considered in this report met that criterion. Criteria 2–5, replication, was quantitated by crediting 1 point for each of the four categories of replication in Table 2, with scores ranging from 1 to 4. Criterion 6, animal-model findings, was quantitated by transforming Table 4 NPASs. NPAS scores of 1–5 were scored as 1; 6–10 as 2; 11–15 as 3; and >15 as 4. Criteria 7 and 8 were factored in together, using the valence score, transformed by applying the same cut-offs to the valence score as to the NPASs. The relative likelihood of the finding translating to clinical neuroprotection is theoretically predicted by its neuroprotective index score (Table 7). The neuroprotective index score was computed by multiplying the replication score for the finding by the transformed animal-model NPAS for the same finding, and multiplying this product by the transformed valence score. The most robust findings (neuroprotective index score ≥16) suggest the usefulness of lithium in AD, tauopathies, and ALS, and valproate in PD.
 |
Provisional Clinical Criteria
The clinical criteria search returned 193 citations, with 54 initially appearing to be of possible relevance. The data that can potentially address neuroprotection, however, were quite limited.
In AD, a randomized, placebo-controlled, 26-week trial of the atypical antipsychotic olanzapine 2.5–7.5 mg/day in non-psychotic and non-agitated patients with moderate AD demonstrated more dramatic cognitive deterioration on the ADAS–Cog at 12 and 26 weeks with active drug, especially in those with greater cognitive impairment at baseline.74 Although a retrospective study of bipolar disorder indicated reduced dementia incidence after 10 years of lithium treatment,75 a 10-week, randomized, single-blind, placebo-controlled trial of lithium (0.5–0.8 mM/liter) revealed no clinical benefit or change in total tau, phospho-tau, GSK, or Aβ,76 although a subgroup with increased serum BDNF showed ADAS–Cog improvement.77 Studies of sertraline in AD78 and citalopram in dementia79 were not designed to assess neuroprotection.
In PD, although no ropinirole studies met our preclinical criteria, a 5-year, multicenter, double-blind study of 288 patients with early PD randomized to either ropinirole or L-dopa found less dyskinesia (20% versus 45%) with ropinirole, but no difference in clinical markers of PD progression.80 In the REAL-PET ropinirole trial in 186 patients with PD randomized to ropinirole or L-dopa, fluorine-18-DOPA positron-emission tomography (PET) scans at 2 years revealed the rate of putamenal 18F-DOPA signal decline to be one-third slower in the ropinirole group.81,82 A smaller, double-blind study of 45 patients failed to show any difference at 2 years.83 It has been argued, however, that lack of a placebo in the REAL-PET study may indicate that PET differences instead reflect greater L-dopa toxicity84 and that the study was underpowered and excluded too many normal baseline scans,85 making the results inconclusive. In the CALM–PD study, a multicenter, double-blind, 24 month trial in 301 patients with PD randomized to either pramipexole-plus-placebo, L-dopa, or L-dopa-plus-placebo, pramipexole demonstrated fewer dopaminergic motor complications with pramipexole but greater UPDRS Parkinson Scale improvement with L-dopa,86 suggesting either a lack of dose-equivalence or intrinsic pharmacological differences between the treatments, rather than neuroprotection. Dopamine transporter imaging (β-CIT) in 82 subjects showed reduced decrement with pramipexole at 2 years;87 however, this may instead indicate greater L-dopa toxicity,84 or may not even reflect disease progression,84 but, rather, biased subject-selection88 or greater transporter down-regulation by pramipexole.89 Open-label extension studies of ropinirole90 and pramipexole91 are further confounded by lack of blinding and the addition of other medications. Thus, neuroprotective findings for dopamine agonists in PD remain inconclusive.
In multiple system atrophy (MSA), an open-label, 3-month trial of amantadine in olivopontocerebellar atrophy (OPCA; N=12) and Friedreich's ataxia (N=17) revealed improvements in movement-time in both groups,92 but the finding could be purely of symptomatic origin. A double-blind, placebo-controlled, crossover study of amantadine in MSA (N=8) was only 3 weeks in duration.93 Eighteen patients with OPCA were randomized to open-label buspirone 15 mg/day (N=9) or buspirone 15 mg/day-plus-estrogen 0.625 mg/day (N=9) for 12 months, revealing symptomatic motor improvement with buspirone alone at 1 month but not at 12 months;94 however, there was substantial rating variability in the buspirone group over time. Thus, neither amantadine nor buspirone can be concluded or excluded as neuroprotective in MSA.
