Neurological Soft Signs in OCD Patients With Early Age at Onset, Versus Patients With Schizophrenia and Healthy Subjects
Abstract
Compelling evidence suggests that both schizophrenia and obsessive compulsive disorder (OCD) are related to deviant neurodevelopment. Neurological soft signs (NSS) have been proposed to be a marker of abnormal brain development in schizophrenia. The purpose of this study is to examine whether NSS are also a marker in patients with OCD, in particular, in early-onset OCD. The authors included 162 subjects and compared patients with OCD, patients with schizophrenia (SCZ), and healthy control subjects. They were all examined for NSS (Krebs' Scale), extrapyramidal symptoms (Simpson-Angus Scale), and were rated on the Abnormal Involuntary Movements Scale (AIMS). The authors found no differences between NSS total scores and subscores in OCD versus controls, whereas total NSS, motor coordination, and motor integration were significantly lower in OCD than in SCZ. OCD patients with early-onset (before age 13) did not differ from those with later-onset OCD. These results support the idea that NSS, as determined by current scales, is relatively specific to schizophrenia, although they do not preclude the existence of a neurological dysfunction in OCD. Further studies are required to determine the type of neurological signs that could be useful trait-markers in the phenotypic characterization of subtype OCD.
Obsessive-compulsive disorder (OCD), with a lifetime prevalence of 2.5% to 4%,1 is one of the most prevalent and disabling psychiatric disorders. It is characterized by obsessions (which cause marked anxiety or distress) and/or compulsions (which serve to neutralize anxiety). Neurological soft signs (NSS), in contrast to hard neurological signs, are subtle neurological and nonlocalizing anomalies, including altered motor coordination, balance, motor integration, and sensory integration. NSS may reflect cerebral dysfunction in distributed neural networks, and they can give additional information on the functional organization that characterizes some psychiatric disorders.2 NSS have been described in several psychiatric disorders, but they are particularly prevalent in schizophrenia.3 Only a few studies have explored NSS in patients with OCD, and their results are contradictory. There is only one previous study, by Bolton et al.,4 that compared NSS (assessed with the Cambridge Neurological Inventory) in patients with OCD and patients with schizophrenia and control subjects. In this study, as compared with patients with schizophrenia, patients with OCD had lower levels of neurological signs, including motor coordination soft-signs, tardive dyskinesia, catatonic signs, and extrapyramidal signs, and identical levels of NSS in other subscales (sensory integration, primitive reflexes, failure of suppression). This study has never been replicated. Other studies compared patients with schizophrenia with or without OCD and healthy controls.5–7 Once again, the results are contradictory.
Sevincok et al., in 2004,5 found that patients with OCD-schizophrenia had significantly higher NSS scores, restricted to sensory-integration items, as compared with controls. However, the same team, 2 years later (Sevincok et al. [2006]6 and Poyurovsky et al. [2007]7) did not find any significant differences in NSS between patients with schizophrenia with or without OCD. However, the number of OCD patients was low in these studies (N <25). Finally, studies examining NSS in patients with OCD, versus controls, also found contradictory results. Some of these studies found a higher prevalence of NSS in OCD patients than in healthy controls,4,8 with impaired motor coordination and visuospatial tasks,9 abnormal involuntary movements,9,10 sensory-integration abnormalities, and motor overflow.11 However, other studies did not report such differences,7,12,13 and, overall, the studies remain inconclusive because of intrinsic limitations; these observations were done in rather small samples,5–7 with no or insufficient information on medication status or history,2–4,14–16 or extrapyramidal symptom assessment,8,12,13,17 and/or without a standardized examination.17,18 making any comparison difficult. It has been suggested that NSS in OCD patients may be used as an early element of diagnosis4,19 or as predictors of good treatment response to selective serotonin reuptake inhibitors20 or response to psychotherapy,21,22 but, here, as well, there are contradictory findings.13,17,22,23 Overall, although some reviews state that NSS are increased in OCD, a closer reading of previous studies reveals that the picture is not very clear.
