Does Tardive Dysmentia Really Exist?
Abstract
Tardive dysmentia has been described as the behavioral equivalent of tardive dyskinesia in patients with schizophrenia. Its association with tardive dyskinesia and psychopathology has been controversial. The authors assessed 123 inpatients with chronic schizophrenia for presence of tardive dysmentia symptoms/signs. Psychopathology and tardive dyskinesia were also assessed. Of the group, 24 patients (19.5%) had at least one tardive dysmentia symptom/sign, whereas only 1 patient (0.8%) fulfilled the syndromal criteria for tardive dysmentia. Those with tardive dysmentia had higher psychopathology scores, higher Abnormal Involuntary Movement Scale total scores, higher number of women and family psychiatric illness, and higher rates of persistent tardive dyskinesia. Tardive dysmentia symptoms/signs are frequently seen in chronic schizophrenia, but the complete syndrome may be rare.
Burnett et al.1 noted that certain chronic schizophrenic patients with severe tardive dyskinesia presented with behavior resembling that of hypomania as well as schizophrenia. These patients tended to be loquacious and to speak in a loud voice. Wilson et al.2 studied this phenomenon in 29 schizophrenia patients and coined the term tardive dysmentia as the behavioral equivalent of tardive dyskinesia. It is described as a behavioral syndrome in schizophrenia characterized by a triad of symptoms/signs: alteration in 1) affect; 2) activation level; and 3) interpersonal interaction.
Since the time term tardive dysmentia was coined by Wilson et al.,2 research papers have been published questioning the validity of the term. Mukherjee3 reassessed the concept of tardive dysmentia and discouraged the use of “dysmentia” on phenomenological grounds. Also, the association with tardive dyskinesia was challenged, based on the observation that such syndrome was absent in bipolar patients with tardive dyskinesia after long-term exposure to antipsychotics.3 Mukherjee and Bilder4 reported that there is no evidence that the syndrome is “tardive” or “iatrogenic” in nature and that it may be a manifestation of schizophrenia. Perenyi et al.5 found this phenomenon to be very rare and suggested retaining the term tardive dysmentia until a better characterization is found.
Tardive dysmentia has been associated with various risk factors. Studies have related tardive dysmentia with the severity of tardive dyskinesia,2 duration of antipsychotic exposure,2 and phenomenology and pathophysiology of schizophrenia.3 There is lack of consensus on whether the tardive dysmentia is primarily related to disease process or is iatrogenic in origin. Furthermore, although various risk factors for tardive dysmentia have been suggested in patients with schizophrenia, only a small proportion develops this condition. Understanding these risk factors would have far-reaching implications on prognosis, treatment, and understanding of the disorder. Only a handful of studies on tardive dysmentia have been systematically done to-date. Therefore, we aimed to assess the prevalence of tardive dysmentia and its relationship with tardive dyskinesia and psychopathology in patients with chronic schizophrenia.
Methods
Sample
This was cross-sectional, hospital-based study conducted at Institute of Mental Health (IMH) and Hospital, Agra, India. The study was approved by the institutional ethics committee. The sample consisted of male and female inpatients fulfilling criteria for schizophrenia according to ICD-10 Diagnostic Criteria for Research,6 having chronic illness (duration of illness of 2 years or more) and giving written informed consent. Those with comorbid mental retardation or major medical or neurological illness were excluded from the study.
