Catatonic Disorder Due to a General Medical or Psychiatric Condition
Abstract
Identification of individuals with catatonic disorder secondary to a general medical condition (CD-GMC) may affect both acute and long-term patient management. The authors performed a 20-year retrospective cohort analysis of all patients meeting DSM-IV-TR criteria for catatonic subtypes seen at our institution. Encephalitis was the most common etiologic diagnosis among patients with CD-GMC, and lumbar puncture the test most likely to affect acute management. Univariate logistic-regression analysis utilizing Bonferroni correction for multiple comparisons yielded absence of a psychiatric history and history of clinical seizure as variables increasing the likelihood of a diagnosis of CD-GMC. Prospective evaluation across a larger patient series will be required to better identify patients with catatonia who would benefit from neurologic evaluation.
Catatonia is a neuropsychiatric syndrome characterized by psychomotor symptoms, which may include mutism, gegenhalten rigidity, catalepsy (“waxy flexibility”), echolalia, and echopraxia.1 Catatonia exists on a spectrum with malignant, life-threatening phenotypes, in which criteria may be met for neuroleptic malignant syndrome.2 Historically, the syndrome was thought to be a unique manifestation of schizophrenia; however, it is now appreciated to be most commonly associated with affective disorders.3 Increasingly, catatonia is being recognized as a feature of primary general-medical and neurologic disease states.4–6 The latter observation has been recognized by the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision (DSM-IV-TR) under the heading of catatonic disorder due to a general medical condition (CD-GMC).7
Contemporary understanding of CD-GMC is derived largely from anecdotal case report literature, of which 257 previously reported cases have been systematically reviewed by Carroll et al.5 However, dedicated, single-center case series are rare, and have only included very small numbers of patients5,8 Reported etiologies are widely varied, with infectious, inflammatory, and structural pathologies of the brain, in addition to systemic toxic-metabolic states, commonly being invoked.4–6 The diagnosis of catatonia and symptomatic treatment are often not influenced by the underlying pathophysiology.5,9–11 However, accurate identification of the catatonic subtype is critical for proper prognostication, identification of disease-specific acute therapies, and long-term therapeutic management of the underlying disease state.5,11–14
By performing a 20-year retrospective cohort analysis of all adult patients at the Mayo Clinic given a diagnosis of catatonia, we sought to describe the clinical and paraclinical features of patients with CD-GMC. Also, we attempted to identify clinical and paraclinical features that might help to identify patients eventually diagnosed with secondary etiologies of catatonia.
Methods
Subject Identification and Selection
With Mayo Clinic Institutional Review Board (IRB) approval, the Mayo Medical Index Registry was searched between the dates January 1, 1990 and December 31, 2010 for all patient charts linked to the search term “catatonia.” Our inclusion criteria were age ≥18 years at the time of diagnosis of catatonia, and fulfillment of DSM-IV-TR criteria for “catatonic disorder due to a general medical condition” (CD-GMC), “catatonic type, schizophrenia”, or “mood disorder with catatonic features.”7 Exclusion criteria included the presence of confounding diagnoses: Parkinson’s disease, neuroleptic malignant syndrome, serotonin syndrome, delirium, selective mutism, and akinetic mutism. Patients meeting the above inclusion and exclusion criteria were further divided into catatonic subtypes within the limits of the DSM-IV-TR criteria. The only modification to these criteria was for the allowance of patients meeting DSM-IV-TR criteria for schizoaffective disorder and catatonia to be included under the realm of the criteria outlined for “catatonic type, schizophrenia.” Neurologic causality was not assumed in the diagnosis of CD-GMC, and patients were classified within a psychiatric category if the catatonia was “better accounted for by another mental disorder,” and there was not evidence of a “direct physiological” relationship to the underlying secondary condition.7 Categorization required agreement of all the authors.
Data Collection
The historic medical records of all patients were exhaustively reviewed. Predetermined study variables (N=36) were recorded, including demographic, past medical, family, and social information. Treatment responses of catatonia to benzodiazepines and/or electroconvulsive therapy (ECT) were recorded in a binary fashion. Vital signs during the hospitalization were recorded as maximum values observed for heart rate, blood pressure, and core body temperature. Paraclinical variables that were recorded included the maximum creatine kinase (CK) value measured during a catatonic episode, in addition to routine indices taken from the complete blood count and basic metabolic profile at the time of presentation. Features of neurodiagnostic testing (electroencephalography [EEG]), magnetic resonance imaging (MRI), and cerebrospinal fluid (CSF) analysis) were also recorded.