Like many psychiatric disorders, OCD is heterogeneous, and previous studies have stressed that children with early onset (before age 7) are more likely to be male and to have a family member with OCD than those with later-onset OCD.24 Also, the age at onset is earlier for boys (prepubertal onset) than for girls (during adolescence), with younger boys experiencing more severe symptoms than younger girls.24 This suggests that early age at onset of OCD reflects more neurodevelopmental loading than a later age at onset. In line with this, early age at onset of OCD is more frequently associated with tic disorders.25–27
These investigations suggest that NSS may be higher in patients with early age at onset of OCD than in patients with later age at onset. However, to the best of our knowledge, no study has directly examined the influence of age at onset on NSS in patients with OCD.
Our primary objective in this study was to examine NSS in patients with OCD as compared with patients with schizophrenia and a control group. A secondary objective was to look at whether OCD patients with an early age at onset (before age 13) have more NSS than other OCD patients or the control group, and less than patients with schizophrenia.
METHOD
Participants
In this study, 162 subjects were recruited by three centers: the Centre Hospitalier Sainte-Anne, in Paris; the Centre Hospitalier Henri Laborit, in Poitiers; and the Centre Hospitalier Guillaume Regnier, in Rennes. This protocol was approved by the ethics committee. All subjects received a complete description of the study and gave written informed consent. All subjects with OCD were consecutively-encountered patients of European Caucasian descent who consented to participate to the study. Patients with schizophrenia were selected in order to match to OCD patients for gender and age, selected from a large cohort of consecutively-encountered European Caucasian patients with schizophrenia. This study was part of a larger clinical and genetic research program and based on a national collaborative research network (ReFaPsy).
These 162 subjects were divided into three groups: patients with OCD (N=54), patients with schizophrenia (N=54), and healthy control subjects (N=54). Patients were selected from both inpatient and outpatient clinical settings (33% and 66%, respectively). The percentage of inpatients versus outpatients was 27% versus 33% for patients with OCD and schizophrenia, respectively (NS). The inpatients were included when stabilized, just before discharge from the hospital. Controls were recruited from the administrative and paramedical staff of the hospital and through advertisement, and received an incentive.
Patients and controls were examined with a standardized interview (DIGS: Diagnostic Interview Genetic Study) leading to lifetime diagnoses according to DSM-IV criteria. For all subjects, exclusion criteria were 1) current or history of neurological illness or severe medical condition; 2) sequelae of trauma that could hinder the neurological examination (e.g., wrist fracture); 3) recent (in the past year) substance abuse or dependence and/or abuse or dependence lasting for more than 5 years; 4) clinically significant extrapyramidal symptoms (Simpson-Angus Scale [SAS] >9 and Abnormal Involuntary Movement Scale [AIMS] >3).
Patients with OCD (18–53 years old) met the DSM-IV criteria for OCD but not those for schizophrenia. Patients with schizophrenia (18–55 years old) met the DSM-IV criteria for schizophrenia, but not those for OCD. The control group comprised healthy volunteers with no history of or current psychiatric illness; they were matched to the OCD group for age, gender, and education level.
Age at onset was further determined in OCD patients as the first time that the presence of symptoms was noticed by the individual and/or a family member.28 Early-onset OCD (EO-OCD) patients were defined as having an age at onset under 13 (and, conversely, late-onset OCD [LO-OCD] patients were defined as having an age at onset at or later than 13), in order to subgroup prepubertal onset, as suggested by Geller et al.29 Moreover, this threshold is consistent with a SPECT study reporting significant differences in brain functional activity between early- and late-onset OCD by use of this cutoff.30
Measures