ALS
A 9-month futility study of r-pramipexole (30–60 mg/day) found no difference in slopes of deterioration for revised ALS Functional Rating Scale scores or functional vital capacity.95 Although an initial 15-month, rater-blind, parallel group investigation with randomization to either lithium 150 mg po bid–tid (lithium levels 0.4–0.8 mEq/liter)-plus-riluzole 100 mg po qd (N=16) or riluzole 100 mg po qd alone (N=28), indicated strikingly less death, progression, and disability in the lithium arm,41 a more recent, placebo-controlled futility study (lithium 150 mg/day, 0.4mEq/liter; with riluzole dose unclear) failed to find any differences in endpoint achievement at 5.4 months.96 Patients were younger, with higher lithium doses, and standardized riluzole doses for a longer duration (albeit without placebo administration) in the positive study; however, a recent multicenter, single-blind, randomized, dose-finding study of lithium 0.2–0.4 mEq/liter (N=84) versus 0.4–0.8 mEq/liter (N=87) found lithium to be poorly tolerated and showed no difference in death rate between the two doses.97 In a randomized trial of valproate 1,500 mg/day versus placebo in ALS (N=163), neither survival nor functional status were different at either 12 or 16 months.98 Thus, r-pramipexole, lithium, and valproate do not appear to be neuroprotective in ALS.
The results of clinical trials do not permit firm clinical conclusions except that there are preliminary data suggesting that olanzapine may promote neurodegeneration in AD, and lithium is likely futile as a neuroprotectant in most ALS patients. (Several forthcoming studies in AD, progressive supranuclear palsy [PSP], and corticobasal degeneration [CBD] are discussed in the next-to-last Discussion paragraph.)
DISCUSSION
This report applies a rigorous inclusion requirement and a presumptively valid quantitative method to produce a broad comprehensive review of commonly-prescribed psychotropic agents that could double as neuroprotective therapies. The findings indicate a potential neuroprotective role for lithium, paroxetine, or lithium-plus-valproate in AD, lithium in frontotemporal lobar degeneration (FTLD) and in combination with valproate in ALS, pramipexole and valproate in PD, amantadine and buspirone in MSA, and antidepressants in HD. This report, limited to psychotropic medicines, should prove of special relevance to neuropsychiatrists and others who use these drugs in NDD with neuropsychiatric symptoms.
The best-documented, most robust, and promising preclinical findings are shown in Table 7 and are considered to have the highest probability of translating to clinical neuroprotection in human patients. The neuroprotective index scores of this table reflect replicated findings in drugs with proven benefit in disease-specific animal models and a net positive neuroprotective valence. Dopamine agonist, neuroleptic, atypical antipsychotic, and the VMAT-2 inhibitor tetrabenazine findings did not fulfill the Table 7 composite criteria. Lithium showed potential neuroprotection in reducing Aβ1–42, tau phosphorylation, and fibrillar tau in aging, amyloidopathic, and tauopathic mouse models (Table 7). In particular, tau phosphorylation was reduced at epitopes relevant to AD (Thr181, Ser199, AT8, Thr231, Ser396) and tauopathies including PSP, FTLD, and FTDP-17 (Tau1, Ser202, AT8, Thr231, Ser396, Ser404), and MSA (Tau1).
In the G93A SOD1 transgenic mouse model of ALS, lithium showed both neuronal and overall neuroprotective properties in most studies. Valproate reduced Aβ and neuritic plaques in amyloidopathic transgenic mouse models of AD and increased αSyn while decreasing nuclear translocation in the rotenone rat model of PD. The antidepressant paroxetine reduced cortical and hippocampal Aβ processing and hippocampal HT7 human tau in a transgenic model of AD. In HD mouse models, nortriptyline and desipramine preserved caudate medium spiny neurons against glutamate apoptosis, and sertraline increased both BDNF and hippocampal and striatal neurogenesis. Thus, the most robust preclinical findings detailed in Table 7 suggest neuroprotective potential for lithium, valproate, and paroxetine in AD, lithium in tauopathies and ALS, valproate in PD and perhaps other synucleinopathies, and several antidepressants in HD.
Although a number of psychotropics have been studied in AD, PD, MSA, and ALS, the only conclusions that can be drawn from these clinical trials are that olanzapine may deteriorate cognition in AD, and lithium is probably not a useful neuroprotectant in most ALS cases, at least when given with riluzole. Initial findings needing replication include lithium neuroprotection in AD with increased BDNF levels and in early ALS, and the futility of r-pramipexole and valproate in ALS. Neuroprotective paradigms are needed to resolve the neuroprotective efficacy of ropinirole and pramipexole in PD, and amantadine and buspirone (using more sensitive measures) in MSA. Clinical studies have relied on a difference in slope-deterioration between treatments to measure progression rather than delayed-start or randomized-withdrawal designs.5 At present, no disease-modifying neuroprotective agents have been conclusively demonstrated in clinical trials.