EEG and MRI findings were analyzed in a dichotomous fashion, and classified as “abnormal” or “normal.” Only EEG abnormalities referable to cerebral dysfunction were classified as “abnormal” for the purpose of this study. Patients with benign EEG variants (i.e., wicket waves, small sharp spikes, and alpha variants), in addition to extracerebral artifacts (i.e., scalp muscle artifact) were considered to be “normal” for the purpose of our study. Radiographic abnormalities generally regarded as incidental were considered “normal,” including small-vessel ischemic white-matter changes, asymptomatic meningiomas, asymptomatic pituitary microadenomas, and asymptomatic chronic lacunar infarcts.
As part of a secondary analysis, patients were selected who had undergone both an ictal EEG, in addition to either a baseline pre-catatonic or a follow-up EEG after resolution of symptoms. Patients were excluded from this secondary observational analysis if they were treated with non-benzodiazepine antiepileptic medications or if there was not sufficient clinical documentation to determine that catatonia was absent during the pre- or post-EEG recording.
Statistical Analysis
All analyses were carried out using the JMP Statistical Software Package (Version 8, SAS Institute Inc., Cary, NC). Descriptive statistics were used to describe the cohort and subgroups. Continuous variables were described as means with standard deviations (SD), or median and interquartile range (IQR) for non-normal variables. Nominal variables were described as counts and percentages. The primary objective of our study was to identify variables associated with an increased risk of an underlying diagnosis of CD-GMC relative to psychiatric catatonia. Univariate logistic-regression modeling was used to test for associations. Because of the low number of CD-GMC subjects, multivariate modeling was not performed.
A Bonferroni-adjusted alpha value was chosen in order to correct for multiple comparisons (N=36). Individual results were therefore considered to be statistically significant at p ≤0.001. For exploratory purposes, unadjusted p values are reported in the Results section.
Results
Clinical and Paraclinical Cohort Features
A group of 236 unique patient charts were reviewed to accrue a final cohort with a total of 95 individuals, all meeting study inclusion and no exclusion criteria (Figure 1). DSM-IV-TR criteria were applied to these 95 patients, as described in the Methods section. The most common etiologic category was affective disorders (N=53; 55.8%), with psychotic disorders (N=22; 23.1%) and CD-GMC (N=20; 21%) representing comparable frequencies within the cohort. Major depressive disorder (MDD) was the single most frequently-occurring specific diagnosis (N=32; 33.7%).
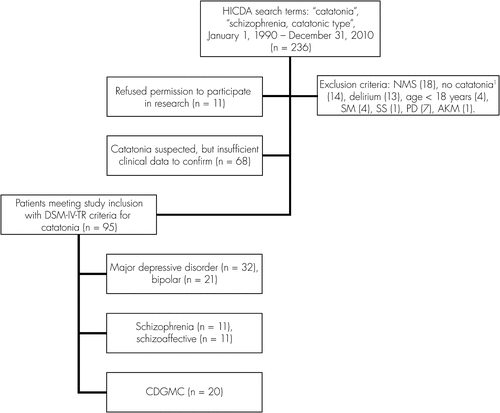
NMS: neuroleptic malignant syndrome; SS: serotonin syndrome; PD: Parkinson’s disease; SM: selective mutism; AKM: akinetic mutism.
aOther diagnoses: Conversion disorder (N=5), conduct disorder (N=2), somatization disorder (N=1), mixed personality disorder (N=1), tardive dyskinesia (N=2), hypomania (N=1), dementia (N=2).
The clinical and paraclinical features of our cohort are summarized in Table 1. The total cohort comprised 95 patients; 40% were male; with a mean age of 51.9 years (SD: 20.9) at the time of presentation. Among the patients presenting with CD-GMC, 9/20 (45%) had a history of psychiatric disease. Depression (N=7) was most commonly reported, with 4 patients having mild depressive symptoms, 2 having had a single major depressive episode, and 1 carrying a diagnosis of major depressive disorder (MDD). One additional patient was thought to have possible bipolar disorder, with insufficient chart documentation to confirm the diagnosis, and the final patient had a history of polysubstance abuse, but had been sober for at least 2 years. A history of a central neurologic problem was seen in 27/75 patients with psychiatric catatonia (36%). The most commonly reported neurologic problems were seizure (N=10), cognitive-domain difficulties (N=7), and traumatic brain injury (N=6). Patients with a history of cognitive problems could be classified as having mental retardation (N=3), probable Alzheimer’s disease (N=2), vascular dementia (N=1), and amnestic mild cognitive impairment (N=1). Less frequently reported neurologic histories included viral encephalitis (N=1), relapsing–remitting multiple sclerosis (N=1), normal-pressure hydrocephalus (N=1), bilateral, chronic subdural hematomas (N=1), and stroke (N=2).