All subjects were interviewed by senior psychiatrists using the French version of the Diagnostic Interview for Genetic Studies, leading to lifespan DSM-IV Axis I disorder.31 The severity of obsessive and compulsive symptoms was measured by the French version of the Yale-Brown Obsessive-Compulsive scale (Y-BOCS).32 A minimum total Y-BOCS score >15 and >1-year duration of obsessive-compulsive symptoms were required. Psychopathology in patients with schizophrenia was assessed by the French version of the Positive and Negative Syndrome Scale (PANSS).33
NSS were assessed with a standardized neurological examination previously described and validated by Krebs et al.34 This examination includes a 23-item scale for NSS as well as SAS for parkinsonism, AIMS for dyskinesia, the Edinburgh, Scotland, Inventory for Lateralization, and the Mini-Mental State Exam (MMSE), for general cognitive functioning. The scale ranges from 0 to 3. It assesses 5 factors: 1) motor coordination (finger opposition, fist-edge-palm, alternate movements of foot and hand); 2) motor integrative function (Romberg test, finger to nose, gait, tandem walk, tongue protrusion, standing, walking heel-to-toe); 3) sensory integration (astereognosia, hand–face, constructive apraxia, graphesthesia, right–left recognition); 4) involuntary movements (mirror movements, abnormal movement); and 5) quality of lateralization (lateral preference, R/L confusion). This scale has good interrater reliability.34
Data Analysis
In the OCD patient group, five patients had tics and had higher AIMS scores (12.8 [SD]: 5.2) than the remaining patients. Because this group of patients with OCD and tics was small, and tics can confound neurological examination, we decided to exclude them from the analysis. Sociodemographic clinical variables were described and compared between the three groups with one-way ANOVA or chi-squared tests for categorical data. Correlation analyses of clinical variables with total scores and subscores of NSS were conducted with the Pearson correlation coefficient. Because of the well-known influence of antipsychotic medication on extrapyramidal symptoms and because the groups differed regarding previous exposure to antipsychotic medication, we decided to adjust NSS on SAS score in our analysis. ANCOVAs were performed for total scores and subscores of NSS, and to evaluate contrasts between OCD patients versus controls and OCD patients versus the schizophrenic group; Bonferroni's correction was applied.
RESULTS
Clinical Description
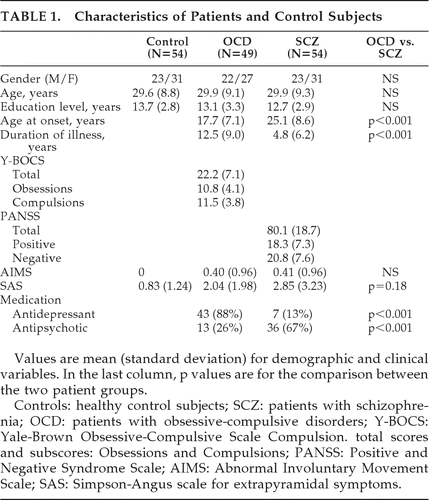
In the OCD group, 88% of patients (N=43) were currently on SSRI antidepressants, and 26% (N=13) were currently being treated with antipsychotics. Age at onset was significantly (p <0.001) earlier in the OCD group than in the schizophrenic group. In the schizophrenic group, 25.9% (N=14) had never received antipsychotic medication, and 7.4% (N=4) had been off antipsychotic medication for at least 3 months. The remaining 66.7% (N=36) were being treated with antipsychotics and 13% (N=7) with antidepressants. There were no significant differences for the remaining descriptive variables between the two patient groups (Table 1).
 |
Compulsion Y-BOCS scores were inversely correlated with age at onset (r = –0.31; p=0.03); the higher the Y-BOCS scores, the lower the age at onset. Thirteen OCD patients (33%; 7 women, 6 men) had an early age at onset (<13 years) of their first symptoms (EO-OCD).
Neurological Examination of Patients With OCD
In the OCD patients, the NSS total score did not correlate either with the severity of the YBOCS total score or with the obsession or compulsion subscores.
EO-OCD patients did not differ from LO-OCD groups on extrapyramidal symptoms: AIMS (EO-OCD: 0.42 [0.9]; LO-OCD: 0.41 [0.99]; NS) or on Simpson-Angus (EO-OCD: 2.70 [2.5]; LO-OCD: 1.83 [1.8]; NS). No significant differences (F[1,46]=0.03; NS) were observed between EO-OCD and LO-OCD groups in the NSS total score (EO-OCD: 5.9 [5.5]; LO-OCD: 6.2 [5.9]) or subscores (Motor Coordination factor, EO-OCD: 1.85 [1.99]; LO-OCD: 2.34 [3.15]; Motor Integrative function, EO-OCD: 1.38 [2.31]; LO-OCD: 1.09 [1.42]; Sensory Integration factor, EO-OCD : 1.85 [1.63]; LO-OCD: 1.63 [1.66]).
When comparing OCD patients with and without treatment with antipsychotic medication, no significant differences were found in NSS, Simpson-Angus, or in AIMS scores. No significant differences (F[1,47]=0.63; NS) were observed in NSS between EO-OCD (5.9 [5.5]) and controls (5.38 [2.98]).