Additional studies in the literature require more detailed discussion. Moderate neuroprotective index scores for motor-neuron survival and motor function with lithium in the ALS G93A SOD1 mutation mouse model (Table 7; scores of 32 and 16, respectively, and an average of 14.4 across ALS findings, out of possible scores of 1 to 64) contrast somewhat with negative clinical findings, although it has been argued that this mutation model is not representative of human disease, and the mutation is present only in a small minority of ALS patients.
Moderate scores in AD models with tau mutations suggest therapeutic potential for lithium in tauopathic diseases, although poor tolerability resulted in premature discontinuation in 13 of 14 patients with progressive supranuclear palsy (PSP) or corticobasal degeneration (CBD) during a 28-week, open-label, safety and tolerability trial (recently published as an abstract).105 For valproate, a low-to-moderate neuroprotective index score was achieved for Aβ neuritic plaque reduction in AD amyloid- and presenilin-mutant mice. Forthcoming results of a 26-month, randomized, placebo-controlled trial in 313 patients with AD with clinical rating, biomarker, and brain-volume outcomes106 (manuscript in review process) indicate no difference in slopes of deterioration on clinical ratings (Tariot PN, personal communication; January 2011). The valproate group showed greater loss in hippocampal and whole-brain volume, accompanied by greater ventricular expansion. It is unclear whether these changes may be due to direct drug effects such as osmotic shifts or metabolic toxicity or influences on AD pathology, and it is unknown whether these effects are reversible or clinically relevant. Although valproate levels averaged 40–50 μg/ml, the levels may not have been sufficient to engage molecular targets (Tariot PN, personal communication). Alternatively, these negative results may reflect a translational predictive failure of Aβ neuritic plaque or amyloid transgenic mouse models. After all, the neuroprotective valence and neuroprotective index score are only as good as the models that they are based upon, and model and tolerability confounds prevent a current assessment of their predictive capability. It is also possible that higher and more robust neuroprotective index scores are needed to predict clinical neuroprotection, since even moderate scores were not consistently obtained across the findings and models of Table 7. Toward this, simultaneous administration of lithium-plus-valproate might potentially produce quantitatively greater and more robust neuroprotective index scores and result in clinical therapeutic effects.
There are several caveats in interpreting how well the present findings generalize and can predict clinical translation. First, we focused on findings in mature neural tissues because of substantial differences in how mature and immature tissues behave with respect to the common intracellular processes considered here, yet it remains possible that some potentially relevant preclinical findings might be excluded by this requirement. Second, the concepts of neuroprotective valence and the composite neuroprotective index score remain to be validated. Although it makes sense that drugs with a multiplicity of different neuroprotective actions and minimal prodegenerative effects (i.e., higher positive valence) will more likely be neuroprotective in human clinical trials, this concept remains to be validated, especially in light of disappointing results for lithium, a drug with a high valence, in ALS. Forthcoming data will also indicate whether the neuroprotective index score is a predictor of clinical neuroprotection in humans.
There are the limitations inherent to a review of the literature. Although the best strategy available, the literature search may have missed some data because of indexing inconsistencies. Several studies were not identified by the preclinical42,69,95,99 and clinical78,80 search strategies, but rather by bibliographic extension. Furthermore, the search strategy targeted the proteins, organelles, and processes specified, and although this produced a number of citations pertaining to additional neurodegenerative processes, the review cannot be considered to be fully comprehensive with respect to processes such as “oxidation.” Obviously, this review also does not consider additional neuroprotective mechanisms, for example, “inflammation.” There are also reporting biases in the literature. Drug actions have been investigated unevenly and unsystematically across and within neuroprotective actions, and animal models vary in their translational predictive power.
There are several perspective-related issues that can make the interpretation of some of the results difficult at this point. For example, an increase in αSyn can lead to proteasomal inhibition, apoptosis, and inclusion formations including Lewy bodies, pathological tau, and Aβ and yet a rise in αSyn can also effect a neuroprotective response. Proteasomal inhibition would seem to be pro-apoptotic, and yet the administration of subtoxic proteasome inhibitors has been associated with neuroprotection.100 In the case of αSyn, mono-ubiquitylation and nuclear translocation predict apoptosis, in contrast to a non-ubiquitylated rise in αSyn concentration.47 Nevertheless, seemingly similar responses can, at times, signal either neuroprotection or neurodegeneration, and whether a particular response is neuroprotective or proapoptotic may also depend on both its extent and whether it is occurring early or late in the disease process.