| All Patients (N=95) | CD-GMC (N=20) | All Psychiatric (N=75) | OR: CD-GMC (95% CI) | p | |
|---|---|---|---|---|---|
| Age, years, mean (SD) | 51.9 (20.9) | 44.7 (19.5) | 53.9 (20.9) | 0.8 (0.62–1.02) | 0.08 |
| Gender (N [% Men]) | 38 (40%) | 12 (60%) | 26 (34.7%) | 2.83 (1.03–8.06) | 0.04 |
| Psychiatric history (N [%]) | 73 (81.1%) | 9 (47.4%) | 64 (90.1%) | 0.09 (0.02–0.31) | 0.0001 |
| Family history psychiatric disease (N [%]) | 50 (60.9%) | 9 (47.4%) | 41 (65.1%) | 0.48 (0.17–1.37) | NS |
| Neurologic history (N [%]) | 40 (43%) | 13 (68.4%) | 27 (36.5%) | 3.77 (1.32–11.81) | 0.016 |
| History of clinical seizure (N [%]) | 18 (19.5%) | 8 (42.1%) | 10 (13.7%) | 4.66 (1.46–14.39) | 0.008 |
| Antidepressant use at presentation (N [%]) | 44 (48.8%) | 6 (32.1%) | 38 (53.5%) | 0.4 (0.13–1.13) | 0.1 |
| Neuroleptic use at presentation (N [%]) | 41 (44.6%) | 3 (15.8%) | 38 (52%) | 0.17 (0.03–0.57) | 0.01 |
| Catatonia improved with benzodiazepines (N [%]) | 39 (76.5%) | 6 (54.5%) | 33 (82.5%) | 0.25 (0.06–1.09) | 0.06 |
| Catatonia improved with ECT (N [%]) | 60 (94.1%) | 10 (83.3%) | 50 (96.1%) | 2.16 (0.02–1.82) | NS |
| Abnormal MRI (all; N [%]) | 31 (37.8%) | 11 (64.7%) | 20 (30.8%) | 4.12 (1.37–13.47) | 0.01 |
| Focal MRI abnormality (N [%]) | 16 (19.5%) | 7 (41.1%) | 9 (13.8%) | 4.36 (1.3–14.63) | 0.02 |
| Abnormal EEG (all; N [%]) | 54 (79.4%) | 15 (93.7%) | 39 (75%) | 5 (0.87–94.7) | NS |
| Diffuse slowing (all; (N [%]) | 44 (86.3%) | 13 (92.8%) | 31 (83.8%) | 2.51 (0.38–50) | NS |
| Focal temporal slowing (N [%]) | 12 (23.5%) | 5 (35.7%) | 7 (19.1%) | 2.4 (0.58–9.44) | NS |
| EEG asymmetry (N [%]) | 12 (23.5%) | 6 (42.8%) | 6 (16.2%) | 3.87 (0.98–15.89) | NS |
| Maximum creatinine kinasea (median [IQR]) | 138 (59–576) (N=56) | 135 (51–397) (N=13) | 156 (60–653) (N=43) | 0.84 (0.51–1.27) | NS |
In an attempt to protect against the identification of false-positive results, given multiple comparisons, a Bonferonni-adjusted significance level of p≤0.001 was utilized. A history of psychiatric disease (OR: 0.09; 95% CI: 0.02–0.31) was associated with a decreased odds ratio for a diagnosis of CD-GMC. A history of a clinical seizure (OR: 4.66; 95% CI: 1.46–14.39) was associated with an increased odds ratio for a diagnosis of CD-GMC, a result of only borderline statistical significance (p=0.008), given the conservative adjustment.
Additional clinical and paraclinical variables were assessed in the univariate logistic-regression model, which yielded a p value ≥0.5. Specifically, these included benzodiazepine use at presentation; histories of venous thrombosis, mental retardation, or head trauma; alcohol or tobacco use at presentation; and maximum heart rate, blood pressure, and temperature recorded during the episode. Additional paraclinical variables included laboratory indices at the time of presentation, and other descriptors of neurodiagnostic evaluation (e.g., “diffuse MRI abnormality,” “focal frontal EEG slowing”).