Comparisons in OCD, Schizophrenia, and Control Subjects
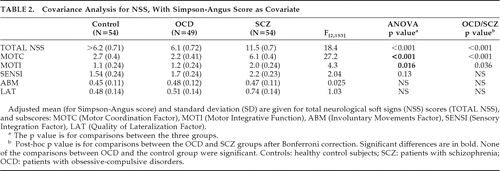
The AIMS scores were comparable in patients with OCD and patients with schizophrenia (Table 1), the two groups being significantly different from controls (respectively, p=0.024, p=0.032). Extrapyramidal symptoms assessed by the Simpson-Angus scale were significantly different among the three groups (p <0.0001): the scores were higher in patients with OCD than in controls (p=0.024), and the scores of OCD patients were not significantly different from patients with schizophrenia (Table 1). Therefore, NSS analyses were adjusted on the Simpson-Angus score.
The total NSS score and the subscores were not significantly different between OCD patients and controls, whereas significant differences were observed between patients with OCD and those with schizophrenia for total score, motor coordination, and motor integration. No differences were seen among groups for sensory integration, involuntary movement, and lateralization factors (Table 2).
 |
DISCUSSION
Both OCD and schizophrenia are thought to reflect abnormal brain development, and NSSs have been demonstrated to be a valid marker of abnormal neurodevelopment in patients with schizophrenia. The main aim of this study was to examine whether NSS could also be a valid marker of deviant neurodevelopment in patients with OCD. We compared OCD patients with carefully-matched patients with schizophrenia and controls and found higher NSS scores in patients with schizophrenia than in those with OCD, which did not differ from controls. Furthermore, we found no differences in the NSS total scores or subscores in patients with early- versus late-onset OCD (age <13 versus ≥13). Taken together, our findings do not support the idea that NSS indicate neurodevelopmental brain anomalies associated with OCD and suggest that NSS could be more specific to the neurodevelopmental anomalies associated with schizophrenia.
Our work has several features. First, the sample size was larger or similar to those of previous reports. Second, the inclusion criteria were very strictly defined, and the populations were well-matched. The two patient groups were well contrasted by excluding OCD in patients with schizophrenia (in contrast to others studies5–7) and, conversely, psychotic symptoms in patients with OCD. These diagnoses were done on the basis of lifespan structured interviews based on DSM-IV criteria, which were used in some,4–10,12,13 but not all2,18 previous reports. Possible confounding factors were avoided by excluding, in particular, neurological disorders and substance abuse, information that is available in some,4–7,12 but not all studies.13,17,22 We also carefully matched the three groups on age and gender. Third, patients were carefully tested, by use of a validated, standardized examination, in contrast to some,2,17,18 but not all9,13,20,22 previous reports. Notably, this comprises semiquantitative rather than presence/absence ratings of NSS and could thus be more accurate, but could also lead to some differences from previous studies.5–7,12 Fourth, the two groups of patients included patients naive for antipsychotic medication, including patients with schizophrenia, which was not the case in previous reports.4–6,9,12,17 Also, we took into account the extrapyramidal status of the patients. In contrast with previous reports,12,17 all subjects were examined not only for NSS, but also for involuntary movements and parkinsonism (using the well-validated AIMS35 and SAS36 scales, respectively). Because differences were observed between the two patient groups for parkinsonism, as measured by SAS, we adjusted the comparison SAS scores. We also made the decision to exclude patients with tics, who may constitute a specific group with regard to neurological condition and possibly underlying pathobiology. In previous studies, it is unclear whether patients with tics were included.4–9,12,13,17,20,22,37
Several limitations to this study should nevertheless be discussed. First, the sample size remained relatively small, despite being larger than in previous studies, and we cannot exclude a lack of power to detect differences, especially when subgrouping the patients with regard to their age at onset. Also, we were not able to analyze separately the patients with tics, although they appear to have specific neurological features. Second, the later age at onset and shorter duration of illness of patients with schizophrenia could potentially bias the results in favor of finding higher NSS scores in OCD patients. Moreover, as would be expected, the groups were different with respect to psychotropic administration. To assess the effects of medication on NSS, it was impossible to introduce, in a linear-regression analysis, either chlorpromazine-equivalents, because most of OCD patients were not under antipsychotic medication, or SSRI dose-equivalents, because most patients with schizophrenia did not take antidepressant medication, and, of course, controls were not taking any medication. However, the relationship between treatment effect and psychotropic NSS remains controversial.8 Also, when we compared patients with versus without antipsychotic and with versus without antidepressant, we did not see any significant differences. These are consistent with several other studies that found no influence of medication on NSS in OCD patients.5,17 or in patients with schizophrenia.14–16 Moreover, Hollander et al.8 report that NSSs were more common in medication-free patients with OCD than in controls. Last, the patients were not necessarily representative of all patients with OCD or schizophrenia. Although consecutively encountered, OCD patients were also selected for European Caucasian descent, and patients with schizophrenia were additionally selected by age- and gender-matching with OCD patients.