Assessment Tools
A datasheet specially designed for the study was used to collect sociodemographic and clinical details of the patients. To diagnose tardive dysmentia, Wilson et al.2 criteria were used. Emotional behavior was rated on five symptoms/signs: 1) depressed-to-euphoric mood; 2) stable-to-labile or unstable mood; 3) quiet-to-loud speech; 4) paucity-to-excess of words; and 5) retreat-to-approach to the examiner, using 100-mm line tests as described by Wilson et al.2 To assess schizophrenic psychopathology, the 30-item Positive and Negative Syndrome Scale (PANSS)7 was used. The PANSS is based on the premise that schizophrenia has two distinct syndromes, a positive syndrome including delusions, hallucinations, and so forth, and a negative syndrome, involving features such as affective flattening and alogia. To characterize tardive dyskinesia, the Abnormal Involuntary Movement Scale (AIMS),8 a 12-item instrument utilized to provide a numeric measure to the observed abnormal movements in different parts of the body, was used. The information is scored on a 5-point scale (0: none; 4: severe). Test–retest reliability ranges from 0.40 to 0.82 for individual items and is 0.71 for overall severity. Research diagnoses for tardive dyskinesia9 were used to classify tardive dyskinesia subtype. These has been suggested to help study the epidemiology, etiology, and treatment of tardive dyskinesia patients. Tardive dyskinesia is divided into six groups: probable tardive dyskinesia, masked probable tardive dyskinesia, transient tardive dyskinesia, withdrawal tardive dyskinesia, persistent tardive dyskinesia, and masked persistent tardive dyskinesia.9
Procedures
Case record files of inpatients of the IMH, Agra were screened to identify patients with schizophrenia. For those fulfilling inclusion criteria, a detailed mental status examination was done to confirm the diagnosis, after obtaining written informed consent. Sociodemographic and clinical details were recorded in the semistructured datasheet. Detailed physical and neurological examination was done to rule out medical comorbidity. Tardive dysmentia was assessed using Wilson et al.2 criteria to characterize two groups: schizophrenia patients with and without tardive dysmentia. All the patients were rated on PANSS, AIMS, and Global Assessment of Functioning (GAF). Tardive dyskinesia was classified using Schooler and Kane criteria.9 The ratings were done by two authors, SL and DMSR. To assess interrater reliability, the first 25 patients were assessed by both raters.
Statistical Analysis
Data obtained were analyzed with the Statistical Package for Social Sciences (SPSS) Version 10.0 for Windows. Normality of data were examined with the Shapiro-Wilk test statistic. To examine interrater reliability, Spearman’s rank correlation coefficient was calculated for 25 patients. To compare patients with or without tardive dysmentia, the Mann-Whitney U test and Pearson’s chi-square test were used for continuous and categorical variables, respectively. A p value of <0.05 (two-tailed) was considered significant.
Results
Sample characteristics as described by the Mann-Whitney U test and Pearson’s chi-square (with effect size) test are summarized in Table 1 and Table 2. The mean age of patients was 35.11 (standard deviation [SD]: 9.42; range: 19–62) years, and mean illness duration was 9.32 (SD: 6.36; range: 2–40) years. Mean level of formal education was 8.23 (SD: 4.69) years. Ninety-one patients (74%) were men; 53 (43.1%) were single; 34 (27.6%) were unemployed before admission to the hospital; 84 (68.3%) were of lower socioeconomic status; and 75 (61%) were from a rural background.
| Without TDm (N=99) | With TDm (N=24) | Mann- Whitney U (Z) | p | |