The etiologies underlying our 20 cases of CD-GMC (see Table 2) included encephalopathies (N=9), dementia (N=4), developmental (N=3), and other (N=4). The specific diagnoses were the following: acute encephalitis (N=4), paraneoplastic limbic encephalitis (N=1), chronic lymphocytic encephalomyelitis of unknown etiology (N=1), Hashimoto’s encephalopathy (N=1), corticosteroid-responsive autoimmune encephalopathy (N=1), relapsing encephalopathy of unknown etiology, suspect metabolic (N=1), probable behavioral-variant frontotemporal dementia (N=3), Lewy-body dementia (N=1), pervasive developmental disorder (N=1), Asperger’s syndrome (N=1), 22q13.3 microdeletion syndrome (periodic catatonia) (N=1), central nervous system lung cancer metastases (N=1), postictal psychosis with catatonia (N=2; one case, with simultaneous cannabinoid intoxication); and treatment-refractory epilepsy (N=1).
| Age/Gender | Case Notes | Response to Therapy | Status at Last Follow-Up | |
|---|---|---|---|---|
| Encephalitis/encephalopathy (N=9) | ||||
| Acute encephalitis: #1 | 45/Male | Prodrome of fever, headache, and myalgias; CSF: leukocytes, 18 (96% lymphocytes); protein, 59; glucose normal | Neither Ativan nor ECT attempted. Improved with supportive measures. | Back to normal at 5-month follow-up. |
| Acute encephalitis: #2 | 18/Male | Aggressive-disinhibited behavior noted. CSF: Leukocytes, 14 (92% lymphocytes); protein and glucose normal. | Ativan not attempted; catatonia responded well to ECT. | Back to normal at hospital dismissal; no long-term follow-up. |
| Acute encephalitis: #3 | 31/Female | CSF: Leukocytes, 53 (98% lymphocytes); protein, 50; glucose normal. | Ativan not attempted; catatonia responded well to ECT. | Full recovery at 5-month follow-up. |
| Acute encephalitis: #4 | 29/Male | CSF: Leukocytes, 55 (96% lymphocytes); protein, 40; glucose normal. | Improved initially with Ativan, and then dramatically with ECT. | Improving at hospital dismissal; no long-term follow-up. |
| Paraneoplastic limbic encephalitis | 37/Female | History of idiopathic generalized epilepsy, controlled on Phenytoin since age 16. Presented during pregnancy at 9 weeks’ gestation; 10-cm adrenal cortical carcinoma found in evaluation of catatonia. | Catatonic state unchanged after tumor removal; catatonia improved with ECT. | Back to baseline; no relapses at 4-year follow-up. |
| Chronic lymphocytic encephalomyelitis of unknown etiology | 23/Male | History of brainstem encephalitis at age 17, with progressive lymphocytic gray- and white-matter inflammatory disorder of unknown etiology. Catatonic syndrome developed after taper of steroids. | ECT was not attempted out of concern for respiratory status. Only minimal improvement noted with high-dose intravenous steroids. | Significant cognitive and motor impairment, without definite catatonia, at 10-year follow-up. |
| Hashimoto’s encephalopathy | 52/Female | Presented with relapsing psychotic encephalopathy with catatonia. CSF: 5 monocytes; protein, 235; glucose normal. Antimicrosomal antibodies: 1:6,400; MRI: Nonspecific white-matter hyperintensities. Brain biopsy showed mild, chronic lymphocytic inflammation, without specific diagnosis. | Improvement with both Ativan and ECT. Catatonia noted to worsen after steroid dose decreased. Improvement with pulse doses of IV methylprednisolone. | No recurrence of catatonia at 1-year follow-up; on chronic immunosuppression. |
| Steroid-responsive encephalopathy with autoimmune thyroiditis | 65/Female | Presentation with catatonia associated with multifocal myoclonus. EEG showed generalized atypical triphasic waves and multifocal spikes. Brain biopsy showed nonspecific, predominantly subcortical gliosis and microglial activation. CSF: protein, 50; leukocytes normal; NSE: 28.5; serum thyroperoxidase: 89 (normal: <40). | Initial improvement with first three ECT treatments, then declining over subsequent five treatments. Ativan not attempted. Catatonia resolved with high-dose methylprednisolone. | Alive at 5 years, without recurrence of catatonia. |
| Relapsing encephalopathy of unknown etiology; suspect metabolic | 44/Male | Familial microcytic anemia and mildly elevated liver enzymes; seizure disorder since age 16. Found on one occasion to have elevated branched chain amino acids, but no diagnosis was made despite extensive evaluation. | Ativan not attempted; improved with ECT. | No long-term follow-up. |
| Dementia (N=4) | ||||