Keeping in mind these limitations,4–6,10,17 we did not replicate previous findings of an increase in NSS in OCD patients as compared with controls and rather found significantly lower NSS scores in those patients than in patients with schizophrenia. Patients with OCD had significantly lower total scores and motor coordination subscores than patients with schizophrenia (Figure 1). Our results are in line with the Bolton's study4 with a larger sample size that compared OCD patients (N=50) to patients with schizophrenia and reported fewer NSS signs, less motor-coordination impairment, tardive dyskinesia, and “extrapyramidal signs” (i.e., parkinsonism) in OCD, including increased tone in limbs, decreased associated movements in walking, shuffling gait, arm-dropping, tremor, or neck rigidity in OCD patients than in patients with schizophrenia. Also in line with their observations, we did not observe any significant difference between the OCD group and the schizophrenia group in terms of sensory integration, involuntary movements, or quality of lateralization.
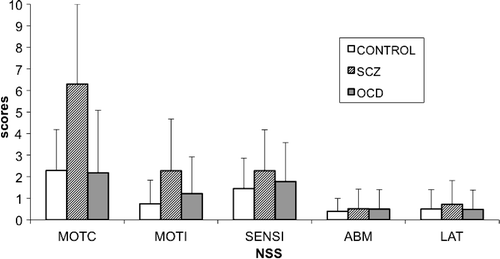
MOTC: Motor Coordination Factor; MOTI: Motor Integrative Function; SENSI: Sensory Integration Factor; ABM: Involuntary Movements Factor; LAT: Quality of Lateralization factor.
Our results suggest that the NSS we assessed in this study are more severe in patients with schizophrenia than in OCD patients; furthermore, we found no differences when comparing patients with OCD to healthy controls. Our results are in line with two others studies in which no differences in terms of NSS between patients with OCD and controls were found.12,13 However, two studies found neurological abnormalities in patients with OCD.4,8 In one of them, the authors8 found more NSS in OCD than in the control group, including impairments in fine motor coordination and visuospatial functions and also involuntary and mirror movements. In the other one, Bolton et al.4 found that OCD patients had higher NSS scores than the control group in motor coordination, sensory integration, and primitive reflexes, but also more extrapyramidal signs and deficient suppression. We did not replicate those findings. Differences in medication status could partly explain these contradictory findings. In Bolton's study, 60% of patients (versus 26% in our study) were on medication,4 with no further details on the type of medication. Noteworthy, although OCD patients had higher parkinsonism scores than controls, comparisons of NSS were not adjusted for this difference.4 In Hollander's study, patients were medication-free for at least 2 weeks (with no further details on the mean duration of withdrawal and or the type of medication). This rather short duration of withdrawal could be too limited to avoid residual neurological side effects induced by antidepressants and even more by antipsychotics.8
Several lines of evidence suggest that early age at onset of OCD could be associated with more brain anomalies.28–30 Nevertheless, to our knowledge, there has been no other study examining the relationship between NSS and age at onset in OCD. Two different definitions of age at onset can be found in the literature: 1) the age at which symptoms were first noticed by the individual and/or family member;28 2) the first time that symptoms altered functioning.29 In this study, we chose to use the former definition, since it is likely to reflect the beginning of the disorder, whereas the latter defines the onset of the syndrome and, as such, reflects the severity of the symptoms.