|---|---|---|---|---|
| Age, years | 34.82 (9.10) | 36.29 (10.76) | 1,127.5 | 0.699 |
| Median (range) | 34 (42) | 34 (38) | (−0.39) | |
| Education years | 8.13 (4.56) | 8.63 (5.31) | 1,108.0 | 0.607 |
| Median (range) | 8 (17) | 9 (17) | (−0.51) | |
| Illness duration, years | 9.09 (6.45) | 10.29 (5.98) | 1,002.5 | 0.235 |
| Median (range) | 7 (38) | 9.50 (23.5) | (−1.19) | |
| PANSS Positive | 9.93 (4.30) | 12.33 (5.47) | 815.5* | 0.014 |
| Median (range) | 8 (20) | 12 (25) | (−2.47) | |
| PANSS Negative | 12.06 (7.77) | 11.29 (6.04) | 1,163.0 | 0.868 |
| Median (range) | 8 (28) | 8.5 (20) | (−0.17) | |
| PANSS General psychopathology | 22.04 (7.67) | 22.83 (5.07) | 919.5 | 0.085 |
| Median (range) | 18 (43) | 20.5 (16) | (−1.72) | |
| PANSS Total | 43.89 (17.80) | 46.46 (14.15) | 928.5 | 0.097 |
| Median (range) | 35 (97) | 43 (56) | (−1.66) | |
| AIMS Total | 0.42 (1.86) | 0.96 (2.49) | 1,054.5 | 0.059 |
| Median (range) | 0 (10) | 0 (9) | (−1.89) | |
| Antipsychotics dose (chlorpromazine equivalent) | 384.34 (214.95) | 333.33 (208.86) | 995.5 | 0.213 |
| Median (range) | 300 (1400) | 300 (850) | (−1.25) |
| Without TDm (N=99) | With TDma (N=24) | χ2 | p | Effect size (φ) | ||
|---|---|---|---|---|---|---|
| Sex | Male | 78 (78.8) | 13 (54.2) | 6.08* | 0.014 | 0.222 |
| Female | 21 (21.2) | 11 (45.8) | ||||
| Marital status | Single | 43 (43.4) | 10 (41.7) | 0.03 | NS | — |
| Married | 56 (56.6) | 14 (58.3) | ||||
| Occupation | Unemployed | 24 (24.2) | 10 (41.7) | 2.93 | 0.087 | 0.154 |
| Employed | 75 (75.8) | 14 (58.3) | ||||
| SES | Lower | 68 (68.7) | 16 (66.7) | 0.04 | NS | — |
| Middle | 31 (31.3) | 8 (33.3) | ||||
| Family type | Nuclear | 25 (25.3) | 6 (25) | 0.01 | NS | — |
| Joint | 74 (74.7) | 18 (75) | ||||
| Domicile | Rural | 59 (59.6) | 16 (66.7) | 0.41 | NS | — |
| Urban | 40 (40.4) | 8 (33.3) | ||||
| Schizophrenia subtype | Paranoid | 58 (58.6) | 15 (62.5) | 0.12 | NS | — |
| Nonparanoid | 41 (41.4) | 9 (37.5) | ||||
| Comorbid substance dependence | Absent | 53 (53.5) | 18 (75) | 3.65 | 0.056 | 0.172 |
| Present | 46 (46.5) | 6 (25) | ||||
| Family psychiatric illness | Absent | 83 (83.8) | 12 (50) | 12.58** | <0.001 | 0.320 |
| Present | 16 (16.2) | 12 (50) | ||||
| TD (Schooler & Kane)9 | Absent | 96 (97) | 20 (83.3) | 6.69* | 0.01 | 0.233 |
| Persistent | 3 (3) | 4 (16.7) | ||||
Interrater reliability, as seen using Spearman’s rank coefficient of correlation, was the following: PANSS Positive score, 0.97 (p<0.001); PANSS Negative score, 0.96 (p<0.001); PANSS General psychopathology score, 0.89 (p<0.001); PANSS Total score, 0.93 (p<0.001); AIMS total score, 0.70 (p<0.001); euphoria, 0.97 (p<0.001); unstable mood, 0.95 (p<0.001); loud speech, 0.90 (p<0.001); excessive words, 0.87 (p<0.001); and approach to examiner, 0.89 (p<0.001).
Mean PANSS Positive scores were significantly higher in patients with tardive dysmentia symptoms/signs than those without (p=0.014), and there was a trend toward higher scores in PANSS Total score and General psychopathology scores. A trend toward higher mean AIMS total scores was also seen in patients with tardive dysmentia symptoms/signs than those without (p=0.059). There was no difference in illness duration and antipsychotics dose between the two groups. Women were significantly more frequent among those with tardive dysmentia symptoms/signs than those without (p=0.014), although the effect size was small. Also, family psychiatric illness was higher in the tardive dysmentia symptoms/signs group (p<0.001), with moderate effect size. Furthermore, trend toward a higher (16.7%) rate of persistent tardive dyskinesia was noted in those with tardive dysmentia symptoms/signs than those without (p=0.1), with small effect size.