| Probable bvFTD: 1 (rapidly progressive) | 73/Male | 3-month progression of diminished speech output, obsessive-compulsive symptoms, and behavioral outbursts. PET (18FDG): moderately-to-severely reduced uptake in the fronto-temporal lobes (left > right). | No improvement with either Ativan or ECT. | Not alive 2 years after onset, with catatonic symptoms persisting. |
| Probable bvFTD 2 | 68/Male | 5-year progression of aphasic dementia and behavioral dyscontrol. PET (18FDG): marked fronto-temporo-parietal hypometabolism (left > right). | Neither Ativan nor ECT attempted; spontaneous resolution of catatonia with supportive measures. | Not alive 1 year after catatonic episode; managed by hospice with comfort measures only. |
| Probable bvFTD 3 (rapidly progressive) | 65/Male | 9-month history of progressive abulia, personality changes, and changes in emotional expression. MRI: bilateral temporal greater than generalized atrophy. | No therapy attempted. | No long-term follow-up. |
| Lewy-body dementia | 77/Male | Preceding history of cognitive decline (greatest difficulty with visuospatial functioning), mild parkinsonism, and REM behavior sleep disorder. | No improvement with Ativan; improved with ECT. | Not living at 7-year follow-up; no documented recurrence. |
| Developmental (N=3) | ||||
| Pervasive developmental disorder | 19/Male | 2-month history of “freezing spells” lasting up to 2 hours, resolving spontaneously, leading to more-protracted episode; had actually developed exposure keratitis as a result of inadequate eye closure. | Ativan not attempted; some improvement noted after 2 ECT treatments; subsequent 13 treatments were not helpful. | No long term follow-up available. |
| Asperger’s syndrome | 19/Male | 2-year history of episodic catatonia. | Improvement with both Ativan and ECT. | No long-term follow-up available. |
| 22q13.3 microdeletion syndrome (periodic catatonia) | 23/Female | Global developmental delay; nonverbal throughout life, but no behavioral problems until age 18, when irritability, aggressive behaviors, and periodic catatonia (every 6–8 weeks) were noted. | Improvement with Ativan; ECT reported not to help in the past. | No long-term follow-up. |
| Other (N=4) | ||||
| CNS lung cancer metastases | 66/Female | Known metastatic non-small cell lung cancer, to right cerebellum and left parietal lobe; both increased in size along with catatonic presentation. | Improved with Ativan. Documentation insufficient to judge clinical effect of steroids on catatonia. | Not alive at 3-year follow-up; no documented recurrence. |
| Postictal psychosis progressing to catatonia | 47/Female | 30-year history of treatment-refractory complex partial epilepsy. | Improved with Ativan; ECT not attempted. | No recurrence at 3-year follow-up. |
| Postictal catatonia; simultaneous cannabinoid intoxication. | 51/Female | Distant history of traumatic brain injury and complex partial epilepsy; not on any seizure medicines because of seizure freedom for 10 years. Brought to hospital after an unwitnessed episode of loss of consciousness with urinary incontinence, amnesia. Toxicology positive for cannabinoids. EEG showed bilateral independent temporal sharp waves (left > right). | Improved with Ativan; ECT not attempted. | No recurrence of catatonia at 3-year follow-up. Seizure free on Keppra. |
| Treatment-refractory epilepsy s/p left-anterior temporal lobectomy. | 42/Male | Catatonia developed in the setting of epilepsy-monitoring unit evaluation while off seizure medicines (phenytoin and carbamazepine) for 2 days. No epileptiform correlate to catatonia. | Epilepsy medications restarted, without acute load. No improvement with Ativan; dramatic improvement after 2 ECT treatments. | No recurrence at 6-year follow-up. |
Neurodiagnostic Testing in Catatonia
In all, 31 patients underwent MRI imaging of the head, which most commonly demonstrated diffuse findings (N=18) of mild-to-moderate generalized atrophy. More focal atrophy was seen in 4 patients, bifrontal in 2, and cerebellar in another 2. Focal encephalomalacia (N=7) was also seen, most frequently in patients with a history of head trauma or stroke. These latter changes were seen in the frontal (N=2), temporal (N=3), and parietal (N=2) cortical distributions. Finally, multifocal changes (N=3) were seen, in 1 patient with non-enhancing multiple sclerosis lesions, in typical periventricular and subcortical locations, without cortical atrophy; in another patient with CNS metastases involving the left parietal lobe and right cerebellum; and, finally, in an individual with chronic encephalomyelitis involving mainly the anterior left parietal lobe, brainstem, and right cerebellum.