There are several drug-related issues that can affect generalizability and translational prediction. Drug dose can make a difference in effects. Low doses of lithium, for example, activate autophagy in extraneuronal models,101 whereas these same doses may be ineffective in activating other neuroprotective functions. On the other hand, in preclinical models of neuroprotection, a bell-shaped rather than open-ended sigmoid-shaped dose-response curve is often encountered. Thus, too high a dose may exceed the therapeutic window of neuroprotection for some mechanisms. This could help explain the diverging effects of low versus standard doses of clozapine69 and diazepam.61,102 Results may also vary with the type of apoptogen, neurotoxin, and locus of action. For example, valproate prevented nigral loss in the rotenone,47 but not the MPTP103 mouse model of PD. Some neuroprotective actions of psychotropics may relate to their pharmacodynamic mechanisms.3 For example, 5HT1a receptor agonists may inhibit apoptosis through nerve growth factor signaling.104 Duration of treatment can affect neuroprotection as well. For example, hippocampal Bcl-2 expression is upregulated by chronic but not acute treatment with antidepressants.58,64 Taken together, a given psychotropic can have plural neuroprotective mechanisms and, simultaneously, have pro-degenerative effects, with the capacity to act as both friend and foe, depending on dosing and context.
In conclusion, despite limitations and other issues in interpreting the literature, several established psychotropic medications have sufficient promise to warrant further study as neuroprotective agents. We need clinical trials using designs adequate to assess disease-modification5,107 as follow-up to these preclinical and clinical data. Priorities include the agents identified by the provisional preclinical neuroprotective criteria and resolution of initial findings in the clinical trials, including dopamine agonists (PD, ALS), amantadine (MSA), lithium (AD with elevated BDNF, tauopathies, early ALS), valproate (AD, PD, synucleinopathies, ALS), lithium-plus-valproate (AD, ALS), nortriptyline (HD), desipramine (HD), sertraline (HD), paroxetine (AD), and buspirone (MSA-C). Secondary priorities include study of the other agents identified by each of the provisional preclinical neuroprotective criteria in isolation. It will be of interest to identify which provisional criteria prove to be most predictive of clinical translational benefit. Equivocal results in clinical neuroprotective trials will signal the need to develop better preclinical models with improved abilities to predict translational treatments in human disease.
1. : Psychopharmacological neuroprotection in neurodegenerative disease: assessing the preclinical data. J Neuropsychiatry Clin Neurosci 2010; 22:8–18Link, Google Scholar
2.
3. : Psychopharmacological neuroprotection in neurodegenerative disease: heuristic clinical applications. J Neuropsychiatry Clin Neurosci 2010; 22:130–154Link, Google Scholar
4. : Forecasting the global burden of Alzheimer's disease. Alzheimer's Dementia 2007; 3:186–191Crossref, Medline, Google Scholar
5. : The evaluation of disease modifying therapies in Alzheimer's disease: a regulatory viewpoint. Stat Med 2004; 23:305–314Crossref, Medline, Google Scholar
6. : Evaluation of the neurotoxic activity of typical and atypical neuroleptics: relevance to iatrogenic extrapyramidal symptoms. Cell Mol Neurobiol 2001; 21:705–716Crossref, Medline, Google Scholar
7. : Lithium induces apoptosis in immature cerebellar granule cells but promotes survival of mature neurons. Exp Cell Res 1994; 211:332–338Crossref, Medline, Google Scholar
8. : Lithium protects rat cerebellar granule cells against apoptosis induced by anticonvulsants, phenytoin and carbamazepine. J Pharmacol Exp Ther 1998; 286:539–547Medline, Google Scholar
9. : Inhibition of complex I by neuroleptics in normal human brain cortex parallels the extrapyramidal toxicity of neuroleptics. Mol Cell Biochem 1997; 174:255–259Crossref, Medline, Google Scholar
10. : Neuroleptic-induced mitochondrial enzyme alterations in the rat brain. J Pharmacol Exp Ther 1997; 280:261–267Medline, Google Scholar
11. : Effect of antipsychotics on succinate dehydrogenase and cytochrome oxidase activities in rat brain. Naunyn Schmiedebergs Arch Pharmacol 2007; 376:127–133Crossref, Medline, Google Scholar
12. : Translocation of AIF in the human and rat striatum following protracted haloperidol, but not clozapine treatment. Apoptosis 2006; 11:663–672Crossref, Medline, Google Scholar
13. : Haloperidol-induced neuronal apoptosis: role of p38 and c-Jun-NH(2)-terminal protein kinase. J Neurochem 2000; 75:2327–2334Crossref, Medline, Google Scholar
