Musical Hallucinations Associated With Pontine Lacunar Lesions
Abstract
Three elderly patients experienced musical hallucinations (MH) in the context of hearing loss. In at least two of the cases, the onset was sudden. All three patients had pontine T2/FLAIR hyperintense foci on MR scan after the onset of the MH.
Musical hallucinations (MH) occur in fewer than 1% of elderly patients with hearing impairment1 and 0.16% of psychiatric patients.2 MH are found most often in the age range of 50–70 and in women (70%).2 Hearing deficits are strongly associated with their appearance, and social isolation seems to be a contributing factor, as well.3 Although the prevalence of MH in psychiatric patients is probably quite low, some authors claim very high rates in obsessive-compulsive disorder and in schizophrenia.4 Other observers note significantly lower frequencies in psychotic populations.5 There are reports of patients who have both MH and the visual hallucinations of Charles Bonnet syndrome, which is considered a visual-system analog.5
Various etiologies are thought to underlie the emergence of MH.6 Auditory sensory deprivation (de-afferentiation) is usually a contributing factor. Epilepsy is noted in a minority of cases. Numerous types of intoxication and withdrawal, as well as other causes of metabolic change (e.g., hypothyroidism), have produced MH. Focal lesions, mainly vascular insults or tumors, may be causative. Within the central auditory pathway, cortical lesions, but not brainstem pathology, are usually considered the likely culprit. However, several cases of pontine lesions have appeared in the literature.7–13
We now report three cases of elderly patients who developed MH and were found to have pontine lesions in the vicinity of the cochlear nucleus and/or other structures and radiations in the auditory pathway. These unusual cases suggest that lesions within the brainstem can, like cortical lesions, lead to MH.
Case Reports
Case #1
This patient, a 79-year-old woman, had the abrupt onset of MH in early 2011 after a transient ischemic attack (TIA). She had longstanding bilateral hearing loss. The MH emanated from her left ear as songs: The Star-Spangled Banner and the French national anthem, and “synagogue chanting.” There was no musical accompaniment. She denied any facial or trigeminal nerve symptoms, any previous history of head trauma, or headaches. She did have a history of an aortic valve replacement 4 years earlier. There was a history of depressive episodes without psychiatric hospitalization. She also had a history of some formed visual hallucinations in the context of macular degeneration and diabetic retinopathy (Charles Bonnet syndrome). Reality-testing was preserved regarding the nature of the hallucinations in both sensory modalities. An MRI after the onset of MH revealed two small cavities in the left pontine tegmentum, compatible with gliosis due to lacunar infarcts (Figure 1). These were not present in an MRI study done 2 years earlier (Figure 2).
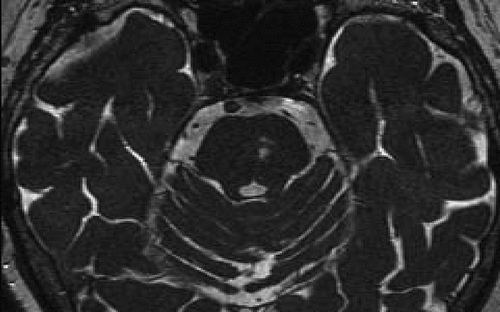
MH: musical hallucinations.
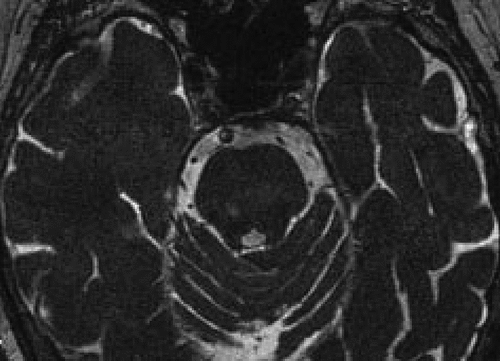
MH: musical hallucinations.
Case #2
This patient suddenly began to hear music and singing at age 84, some months after a coronary bypass procedure. He had experienced a postoperative delirium after the bypass surgery. His MH were complex, at times operatic. He heard Dvorak symphonies, “Ol’ Man River,” and a variety of songs, chants, and phrases. The patient had premorbid obsessive-compulsive disorder (OCD), as noted by his daughter and his internist, but he was only treated with psychotherapy previously. He had also been hard-of-hearing for years. His hallucinations went on to include accusatory and threatening voices, prompting him to call the police. An MRI showed patchy hyperintense lesions in the pontine tegmentum (Figure 3).
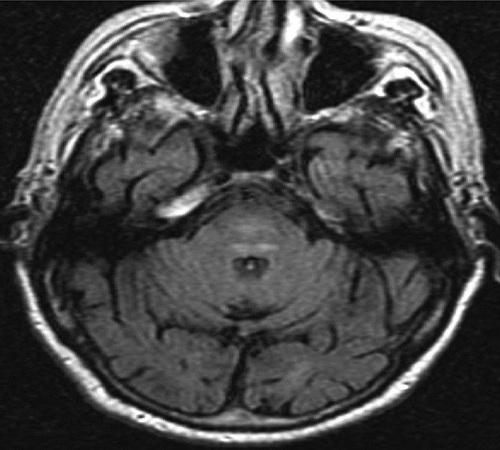
MH: musical hallucinations.
Case #3
The third patient, an 85-year-old woman with longstanding hearing loss, reported that she had been hearing MH for an indeterminate period of time (onset somewhere between 6 weeks and 1 year before). She heard a male voice singing “My Old Kentucky Home,” “God Bless America,” “White Christmas,” and other Christmas carols. The patient had Parkinson’s disease and early cognitive loss, breast surgery (cancer), and shoulder and knee surgery, all in the past few years. She was also in mourning after the loss of a child. She denied any facial or trigeminal nerve symptoms. An MRI demonstrated two punctate foci of hyperintense signal in the dorsal pons bilaterally (Figure 4).
