Manic Psychosis After Sertraline and Transcranial Direct-Current Stimulation
Case Report
“Ms. R” presented to our research center to participate in our depression trial2 in May 2010. She was on S-citalopram 20 mg/day for 3 months without improvement; and, at her initial evaluation, she fulfilled eligibility criteria for enrollment, which includes no previous history of bipolar disorder and moderate-to-severe depression (Figure 1). S-citalopram was tapered down during 1 week, and she was then randomized to receive tDCS (2mA/30 minute per day with the anode over the left and cathode over the right dorsolateral prefrontal cortex; current density of 0.06 m2/A) and sertraline 50 mg/day. After 5 days of combined treatment and a weekend interval, she returned agitated and restless. During the interview she was delusional, with auditory hallucinations, claiming that God told her she was protected and could spend any amount of money. Her mood, although excited, quickly swung to hostility and irritability when we explored her delusional content. Her mood scores changed accordingly (Figure 1), with maximum scoring on thought content (delusional), sleep (denying need for sleeping), and elevated mood (euphoric). At that time, we diagnosed her with Bipolar Disorder type I, acute manic episode with psychotic features; she was dropped out of the trial, broke blinding, and started on risperidone 2 mg/day and lithium 900 mg/day. Two weeks later, Ms. R had a serum lithium level of 0.6 meq/liter and presented significant improvement of symptoms (Figure 1).
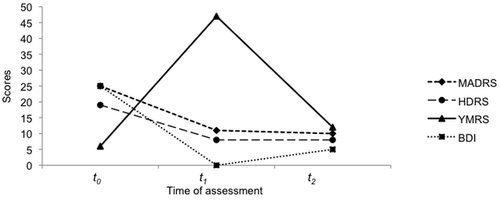
BDI: Beck Depression Inventory; Ham-D: Hamilton Rating Scale for Depression; MADRS: Montgomery-Asberg Depression Rating Scale; YMRS: Young Manic Rating Scale.
To the best of our knowledge, this is the first report of psychotic mania induced by tDCS. Other reports have described transient hypomania after tDCS, which resolved spontaneously4 or after tDCS withdrawal.5 Along these lines, in a transcranial magnetic stimulation (TMS) review,6 only 13 cases of treatment-emergent mania (TEM) were reported, which was less than 1% of patients stimulated. Most had previous diagnosis of bipolar disorder, and psychotic/delusional symptoms were not reported; in fact, the most similar case to ours was from a 79-year-old woman with recurrent depression who developed mania after 3 days of TMS; however, she was already using tranylcipromine and haloperidol, and had a less severe episode of mania that resolved spontaneously in 2 days. Importantly, in our case, sertraline was combined with tDCS; therefore it is not possible to detangle which intervention (or the combination) was responsible for the symptoms. Also, other interventions could have triggered this episode, although S-citalopram did not induce any adverse effects. Besides these limitations, this report presents valuable safety information and raises important points: 1) combining this report with others, TEM rates with tDCS might be higher than TMS, and perhaps similar than tricyclics (7%–11%)7,8 and selective serotonin reuptake inhibitors (3%–6%);8,2 likewise, tDCS might induce more severe manic episodes than TMS; thus: 2) further tDCS clinical trials for depression should consider protecting patients at high risk of TEM with mood stabilizers9,10 and screening and accessing this risk using appropriate scales.4,11 Future research should attempt to identify whether and which tDCS parameters, such as dosage, electrode positioning, period of treatment, and augmentation with antidepressants are associated with TEM;5 the combination of tDCS and pharmacotherapy should also be carefully investigated.
1. : Treatment of depression with transcranial direct current stimulation (tDCS): a review. Exp Neurol 2009; 219:14–19Crossref, Medline, Google Scholar
2. Efficacy Study of Transcranial Direct Current Stimulation to Treat Major Depressive Disorder. 2009; from: clinicaltrials.gov.http://clinicaltrials.gov/ct2/show/NCT01033084Google Scholar
3. : A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56–62Crossref, Medline, Google Scholar
4. : Induction of hypomanic episode with transcranial direct current stimulation. J ECT 26:68–69Crossref, Google Scholar
5. : Hypomanic episode in unipolar depression during transcranial direct current stimulation Acta Neuropsychiatr (in press)Google Scholar
6. : Treatment-emergent mania in unipolar and bipolar depression: focus on repetitive transcranial magnetic stimulation. Int J Neuropsychopharmacol 2008; 11:119–130Crossref, Medline, Google Scholar
7. : Double-blind, placebo-controlled comparison of imipramine and paroxetine in the treatment of bipolar depression. Am J Psychiatry 2001; 158:906–912Crossref, Medline, Google Scholar
8. : Induction of mania with selective serotonin re-uptake inhibitors and tricyclic antidepressants. Br J Psychiatry 1994; 164:549–550Crossref, Medline, Google Scholar
9. : Bipolar depression: issues in diagnosis and treatment. Harv Rev Psychiatry 2005; 13:257–271Crossref, Medline, Google Scholar
10. : Maintenance therapies in bipolar disorder: focus on randomized, controlled trials. Aust N Z J Psychiatry 2005; 39:652–661Crossref, Medline, Google Scholar
11. . Neuromodulation approaches for the treatment of major depression: challenges and recommendations from a working group meeting. Arq Neuropsiquiatr 68:433–451Crossref, Google Scholar