Features of 70 independent EEG recordings from 53 patients during clinical catatonia are summarized in Table 3. The most common abnormality seen was generalized, symmetric slowing, most often in the theta frequency, but not uncommonly involving mixed theta/delta slowing. Superimposed focal slowing was not uncommon, and involved the temporal lobes more commonly than the frontal lobes. Generalized triphasic waves were more rarely seen. Asymmetry was seen in 9 recordings, most often involving left greater than right slowing. Finally, epileptiform abnormalities were seen in 6 patients, 2 of whom developed status epilepticus after ECT, and the remainder in patients with a history of a clinical seizure disorder.
| Generalized slowing (N=57) |
| Theta (N=26) |
| Mixed theta/delta (N=23) |
| Delta (N=8) |
| Focal slowing (N=26) |
| Temporal (N=16), including TIRDA (N=4), and bitemporal slowing (N=7) |
| Frontal (N=8), including FIRDA (N=3) |
| Occipital (N=2) |
| Generalized triphasic waves (N=3) |
| Typical (N=1), atypical (N=2) |
| Asymmetry (N=9) |
| Left > right slowing (N=8) |
| Epileptiform activity (N=6) |
| Generalized spike and wave (N=2) |
| Focal sharp waves and/or spikes (N=4); frontal (N=1), temporal (N=3), parietal (N=1), central (N=2) |
Nine patients were identified who had undergone an ictal EEG recording, in addition to having had either a baseline or follow-up EEG study. Baseline EEGs had been documented in only 3 patients at a mean time lapse of 3.3 years preceding the catatonia (range: 1 month to 82 months). At baseline, 1 patient had diffuse mixed theta/delta slowing; 1 had focal bi-temporal slowing; and 1 had a normal EEG. Follow-up EEGs were obtained in 6 patients at a mean time of 5.2 months after the ictal catatonic EEG recording (range: 5 days to 16 months). Overall, with the clinical development of catatonia, either worsening (N=2), or persistent (N=1) changes were seen. An improved EEG pattern was not seen in any of the 3 patients. With the clinical resolution of catatonia, all 6 patients showed improvement in at least 1 EEG abnormality, most commonly generalized slowing, but also resolution of frontal intermittent rhythmic delta activity (FIRDA) in 1 patient. However, complete improvement was not the rule, and persistent bi-temporal slowing was seen in 1 patient, and generalized slowing was seen in another.
Discussion
The correct diagnostic subcategorization of catatonia is critical for proper prognostication, identification of disease-specific acute therapies, and long-term therapeutic management of the underlying disease state.5,11–14 Clinically, the identification of individuals with a diagnosis of CD-GMC versus psychiatric catatonia is challenging because of the lack of a standardized approach and the marked clinical overlap, which may be seen in both clinical presentation and response to symptomatic catatonia therapy.5,9,12,15 Previously proposed discriminating features include response to an amobarbital interview and an initially abnormal EEG.9,16 In our study, we identified the absence of a psychiatric history and a history of clinical seizure as candidate covariates that may be helpful in identifying individuals to screen for CD-GMC. Unfortunately, no clinical or paraclinical variable appeared to have sufficient discriminating value to rely upon. However, a major limitation to our study was the small number of patients analyzed, and our analysis should be therefore viewed as exploratory.
Additional limitations of our study included the use of a retrospective methodology, selection bias from analysis of a cohort from a tertiary-referral center, and the fact that diagnostic testing was not uniform across our cohort (i.e., patients with psychiatric catatonia may have had unrecognized neurologic disease). An additional limitation imposed by the retrospective methodology is the difficulty in accounting for all variables that may have confounded interpretation of the EEG findings (i.e., concurrent medication exposures and recent electroconvulsive therapy). Finally, our study was insufficiently powered to perform more detailed multivariate analysis and to analyze patients with different psychiatric diagnoses as distinct pathologic entities.