14. : Cystamine prevents haloperidol-induced decrease of BDNF/TrkB signaling in mouse frontal cortex. J Neurochem 2008; 107:941–951Medline, Google Scholar
15. : Neurotoxic potential of haloperidol in comparison with risperidone: implication of Akt-mediated signal changes by haloperidol. J Neural Transm 2004; 111:667–681Crossref, Medline, Google Scholar
16. : GSK-3alpha regulates production of Alzheimer's disease amyloid-beta peptides. Nature 2003; 423:435–439Crossref, Medline, Google Scholar
17. : Neuroprotective effects of regulators of the glycogen synthase kinase-3beta signaling pathway in a transgenic model of Alzheimer's disease are associated with reduced amyloid precursor protein phosphorylation. J Neurosci 2007; 27:1981–1991Crossref, Medline, Google Scholar
18. : Lithium down-regulates tau in cultured cortical neurons: a possible mechanism of neuroprotection. Neurosci Lett 2008; 434:93–98Crossref, Medline, Google Scholar
19. : Chronic lithium treatment decreases mutant tau protein aggregation in a transgenic mouse model. J Alzheimers Dis 2003; 5:301–308Crossref, Medline, Google Scholar
20. : Chronic lithium administration to FTDP-17 tau and GSK-3beta overexpressing mice prevents tau hyperphosphorylation and neurofibrillary tangle formation, but pre-formed neurofibrillary tangles do not revert. J Neurochem 2006; 99:1445–1455Crossref, Medline, Google Scholar
21. : Lithium reduces tau phosphorylation: effects in living cells and in neurons at therapeutic concentrations. Biol Psychiatry 1999; 45:995–1003Crossref, Medline, Google Scholar
22. : GSK-3 activity in neocortical cells is inhibited by lithium but not carbamazepine or valproic acid. Bipolar Disord 2005; 7:260–265Crossref, Medline, Google Scholar
23. : Lithium treatment decreases activities of tau kinases in a murine model of senescence. J Neuropathol Exp Neurol 2008; 67:612–623Crossref, Medline, Google Scholar
24. : Lithium reduces tau phosphorylation but not A beta or working memory deficits in a transgenic model with both plaques and tangles. Am J Pathol 2007; 170:1669–1675Crossref, Medline, Google Scholar
25. : Lithium inhibits Alzheimer's disease-like tau protein phosphorylation in neurons. FEBS Lett 1997; 411:183–188Crossref, Medline, Google Scholar
26. : Lithium protects cultured neurons against beta-amyloid-induced neurodegeneration. FEBS Lett 1999; 453:260–264Crossref, Medline, Google Scholar
27. : Linking alterations in tau phosphorylation and cleavage during neuronal apoptosis. J Biol Chem 2004; 279:54518–54528Crossref, Medline, Google Scholar
28. : Inhibition of glycogen synthase kinase-3beta downregulates total tau proteins in cultured neurons and its reversal by the blockade of protein phosphatase-2A. Brain Res 2009; 1252:66–75Crossref, Medline, Google Scholar
29. : Lithium inhibits neurite growth and tau protein kinase I/glycogen synthase kinase-3beta-dependent phosphorylation of juvenile tau in cultured hippocampal neurons. J Neurochem 1999; 73:2073–2083Medline, Google Scholar
30. : Lithium blocks the c-Jun stress response and protects neurons via its action on glycogen synthase kinase 3. Mol Cell Biol 2003; 23:6027–6036Crossref, Medline, Google Scholar
31. : Opposite effects of lithium and valproic acid on trophic factor deprivation-induced glycogen synthase kinase-3 activation, c-Jun expression and neuronal cell death. Neuropharmacology 2005; 48:576–583Crossref, Medline, Google Scholar
32. : Tau overexpression in transgenic mice induces glycogen synthase kinase 3beta and beta-catenin phosphorylation. Neuroscience 2007; 146:730–740Crossref, Medline, Google Scholar
33. : Inhibition of glycogen synthase kinase-3 by lithium correlates with reduced tauopathy and degeneration in vivo. Proc Natl Acad Sci U S A 2005; 102:6990–6995Crossref, Medline, Google Scholar
34. : Induction of hyperphosphorylated tau in primary rat cortical neuron cultures mediated by oxidative stress and glycogen synthase kinase-3. J Alzheimers Dis 2004; 6:659–671Crossref, Medline, Google Scholar
35. : A myocyte enhancer factor 2D (MEF2D) kinase activated during neuronal apoptosis is a novel target inhibited by lithium. J Neurochem 2003; 85:1488–1499Crossref, Medline, Google Scholar
36. : Long term lithium treatment suppresses p53 and Bax expression but increases Bcl-2 expression. A prominent role in neuroprotection against excitotoxicity. J Biol Chem 1999; 274:6039–6042Crossref, Medline, Google Scholar
37. : Lithium inhibits aluminum-induced apoptosis in rabbit hippocampus, by preventing cytochrome c translocation, Bcl-2 decrease, Bax elevation and caspase-3 activation J Neurochem 2002; 82:137–145Crossref, Medline, Google Scholar
