Disorders of Consciousness and N400 ERP Measures in Response to a Semantic Task
Abstract
Disorders of consciousness (DOC) have been studied in recent years. In the present research, electrophysiological measures (ERPs) were used to verify the preservation of semantic linguistic processes in vegetative states (VS) and minimal-consciousness state (MCS). Eighteen patients classified as VS or MCS, and 20 controls were submitted to a semantic associative task with congruous or incongruous word sequences (auditory stimuli). An increased N400 peak amplitude within the fronto-central cortical areas was shown in response to incongruous sequences for both patients and controls. Thus, semantic processing was partially preserved in both VS and MCS patients.
An increasing number of studies have focused their attention on disorders of consciousness (DOC).1 These disorders were classified in terms of awareness and wakefulness.2,3 Awareness refers to a state in which people have complete experiences, such as thoughts, memories, and emotions. Wakefulness refers to the state in which people can open their eyes and perform motor responses. Three different typologies of distinct disorders of consciousness can be defined, based on these two states: coma (C), where patients show absence of both awareness and wakefulness; persistent vegetative state (VS), where there is wakefulness without awareness; and minimally-conscious state (MCS), where both awareness and wakefulness are partially preserved. In contrast to patients in VS, they also reveal discernible evidence of awareness: for example they may speak a word or make a gesture in response to a command.1 A diagnosis of VS or MCS is difficult to make, because clinical examination is limited in these unresponsive patients. In fact, clinical tests rely on a patient’s demonstrating awareness by means of overt behaviors, as does the Coma/Near Coma (CNC), the Glasgow Coma Scale (GCS), and the Disability Rating Scale (DRS).4,5 Some of these scales may offer a more “descriptive” view about the level of general responsiveness of patients to external cues; other scales allow capture of the subtle changes that are indicative of possible development, monitoring the observable behavior over time to find a delayed recovery of consciousness. Specifically, the GCS, the most widely used scale for measuring the level of coma, allows us to describe the level of vigilance (wakefulness) based on the observation of eye-opening, motor response, and verbal behavior. On the other hand, the DRS furnishes a quantitative measure of the disability outcomes for patients with severe head injury, documenting evolution from initial coma to various stages of impaired levels of consciousness. However, it allows a monitoring of patients’ progress, and it is predictive of long-term outcomes, but it cannot furnish a full evaluation of disorders of consciousness by itself, and it is usually used in association with other scales, such as the CNC. The latter furnishes measures of responsiveness of patients with severe brain lesions. It measures patients’ response to stimulation in different sensory modalities. Compared with the DRS, it shows a higher sensitivity in detecting small changes in degree of responsiveness. However, these scales are more qualitative, and they are mainly based on overt behavior, since they include signs of consciousness that are subjectively defined by an external observer. Moreover, the ability to perform these overt behaviors is often decoupled from consciousness as a direct outcome of the brain injury.
It is therefore important to obtain integrative and more quantitative measures of evaluating brain functions and cognitive responsiveness in these patients, as compared with controls, measures such as functional brain imaging, like positron emission tomography (PET) or functional magnetic resonance imaging (fMRI), as well as electroencephalography (EEG) and event-related potentials (ERP) measures. The relevance of these neuroscientific measures to testing the preservation of cognitive processing was recently demonstrated.6,7 Functional brain imaging and ERPs were previously used to elucidate cognitive processes in MCS and VS. These studies showed that the brain of such patients can respond to very complex aspects of stimulation.8 Functional imaging allowed us to track fluctuations in blood oxygenation to examine activation differences between normal and dysfunctional responses.6 However, there is a critical need to improve the clinical evaluation of the consciousness state by using alternative measures, that is physiological measures.9
Based on the evidence to-date, EEG assessments of consciousness provide valuable information for evaluation of residual functioning, formation of differential diagnoses, and estimation of prognosis. EEG-based paradigms have many advantages over fMRI for monitoring patients with DOC for these main reasons: the millisecond-range resolution, the non-invasiveness of the technique, and the possibility of designing dedicated systems for clinical use.10