We found no differences in NSS scores between EO-OCD and LO-OCD groups or between EO-OCD and healthy subjects, even when adjusting for age at time of assessment (data not shown). Furthermore, no correlations were found between the severity of NSS and demographic variables such as educational level or severity of disease in the OCD group. These results are in line with several other studies,6,12,37,38 although one study has reported a positive correlation between the severity of obsession and high levels of NSS.9 However, they did not find any correlation between severity of disease (Y-BOCS score total) and NSS.
Although NSS reflect dysconnectivity in distributed networks, involving fronto-thalamo-cerebellar loops,39 the prevailing hypothesis regarding OCD suggests hyperactivity in the orbito-frontal cortex,29,40 medial caudate nucleus, thalamus, and anterior cingulate gyrus, with no argument supporting dysconnectivity.
To conclude, NSS as assessed by the current scales, comprising items that discriminate patients with schizophrenia from controls, appear to be relatively specific to schizophrenia, whereas other neurological “subtle signs,” not present in these scales, could be worth exploring in OCD patients, in particular those assessing the frontal-striatal loops. Further studies are needed to explore neurological features of OCD in more depth, using other neurological “soft” or “hard” signs and extrapyramidal symptoms, and with a closer look at the subgroup of OCD patients with tics.
1. : The epidemiology of obsessive-compulsive disorder in five U.S. communities. Arch Gen Psychiatry 1988; 45:1094–1099Crossref, Medline, Google Scholar
2. : Neurological signs and involuntary movements in schizophrenia: intrinsic to and informative on systems pathobiology. Schizophr Bull 2009; 35:415–424Crossref, Medline, Google Scholar
3. : Neurological soft signs in schizophrenia: a meta-analysis. Schizophr Bull 2010; 36:1089–1104Crossref, Medline, Google Scholar
4. : Neurological soft signs in obsessive-compulsive disorder: standardised assessment and comparison with schizophrenia. Behav Neurol 1998; 11:197–204Crossref, Medline, Google Scholar
5. : Neurological soft signs in schizophrenic patients with obsessive-compulsive disorder. Psychiatry Clin Neurosci 2004; 58:274–279Crossref, Medline, Google Scholar
6. : Schizo-obsessive and obsessive-compulsive disorder: comparison of clinical characteristics and neurological soft signs. Psychiatry Res 2006; 145:241–248Crossref, Medline, Google Scholar
7. : Neurological soft signs in schizophrenia patients with obsessive-compulsive disorder. J Neuropsychiatry Clin Neurosci 2007; 19:145–150Link, Google Scholar
8. : Signs of central nervous system dysfunction in obsessive-compulsive disorder. Arch Gen Psychiatry 1990; 47:27–32Crossref, Medline, Google Scholar
9. : A pilot follow-up study of childhood soft signs and the development of adult psychopathology. J Neuropsychiatry Clin Neurosci 1991; 3:186–189Link, Google Scholar
10. : Neurologic soft signs in obsessive-compulsive disorder. Arch Gen Psychiatry 1991; 48:278–279Crossref, Medline, Google Scholar
11. : A. E. Bennett Research Award: Toward a Neurodevelopmental Model of Obsessive-Compulsive Disorder. Biol Psychiatry 1998; 43:623–640Crossref, Medline, Google Scholar
12. : Neurological soft signs in obsessive-compulsive disorder. Neurol India 2004; 52:72–75Medline, Google Scholar
13. : Neurological soft signs in female trichotillomania patients, obsessive-compulsive disorder patients, and healthy control subjects. J Neuropsychiatry Clin Neurosci 1994; 6:184–187Link, Google Scholar
14. : Schizophrenia with good and poor outcome, III: neurological soft signs, cognitive impairment, and their clinical significance. Br J Psychiatry 1985; 46:348–357Crossref, Google Scholar
15. : Significance and meaning of neurological signs in schizophrenia. Am J Psychiatry 1988; 145:11–18Crossref, Medline, Google Scholar
16. : Schizophrenia and neurological soft signs: gender differences in clinical correlates and antecedent factors. Psychiatry Res 1996; 64:105–114Crossref, Medline, Google Scholar