38. : GSK-3 beta inhibition and prevention of mitochondrial apoptosis inducing factor release are not involved in the antioxidant properties of SB-415286. Eur J Pharmacol 2008; 588:239–243Crossref, Medline, Google Scholar
39. : Evaluation of acute antiapoptotic effects of Li+ in neuronal cell cultures. J Neural Transm 2007; 114:405–416Crossref, Medline, Google Scholar
40. : Concurrent administration of Neu2000 and lithium produces marked improvement of motor neuron survival, motor function, and mortality in a mouse model of amyotrophic lateral sclerosis. Mol Pharmacol 2007; 71:965–975Crossref, Medline, Google Scholar
41. : Lithium delays progression of amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A 2008; 105:2052–2057Crossref, Medline, Google Scholar
42. : Combined lithium and valproate treatment delays disease onset, reduces neurological deficits and prolongs survival in an amyotrophic lateral sclerosis mouse model. Neuroscience 2008; 155:567–572Crossref, Medline, Google Scholar
43. : Carbamazepine induction of apoptosis in cultured cerebellar neurons: effects of N-methyl-D-aspartate, aurintricarboxylic acid and cycloheximide. Brain Res 1995; 703:63–71Crossref, Medline, Google Scholar
44. : Neurotoxic/neuroprotective profile of carbamazepine, oxcarbazepine and two new putative antiepileptic drugs, BIA 2–093 and BIA 2–024 Eur J Pharmacol 2000; 406:191–201Crossref, Medline, Google Scholar
45. : Endogenous alpha-synuclein is induced by valproic acid through histone deacetylase inhibition and participates in neuroprotection against glutamate-induced excitotoxicity. J Neurosci 2006; 26:7502–7512Crossref, Medline, Google Scholar
46. : Alpha-synuclein protects cerebellar granule neurons against 6-hydroxydopamine-induced death. J Neurochem 2007; 103:518–530Crossref, Medline, Google Scholar
47. : Valproic acid is neuroprotective in the rotenone rat model of Parkinson's disease: involvement of alpha-synuclein. Neurotox Res 2010; 17:130–141Crossref, Medline, Google Scholar
48. : Valproic acid inhibits Abeta production, neuritic plaque formation, and behavioral deficits in Alzheimer's disease mouse models. J Exp Med 2008; 205:2781–2789Crossref, Medline, Google Scholar
49. : Synergistic neuroprotective effects of lithium and valproic acid or other histone deacetylase inhibitors in neurons: roles of glycogen synthase kinase-3 inhibition. J Neurosci 2008; 28:2576–2588Crossref, Medline, Google Scholar
50. : Valproate inhibits oxidative damage to lipid and protein in primary cultured rat cerebrocortical cells. Neuroscience 2003; 116:485–489Crossref, Medline, Google Scholar
51. : Activation of glycogen synthase kinase 3 beta (GSK-3beta) by platelet activating factor mediates migration and cell death in cerebellar granule neurons. Eur J Neurosci 2001; 13:1913–1922Crossref, Medline, Google Scholar
52. : Brain-derived neurotrophic factor and antidepressant drugs have different but coordinated effects on neuronal turnover, proliferation, and survival in the adult dentate gyrus. J Neurosci 2005; 25:1089–1094Crossref, Medline, Google Scholar
53. : Cell proliferation is influenced by bulbectomy and normalized by imipramine treatment in a region-specific manner. Neuropsychopharmacology 2006; 31:1165–1176Crossref, Medline, Google Scholar
54. : Disturbed Ca2+ signaling and apoptosis of medium spiny neurons in Huntington's disease. Proc Natl Acad Sci U S A 2005; 102:2602–2607Crossref, Medline, Google Scholar
55. : Light deprivation damages monoamine neurons and produces a depressive behavioral phenotype in rats. Proc Natl Acad Sci U S A 2008; 105:4898–4903Crossref, Medline, Google Scholar
56. : Nortriptyline delays disease onset in models of chronic neurodegeneration. Eur J Neurosci 2007; 26:633–641Crossref, Medline, Google Scholar
57. : Fluoxetine up-regulates expression of cellular FLICE-inhibitory protein and inhibits LPS-induced apoptosis in hippocampus-derived neural stem cell. Biochem Biophys Res Commun 2006; 343:391–400Crossref, Medline, Google Scholar
58. : Antidepressant administration modulates neural stem cell survival and serotoninergic differentiation through bcl-2. Curr Neurovasc Res 2007; 4:19–29Crossref, Medline, Google Scholar
59. : The antidepressant sertraline improves the phenotype, promotes neurogenesis and increases BDNF levels in the R6/2 Huntington's disease mouse model. Exp Neurol 2008; 210:154–163Crossref, Medline, Google Scholar
60. : Prophylactic treatment with paroxetine ameliorates behavioral deficits and retards the development of amyloid and tau pathologies in 3xTgAD mice. Exp Neurol 2007; 205:166–176Crossref, Medline, Google Scholar