Caution must be emphasized in the interpretation of our findings, as clinical overlap is often the rule. We, as other authors have appreciated previously, note the common occurrence of a psychiatric history in those presenting with CD-GMC. We have also found the reciprocal observation to be equally likely, that of a neurologic history in those with psychiatric catatonia. Short of a medical evaluation of every patient presenting with catatonia, longitudinal clinical follow-up may be the single most helpful discriminating tool available to the clinician. In our own cohort, analysis of the spinal fluid was the test most likely to affect the therapy of the patient in the acute hospital setting, reflecting the relatively high frequency of encephalitis, which is concordant with the observations of other authors.5 MRI and EEG abnormalities were common, but only changed the immediate diagnostic/therapeutic strategy in a minority of patients.
Hundreds of diverse etiologies have been reported in the case report literature, without insight into what the relative frequency of diagnoses might be among a large, single-institution cohort.5,8 The percentage of CD-GMC among all patients with catatonia in our series was 21% (20/95), comparable to what has been reported by other authors.8,17,18 Etiologies were diverse, generally diffuse cortical processes, and encephalitis was the most common. Encephalitis was also observed to be among the most common etiologies of CD-GMC in the systematic literature review by Carroll, et al,5 and catatonia appears to occur independently of the specific inciting pathophysiology, which may include infectious, paraneoplastic, and inflammatory mechanisms.4
An association with behavioral-variant frontotemporal dementia was noted in three individuals, which is of interest, as frontal lobe dysfunction has been cited as an anatomic substrate underlying several clinical sequelae of catatonia.19–21 We note that two of these individuals had a seemingly rapid progression of their syndrome. Cases have previously been reported in the literature of frontal lobe atrophy without identification of an etiology, in addition to frontal lobe meningiomas, associated with the catatonic syndrome.22,23 The association of the 22q13.3 chromosomal microdeletion and periodic catatonia in one patient is notable, as polymorphisms in the MLC1 gene located in this region have been linked to the syndrome.24 Postictal psychosis and catatonia after a seizure were seen in two individuals, but in no patient was catatonia a direct manifestation of epileptiform activity. Other authors have observed a syndrome indistinguishable from catatonia as an ictal epileptic phenomenon.25,26 The therapeutic response of catatonia to benzodiazepines and ECT has prompted consideration that seizure activity may underlie catatonia; however; the therapeutic mechanisms of action are still not well understood.1 Recent data from Richter et al., utilizing BOLD-fMRI, suggest that lorazepam may modulate GABAergic activity in the orbitofrontal cortex during the processing of emotional stimuli by patients with catatonia.27
We report the spectrum of ictal catatonic EEG results that may be encountered in the largest series to-date. Generalized theta to delta range slowing was the most common abnormality, although superimposed focal slowing was also common in the frontal and/or temporal lobes. Carroll and Boutros9 have noted that the initial EEG may be normal in catatonia, and become abnormal along with the clinical evolution of the episode. Our study did not confirm the previously reported observation that CD-GMC patients would harbor an increased likelihood of an initial EEG abnormality.9
Our observational-serial EEG analysis in a small number of patients with ictal catatonic EEG abnormalities supports EEG abnormalities as a biomarker of catatonia itself, and not just the underlying disease state (i.e., schizophrenia). As reviewed by Abenson,15 EEG abnormalities have been occasionally noted to occur more commonly in catatonic relative to paranoid schizophrenia. Furthermore, in the progression of periodic catatonia, the alpha frequency has been noted to increase, along with a dampening of the amplitude.28,29
Conclusions
Clinical and paraclinical features may help clinicians identify individuals presenting with catatonia who should be evaluated further for a secondary medical or neurologic process. In our exploratory analysis, absence of a psychiatric history and a history of clinical seizure were associated with an increased risk for a diagnosis of CD-GMC relative to psychiatric catatonia. Encephalitis occurred at a high frequency among patients with CD-GMC, and spinal fluid analysis proved the most useful test augmenting the therapeutic strategy in the acute setting. Our data support the use of EEG abnormalities as a biomarker of the catatonic state, and not just the underlying etiology.