The frequency of tardive dysmentia symptoms/signs were the following: 9 (7.3%) had euphoria; 7 (5.7%) had unstable mood; 17 (13.8%) had loud speech; 12 (9.8%) had excessive words; and 3 (2.4%) had approach to examiner. Ninety-nine (80.5%) had no symptoms/signs; 24 (19.5%) had at least one symptom/sign; 16 (13%) had at least two symptoms/signs; 6 (4.9%) had at least three symptoms/signs; 2 (1.6%) had four symptoms/signs; and none had all five symptoms/signs. Only one patient (0.8%) fulfilled the Wilson et al.2 criteria for tardive dysmentia, that is, having all three symptoms/signs: unstable mood, loud speech, and approach to the examiner.
Spearman’s rank correlation between individual tardive dysmentia symptoms/signs with psychopathology showed a significant positive correlation of PANSS Positive symptoms with unstable mood (rs=0.57; p=0.004) and excessive words (rs=0.52; p=0.009; Figure 1[A] and Figure 1[B]), and a trend toward positive correlation with loud speech (rs=0.39; p=0.06) and approach to examiner (rs=0.37; p=0.074). There was no correlation with PANSS Negative, General psychopathology, or Total score, and AIMS total score with any of tardive dysmentia symptoms/signs. There was no correlation of tardive dysmentia symptoms/signs with duration of illness or antipsychotics dose.
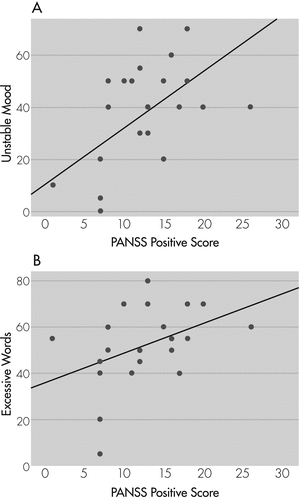
[A] With Unstable Mood; [B] With Excessive Words
Discussion
Results of our study show that tardive dysmentia symptoms/signs are fairly common in chronic schizophrenia patients, whereas, tardive dysmentia, as defined by Wilson et al.,2 is uncommon. Our study supports the hypothesis that these symptoms/signs are related to positive symptoms of schizophrenia,3 with moderate-to-high effect sizes. Although these symptoms/signs were not related to antipsychotic dose, a trend toward higher symptoms/signs was noted in those with tardive dyskinesia, with small effect size. Moreover, these symptoms/signs correlated with higher level of persistent tardive dyskinesia by Schooler and Kane9 criteria. These findings are in contradiction to the reports of association of tardive dyskinesia with negative symptoms of schizophrenia,10,11 although, in recent studies,12 they show correlation with positive symptoms, as well. It is also interesting to note that the symptoms/signs of tardive dysmentia have a superficial resemblance to hypomanic or manic symptoms. There have been reports of a possible association between tardive dyskinesia and affective disorders.13 It also raises the possibility of the usefulness of mood stabilizers in the treatment of such symptoms/signs. Furthermore, tardive dysmentia symptoms/signs were more common in women and those with a family history of psychiatric illness, with small-to-moderate effect sizes, respectively. Similar female predominance has also been reported in tardive dyskinesia studies.14
The mechanisms of these symptoms/signs are poorly understood. Dopamine receptor supersensitivity after prolonged antipsychotics exposure, as proposed for tardive dyskinesia, may be an underlying mechanism for tardive dysmentia.2,15,16 Jones17 argued against such hypothesis and introduced the concept of cholinergic system dysfunction in tardive dysmentia. Correlation with positive symptoms suggests a common psychopathological mechanism for tardive dysmentia symptoms/signs. The common pathway for expression of both these conditions could probably be through involvement of cortico–subcortical circuits.18,19 Interestingly, dopamine receptor supersensitivity has been suggested as the final common state resulting from multiple pathways that converge to elevate the dopamine levels in various brain areas.20 Alternatively, the etiology of tardive dysmentia may be multifactorial, with one mechanism predominating in a subset of such patients. Neurobiological studies in these patients would reveal further insights into this phenomenon and the possible treatment strategies.