61. : Diazepam neuroprotection in excitotoxic and oxidative stress involves a mitochondrial mechanism additional to the GABAAR and hypothermic effects. Neurochem Int 2009; 55:164–173Crossref, Medline, Google Scholar
62. : Effects on free radical generation by ligands of the peripheral benzodiazepine receptor in cultured neural cells. J Neurochem 2002; 83:1226–1234Crossref, Medline, Google Scholar
63. : Desipramine activated Bcl-2 expression and inhibited lipopolysaccharide-induced apoptosis in hippocampus-derived adult neural stem cells. J Pharmacol Sci 2007;104:61–72Crossref, Medline, Google Scholar
64. : Neuroprotection by imipramine against lipopolysaccharide-induced apoptosis in hippocampus-derived neural stem cells mediated by activation of BDNF and the MAPK pathway. Eur Neuropsychopharmacol 2008; 18:128–140Crossref, Medline, Google Scholar
65. : MPTP-induced neuroblast apoptosis in the subventricular zone is not regulated by dopamine or other monoamine transporters. Neurotoxicology 2009; 30:1036–1044Crossref, Medline, Google Scholar
66. : Neuroprotective effect of the antiparkinsonian drug pramipexole against nigrostriatal dopaminergic degeneration in rotenone-treated mice. Neurochem Int 2009; 55:760–767Crossref, Medline, Google Scholar
67. : Pramipexole reduces reactive oxygen species production in vivo and in vitro and inhibits the mitochondrial permeability transition produced by the parkinsonian neurotoxin methylpyridinium ion. J Neurochem 1998; 71:295–301Crossref, Medline, Google Scholar
68. : Beneficial effects of quetiapine in a transgenic mouse model of Alzheimer's disease. Neurobiol Aging 2009; 30:1205–1216Crossref, Medline, Google Scholar
69. : Opposing effects of low and high-dose clozapine on survival of transgenic amyotrophic lateral sclerosis mice. J Neurosci Res 2003; 74:605–613Crossref, Medline, Google Scholar
70. : Dopaminergic signaling and striatal neurodegeneration in Huntington's disease. J Neurosci 2007; 27:7899–7910Crossref, Medline, Google Scholar
71. : Short-term lithium treatment promotes neuronal survival and proliferation in rat striatum infused with quinolinic acid, an excitotoxic model of Huntington's disease. Mol Psychiatry 2004; 9:371–385Crossref, Medline, Google Scholar
72. : Prevention of MPTP (N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) dopaminergic neurotoxicity in mice by chronic lithium: involvements of Bcl-2 and Bax. Neuropharmacology 2004; 46:1130–1140Crossref, Medline, Google Scholar
73. : Multiple therapeutic effects of valproic acid in spinal muscular atrophy model mice. J Mol Med 2008; 86:1243–1254Crossref, Medline, Google Scholar
74. : Olanzapine does not enhance cognition in non-agitated and non-psychotic patients with mild to moderate Alzheimer's dementia. Int J Geriatr Psychiatry 2005; 20:1020–1027Crossref, Medline, Google Scholar
75. : Does lithium protect against dementia? Bipolar Disord 2010; 12:87–94Crossref, Medline, Google Scholar
76. : Lithium trial in Alzheimer's disease: a randomized, single-blind, placebo-controlled, multicenter 10-week study. J Clin Psychiatry 2009; 70:922–931Crossref, Medline, Google Scholar
77. : Increase of BDNF serum concentration in lithium treated patients with early Alzheimer's disease. J Alzheimers Dis 2009; 16:649–656Crossref, Medline, Google Scholar
78. : Cognitive response to pharmacological treatment for depression in Alzheimer disease: secondary outcomes from the depression in Alzheimer's disease study (DIADS). Am J Geriatr Psychiatry 2004; 12:491–498Crossref, Medline, Google Scholar
79. : The clinical efficacy of citalopram in treatment of emotional disturbances in dementia disorders. A Nordic multicentre study. Br J Psychiatry 1990; 157:894–901Crossref, Medline, Google Scholar
80. : A five-year study of the incidence of dyskinesia in patients with early Parkinson's disease who were treated with ropinirole or levodopa. 056. Study Group N Engl J Med 2000; 342:1484–1491Crossref, Medline, Google Scholar
81. : REAL-PET study group: slower progression of Parkinson's disease with ropinirole versus levodopa: The REAL-PET study. Ann Neurol 2003; 54:93–101Crossref, Medline, Google Scholar
82. : A technique for standardized central analysis of 6-(18)F-fluoro-L-DOPA PET data from a multicenter study. J Nucl Med 2004; 45:1135–1145Medline, Google Scholar
83. : A comparison of the progression of early Parkinson's disease in patients started on ropinirole or L-dopa: an 18F-dopa PET study. J Neural Transm 2002; 109:1433–1443Crossref, Medline, Google Scholar
84. : Neuroprotection in idiopathic Parkinson's disease. J Neurol 2002; 249. Suppl 3:III/21–23Google Scholar
85. : Real and calm: what have we learned? Mov Disord 2003; 18:839–840Crossref, Medline, Google Scholar
86.