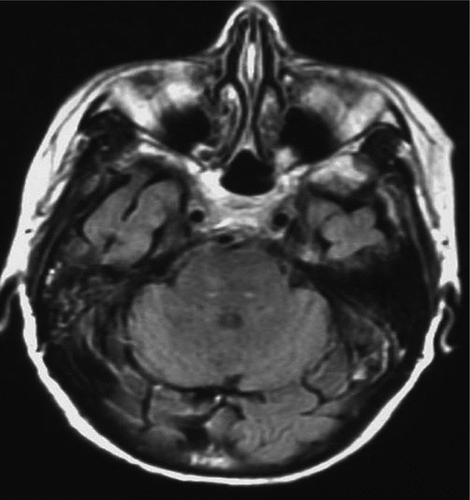
MH: musical hallucinations.
Discussion
The three cases share certain characteristics with those of MH patients in earlier reports. The patients are all elderly and had had significant hearing loss years before the onset of MH. An association with OCD has been noted, but this is based on one study that used a retrospective questionnaire.4 The co-occurrence of MH with Charles Bonnet syndrome in Patient #2 is in keeping with a few other case reports.3,6 It is intriguing to note increased internal sensory “noise” in the face of reduced external sensory input in multiple modalities. All three patients seem to have heard familiar music. The two women heard simple tunes, presumably learned early in life. The male patient’s auditory experiences were much more complex and diverse.
In contrast to traditional dogma, these three cases provide further evidence for brainstem lesions causing MH and may be added to the seven previously published pontine-lesion reports in the MH literature. The first patient had sudden onset of hallucinated songs after a TIA. An MRI taken 2 years earlier was normal, but an MRI just after the TIA demonstrated new lacunar infarcts in the pons. The second patient also had sudden-onset MH, and he had subsequent patchy hyperintense pontine lesions. The nature of the onset of MH in Patient #3 is not as clear, because she was not a consistent historian. Her MRI also demonstrated pontine hyperintense foci. The hyperintense foci in the latter two patients are nonspecific, but most frequently associated with microvascular ischemic disease.
It seems clear that anatomical lesions at any point in the auditory pathways may yield MH under the right circumstances, presumably in the context of pre-existing hypacusis. A major focus of previous reports has been the association of MH with superior temporal gyrus pathology.7 There are case studies of patients who developed MH after left temporal lobectomy14 and after insular glioma resection.15 However, the auditory system is by no means limited to the temporal cortex, and it includes networks of cortical, subcortical, cerebellar, and brainstem elements. The pons is a vital site of those pathways; it includes the cochlear nucleus, the olivocochlear bundle, the superior olivary complex, and the acoustic stria, among other relevant structures. Inhibitory and excitatory auditory pathways to the cortex emanate from the pons.16 The resultant disruption must lead to an increase in “noise,” which, coupled with reduced signal from external sources, produces MH.
1 : The prevalence and phenomenology of auditory hallucinations among elderly subjects attending an audiology clinic. Int J Geriatr Psychiatry 2002; 17:444–452Crossref, Medline, Google Scholar
2 : Prevalence rates of musical hallucinations in a general hospital setting. Adv Psychosom Med 1998; 39:175Crossref, Google Scholar
3 : Hymns and arias: musical hallucinations in older people in Wales. Int J Geriatr Psychiatry 2005; 20:658–660Crossref, Medline, Google Scholar
4 : Musical hallucinations: prevalence in psychotic and nonpsychotic outpatients. J Clin Psychiatry 2004; 65:191–197Crossref, Medline, Google Scholar
5 : Musicophilia: Tales of Music and the Brain. New York, Vintage Books, 2007, p 67Google Scholar
6 : Charles-Bonnet syndrome and musical hallucination. Int Psychogeriatr 2004; 16:489–491Crossref, Medline, Google Scholar
7 : The clinical spectrum of musical hallucinations. J Neurol Sci 2004; 227:55–65Crossref, Medline, Google Scholar
8 : Musical hallucinosis in acquired deafness: phenomenology and brain substrate. Brain 2000; 123:2065–2076Crossref, Medline, Google Scholar
9 : Complex musical hallucinosis in a professional musician with a left subcortical hemorrhage. J Neurol Neurosurg Psychiatry 2001; 71:278–283Crossref, Medline, Google Scholar
10 : Musical hallucinations with dorsal pontine lesions. Neurology 2000; 55:454–455Crossref, Medline, Google Scholar
11 : Musical hallucinations: interplay of degenerative brain disease, psychosis, and culture in a Chinese woman. J Nerv Ment Dis 1996; 184:59–61Crossref, Medline, Google Scholar
12 : Musical auditory hallucinations caused by a brainstem lesion. Neurology 1994; 44:156–158Crossref, Medline, Google Scholar
13 : Brainstem auditory hallucinosis. Neurology 1986; 36:1042–1047Crossref, Medline, Google Scholar
14 : Musical hallucinations after left temporal lobectomy. Cogn Behav Neurol 2008; 21:38–40Crossref, Medline, Google Scholar
15 : Musical hallucinations following insular glioma resection. Neurosurg Focus 2010; 28:E9Crossref, Medline, Google Scholar
16 : Cholinergic cells of the pontomesencephalic tegmentum: connections with auditory structures from cochlear nucleus to cortex. Hearing Res 2011; 279:85–95Crossref, Medline, Google Scholar