1 : Catatonia: clinical aspects and neurobiological correlates. J Neuropsychiatry Clin Neurosci 2009; 21:371–380Link, Google Scholar
2 : Malignant catatonia. J Neuropsychiatry Clin Neurosci 1994; 6:1–13Link, Google Scholar
3 : Catatonia is not schizophrenia: Kraepelin’s error and the need to recognize catatonia as an independent syndrome in medical nomenclature. Schizophr Bull 2010; 36:314–320Crossref, Medline, Google Scholar
4 : Organic catatonia. J Am Acad Child Adolesc Psychiatry 2000; 39:1464Crossref, Medline, Google Scholar
5 : Catatonic disorder due to general-medical conditions. J Neuropsychiatry Clin Neurosci 1994; 6:122–133Link, Google Scholar
6 : The catatonic syndrome. Lancet 1976; 1:1339–1341Crossref, Medline, Google Scholar
7 First MB, Tasman A: DSM-IV-TR Mental Disorders: Diagnosis, Etiology, and Treatment. Hoboken, NJ, Wiley, 2004.Google Scholar
8 : The syndrome of Karl Ludwig Kahlbaum. J Neurol Neurosurg Psychiatry 1986; 49:991–996Crossref, Medline, Google Scholar
9 : Clinical electroencephalograms in patients with catatonic disorders. Clin Electroencephalogr 1995; 26:60–64Crossref, Medline, Google Scholar
10 : Catatonic signs in medical and psychiatric catatonias. CNS Spectr 2000; 5:66–69Crossref, Medline, Google Scholar
11 : Multidisciplinary approach of organic catatonia in children and adolescents may improve treatment decision-making. Prog Neuropsychopharmacol Biol Psychiatry 2008; 32:1393–1398Crossref, Medline, Google Scholar
12 : Catatonia on the consultation–liaison service. Psychosomatics 1992; 33:310–315Crossref, Medline, Google Scholar
13 : Association of adolescent catatonia with increased mortality and morbidity: evidence from a prospective follow-up study. Schizophr Res 2009; 113:233–240Crossref, Medline, Google Scholar
14 : Catatonic schizophrenia: epidemiology and clinical course: a 7-year register study of 798 cases. J Nerv Ment Dis 1974; 158:291–305Crossref, Medline, Google Scholar
15 : EEGs in chronic schizophrenia. Br J Psychiatry 1970; 116:421–425Crossref, Medline, Google Scholar
16 : The amobarbital interview in the differential diagnosis of catatonia. Psychosomatics 1982; 23:437–438Crossref, Medline, Google Scholar
17 : Organic factors in catatonia. Br J Psychiatry 1986; 149:782–784Crossref, Medline, Google Scholar
18 : Catatonia, I: rating scale and standardized examination. Acta Psychiatr Scand 1996; 93:129–136Crossref, Medline, Google Scholar
19 : Orbitofrontal cortical dysfunction in akinetic catatonia: a functional magnetic resonance imaging study during negative emotional stimulation. Schizophr Bull 2004; 30:405–427Crossref, Medline, Google Scholar
20 : Catatonia in the brain: a localization study. Int J Neuropsychiatry 1965; 1:395–403Medline, Google Scholar
21 : Disturbed neural circuits in a subtype of chronic catatonic schizophrenia demonstrated by F-18-FDG-PET and F-18-DOPA-PET. J Neural Transm 2001; 108:661–670Crossref, Medline, Google Scholar
22 : Catatonia with frontal lobe atrophy. J Neurol Neurosurg Psychiatry 1980; 43:185–187Crossref, Medline, Google Scholar
23 : Three cases of frontal meningiomas presenting psychiatrically. BMJ 1968; 3:9–16Crossref, Medline, Google Scholar
24 : MLC1 polymorphisms are specifically associated with periodic catatonia, a subgroup of chronic schizophrenia. Biol Psychiatry 2007; 61:1211–1214Crossref, Medline, Google Scholar
25 : Ictal catatonia as a manifestation of nonconvulsive status epilepticus. J Neurol Neurosurg Psychiatry 1986; 49:833–836Crossref, Medline, Google Scholar
26 : Ictal catatonia as a manifestation of de novo absence status epilepticus following benzodiazepine withdrawal. Seizure 1999; 8:364–366Crossref, Medline, Google Scholar
27 : Lorazepam modulates orbitofrontal signal changes during emotional processing in catatonia. Hum Psychopharmacol 2010; 25:55–62Crossref, Medline, Google Scholar
28 : Electroencephalographic changes in a typical case of periodic catatonia. Acta Psychiatr Neurol Scand 1957; 32:50–57Crossref, Medline, Google Scholar
29 : The EEG in three cases of periodic catatonia. Br J Psychiatry 1967; 113:1271–1282Crossref, Medline, Google Scholar