17. : Neurological examination in obsessive-compulsive disorder. Sao Paulo Med J 1996; 114:1255–1258Crossref, Medline, Google Scholar
18. : Neurological soft signs in neuroleptic-naive and neuroleptic-treated schizophrenic patients and normal comparison subjects. Am J Psychiatry 1995; 152:191–196Crossref, Medline, Google Scholar
19. : Neurological examination, in Obsessive-Compulsive Disorder in Children and Adolescents. Edited by Rapoport JL. Washington, DC, American Psychiatric Press, Inc., 1989, pp 107–115Google Scholar
20. : Neurological soft signs as predictors of treatment response to selective serotonin reuptake inhibitors in obsessive-compulsive disorder. J Neuropsychiatry Clin Neurosci 2005; 17:472–477Link, Google Scholar
21. : Can a subgroup of OCD patients with motor abnormalities and poor therapeutic response be identified? Psychopharmacology (Berl) 2005; 179:826–837Crossref, Medline, Google Scholar
22. : Neurological and neuropsychological signs in obsessive-compulsive disorder: interaction with behavioural treatment. Behaviour Res Ther 2000; 38:695–708Crossref, Medline, Google Scholar
23. : Do soft signs predict treatment outcome in obsessive-compulsive disorder? J Neuropsychiatry Clin Neurosci 1995; 7:218–222Link, Google Scholar
24. : Obsessive-compulsive disorder in adolescence: an epidemiological study. J Am Acad Child Adolesc Psychiatry 1988; 27:764–771Crossref, Medline, Google Scholar
25. : Adults with early-onset obsessive-compulsive disorder. Am J Psychiatry 2001; 158:1899–1903Crossref, Medline, Google Scholar
26. : Early- versus late-onset obsessive-compulsive disorder: investigating genetic and clinical correlates. Psychiatry Res 2004; 128:175–182Crossref, Medline, Google Scholar
27. : Phenomenological and comorbid features associated in obsessive-compulsive disorder: influence of age at onset. J Affect Disord 2004; 79:241–246Crossref, Medline, Google Scholar
28. : Is juvenile obsessive-compulsive disorder a developmental subtype of the disorder? findings from an Indian study. Eur Child Adolescent Psychiatry 2003; 12:290–297Crossref, Medline, Google Scholar
29. : Is juvenile obsessive-compulsive disorder a developmental subtype of the disorder? a review of the pediatric literature. J Am Acad Child Adolesc Psychiatry 1998; 37:420–427Crossref, Medline, Google Scholar
30. : Regional cerebral blood flow abnormalities in early-onset obsessive-compulsive disorder: an exploratory SPECT study. J Am Acad Child Adolesc Psychiatry 2001; 40:347–354Crossref, Medline, Google Scholar
31. : Diagnostic interview for genetic studies: rationale, unique features, and training: NIMH Genetics Initiative. Arch Gen Psychiatry 1994; 51:849–859, 863–864Crossref, Medline, Google Scholar
32. : [French Version of the Yale-Brown Obsessive-Compulsive Scale]. Encephale 1989; 15:335–341Medline, Google Scholar
33. : The Positive and Negative Syndrome Scale (PANSS) for Schizophrenia. Schizophr Bull 1987; 13:261–276Crossref, Medline, Google Scholar
34. : Validation and factorial structure of a standardized neurological examination assessing neurological soft signs in schizophrenia. Schizophr Res 2000; 45:245–260Crossref, Medline, Google Scholar
35. : AIMS: ECDEU Assessment Manual for Psychopharmacology (ADM 76–338). U.S. Dept. of Health and Human Services, Washington, DC, 1976, pp 534–537Google Scholar
36. : A rating scale for extrapyramidal side effects. Acta Psychiatr Scand 1970; 212(suppl):11–19Crossref, Google Scholar
37. : Obsessive-compulsive disorder in hospitalized patients with chronic schizophrenia. Psychiatry Res 2001; 102:49–57Crossref, Medline, Google Scholar
38. : Phenotypic differences in early- and late-onset obsessive-compulsive disorder. Compre Psychiatry 2000; 41:373–379Crossref, Medline, Google Scholar
39. : Correlations of cerebello-thalamo-prefrontal structure and neurological soft signs in patients with first-episode psychosis. Acta Psychiatr Scand 2011; 123:451–458Crossref, Medline, Google Scholar
40. : Correlates between neurological soft signs and saccadic parameters in schizophrenia. Prog Neuro-Psychopharmacol Biol Psychiatry 2009; 15:676–681Crossref, Google Scholar