87.
88. : Dopamine agonist monotherapy in Parkinson's disease. Lancet 2002; 360:1767–1769Crossref, Medline, Google Scholar
89. : Influence of L-dopa and pramipexole on striatal dopamine transporter in early PD. Neurol 2001; 56:1559–1564Crossref, Medline, Google Scholar
90. : Ten-year follow-up of Parkinson's disease patients randomized to initial therapy with ropinirole or levodopa. Mov Disord 2007; 22:2409–2417Crossref, Medline, Google Scholar
91.
92. : Treatment of heredo-degenerative ataxias with amantadine hydrochloride. Can J Neurol Sci 1991; 18:307–311Crossref, Medline, Google Scholar
93. ;
94. : The efficacy of combined estrogen and buspirone treatment in olivopontocerebellar atrophy. J Neurol Sci 2008; 271:87–90Crossref, Medline, Google Scholar
95. : R+ pramipexole as a mitochondrially focused neuroprotectant: initial early phase studies in ALS. Amyotroph Lateral Scler 2008; 9:50–58Crossref, Medline, Google Scholar
96. : Northeast and Canadian Amyotrophic Lateral Sclerosis Consortia: safety and efficacy of lithium in combination with riluzole for treatment of amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trial. Lancet Neurol 2010; 9:481–488Crossref, Medline, Google Scholar
97. : LITALS Study Group: lithium carbonate in amyotrophic lateral sclerosis: lack of efficacy in a dose-finding trial. Neurol 2010; 75:619–625Crossref, Medline, Google Scholar
98. : Randomized sequential trial of valproic acid in amyotrophic lateral sclerosis. Ann Neurol 2009; 66:227–234Crossref, Medline, Google Scholar
99. : The antidepressant sertraline improves the phenotype, promotes neurogenesis and increases BDNF levels in the R6/2 Huntington's disease mouse model. Exp Neurol 2008; 210:154–163Crossref, Medline, Google Scholar
100. : A proteasomal stress response: pre-treatment with proteasome inhibitors increases proteasome activity and reduces neuronal vulnerability to oxidative injury. J Neurochem 2004; 91:996–1006Crossref, Medline, Google Scholar
101. : Autophagy, lithium, and amyotrophic lateral sclerosis. Muscle Nerve 2009; 40:173–194Crossref, Medline, Google Scholar
102. : Evidence in favour of a role for peripheral-type benzodiazepine receptor ligands in amplification of neuronal apoptosis. Apoptosis 2005; 10:91–104Crossref, Medline, Google Scholar
103. : Lamotrigine is neuroprotective in the energy deficiency model of MPTP intoxicated mice. Pediatr Res 2007; 62:14–19Crossref, Medline, Google Scholar
104. : The 5-HT1A receptor agonist Bay x 3702 inhibits apoptosis induced by serum deprivation in cultured neurons. Eur J Pharmacol 1999; 370:211–216Crossref, Medline, Google Scholar
105. ,
106. : Can lithium or valproate untie tangles in Alzheimer's disease? J Clin Psychiatry 2009; 70:919–921Crossref, Medline, Google Scholar
107. : Defining and labeling disease-modifying treatments for Alzheimer's disease. Alzheimers Dement 2009; 5:406–418Crossref, Medline, Google Scholar