In summary, tardive dysmentia symptoms/signs are frequently seen in patients with schizophrenia, but the complete syndrome may be rare. Besides correlation with psychopathology and tardive dyskinesia, which may provide clues to the neurobiological mechanisms, there are clinical implications also. The symptoms/signs of tardive dysmentia may lead to misdiagnosis of patients with schizophrenia by an unwary examiner as bipolar or schizoaffective disorder. Although our study had a large sample size, it may not be sufficient to characterize the true prevalence of this syndrome, and further studies are required. Another limitation is that all patients were chronically institutionalized, and, hence, these findings cannot be generalized to other population. Future studies may be designed to include schizophrenia patients from the community to overcome the effects of institutionalization and characterize the true prevalence of these symptoms/signs.
1 : Adverse effects of anticholinergic antiparkinsonian drugs in tardive dyskinesia: an investigation of mechanism. Neuropsychobiology 1980; 6:109–120Crossref, Medline, Google Scholar
2 : Is there a tardive dysmentia? Schizophr Bull 1983; 9:187–192Crossref, Medline, Google Scholar
3 : Tardive dysmentia: a reappraisal. Schizophr Bull 1984; 10:151–153Crossref, Medline, Google Scholar
4 : Tardive dysmentia: further comments. Schizophr Bull 1985; 11:189–190Crossref, Medline, Google Scholar
5 : Tardive dysmentia in Hungary. Biol Psychiatry 1988; 23:429–430Crossref, Medline, Google Scholar
6 : The ICD-10 Classification of Mental and Behavioural Disorders Diagnostic Criteria for Research. Geneva, WHO, 1993Google Scholar
7 : The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull 1987; 13:261–276Crossref, Medline, Google Scholar
8 Guy D: Abnormal Involuntary Movement Scale, in ECDEU Assessment Manual for Psychopharmacology. Rev. Ed. (DHEW publication (ADM) 76-338). Rockville, MD: National Institute of Mental Health; 1976Google Scholar
9 : Research diagnoses for tardive dyskinesia. Arch Gen Psychiatry 1982; 39:486–487Crossref, Medline, Google Scholar
10 : Late-onset involuntary movements in chronic schizophrenia: relationship of "tardive" dyskinesia to intellectual impairment and negative symptoms. Br J Psychiatry 1986; 149:616–620Crossref, Medline, Google Scholar
11 : Cognitive dysfunction, negative symptoms, and tardive dyskinesia in schizophrenia: their association in relation to topography of involuntary movements and criterion of their abnormality. Arch Gen Psychiatry 1987; 44:907–912Crossref, Medline, Google Scholar
12 : Clinical correlates of tardive dyskinesia in schizophrenia: baseline data from the CATIE Schizophrenia Trial. Schizophr Res 2005; 80:33–43Crossref, Medline, Google Scholar
13 : Tardive dyskinesia: relationship with a primary affective disorder. Dis Nerv Syst 1977; 38:423–427Medline, Google Scholar
14 : Gender differences in tardive dyskinesia: a critical review of the literature. Schizophr Bull 1992; 18:701–715Crossref, Medline, Google Scholar
15 : Clinical relationship of extrapyramidal symptoms and tardive dyskinesia. Can J Psychiatry 1994; 39(Suppl 2):S76–S80Medline, Google Scholar
16 : Dopamine receptor supersensitivity: development, mechanisms, presentation, and clinical applicability. Neurotox Res 2008; 14:121–128Crossref, Medline, Google Scholar
17 : Tardive dysmentia : further comments. Schizophr Bull 1985; 11:187–189Crossref, Medline, Google Scholar
18 : Prefrontal cortical dopamine systems and the elaboration of functional corticostriatal circuits: implications for schizophrenia and Parkinson’s disease. J Neural Transm 1993; 91:197–221Crossref, Google Scholar
19 : Frontal-subcortical circuitry and behavior. Dialogues Clin Neurosci 2007; 9:141–151Medline, Google Scholar
20 : Psychosis pathways converge via D2 high dopamine receptors. Synapse 2006; 60:319–346Crossref, Medline, Google Scholar