The current contribution focuses on electrophysiology, as this measure is readily available across a range of clinical settings and thus provides an important tool for the consciousness assessment problem. Such electrophysiological markers have emerged from both diagnostic and prognostic levels of analysis, pointing out the possible interplay of these two clinical aspects.6 Nevertheless, only recently, the relevance of different ERP components in large samples of VS and MS patients was evaluated.11 Cognitive ERPs are used to evaluate higher-level functions like attention, memory, and language. These long-latency potentials are considered well-suited to assessing functions of consciousness. Four specific components were considered to focus the analysis of conscious awareness, they are 1) N100; 2) the mismatch negative (MMN);12 3) the P300;13,14 and 4) the N400.15 About the MCS, a significant amount of data confirmed the presence of some cognitive functions, such as was shown by analysis on P300 and N400 ERP deflections.10,14 Recent research found that MMN response was a strong predictor of functional recovery, since it can predict recovery toward improved levels of consciousness. Moreover, MMN and P300 were found to be more accurate than the N100 at predicting a progressive awake state.14
In the N400, deflection is observed after sentences that end with semantically inappropriate words or, more generally, significant stimuli.16,17 Its amplitude was found to be related to the semantic congruence, semantic relatedness or proximity, meaning probability, and contextual constraint. Thus, the linguistic marker, such as N400 effect, may be used to test preserved abilities to respond to linguistic stimuli. Specifically, this marker may highlight the ability to evaluate intention in DOC patients. In fact, the semantic significance of N400 and its value in terms of higher-order cognitive processes may integrate the general spectrum of the cognitive functions that may be preserved in DOC, with specific attention to the linguistic-semantic level. Some previous research focused on semantic processing by using classical linguistic tasks.15,18,19 Preserved abilities were found in DOC in response to linguistic stimuli. Functional skills to process semantic meaning were observed in some conditions, that is, in MCS, and sometimes in single-case reports of VS.15 However, because of the complexity of the stimulation conditions (whole sentences to be processed, or visual stimulation), it may be supposed that, in some cases, the absence of significant responses by some patients may be related to the experimental paradigm more than to the real absence of semantic abilities in DOC.
For these reasons, the present research explored the contribution of the N400 ERP effect induced by a semantic auditory task, where patients were implicitly required to process congruous versus incongruous linguistic semantic associations. In the experimental paradigm used in the present research, for the first time, a specific preserved ability to evaluate semantic associations based on word meaning was tested in MCS and VS.
The main goal of the present research was to test patients with DOC (previously diagnosed as VS or MCS) for their ability to comprehend congruous versus incongruous semantic associations, related to implicit word categorization ability based on meaning associations. A significant N400 higher deflection was expected in response to incongruous patterns in comparison with congruous patterns in DOC patients, since their semantic abilities may be partially preserved. Second, we expected a more fronto-central peak localization in response to auditory stimuli, as shown in previous research. Third, a homogeneous cortical response is expected for both VS and MCS in cases of semantic processing preservation, since we expected a partial ability to comprehend semantic associations in both categories. In fact, as shown in previous research, these semantic abilities may also be preserved in VS, although the empirical evidence was not always consistent. Thus, we aim to evaluate the degree of functional semantic responsiveness in VS and MCS.
Methods
Subjects
The sample included 18 patients (10 men and 8 women) between 25 and 69 years old (mean age [SD]: 50 [10.11] years). The educational level was a mean of 12.94 years. The VS/MCS followed a coma due to anoxia (10), traumatic brain injury (5), or stroke (3). The VS/MCS distinction took into account the CNC, DRS, and GCS measures. Based on these scores, patients were subdivided into two subgroups: VS and MCS. The first included 10 patients who scored more than 2.00 on the CNC, more than 22 (range: 22–24) on the DRS, and between 0–10 on the GCS (range: 4–10); the second group was 8 patients, who scored between 0.00–2.00 on the CNC, <22 (range: 12–20) on the DRS, and >10 on the GCS (range: 11–13). This classification was based on previous cut-offs, as suggested by the literature, to classify MCs and VS.4,5 The patients had no history of neurologic disorder before coma, and no hearing deficit was observed, based on clinical screening and medical profile analysis conducted by a neurologist. All patients underwent at least a CT or MRI. The time between coma onset and the experiment ranged between 6 and 70 months (mean: 52 months).
There were 20 control subjects (11 men and 9 women) included in the study. They were matched for age (range: 24–69; mean age [SD]: 46 [7.13]), gender, and education (mean: 12.90 years) with the patient group. In fact, the direct comparison between patients and controls did not reveal significant statistical differences based on age (t[37]=0.87, NS; η2=0.11), educational level (t[37]=0.95, NS; η2=0.14), and gender (χ2=1.02, NS). They had no history of neurological disorders and no hearing deficit (see previous criteria). To exclude functional and cognitive deficits or other psychiatric factors, the control group was submitted to a specific screening (the Mini-Mental State Exam, the State–Trait Anxiety Inventory, and the Beck Depression Inventory).
The study did not modify the usual medical practice, and it complies with the Declaration of Helsinki relative to patients’ rights. It was approved by the Ethic Committee of the Catholic University of Milan.
Stimulus and Procedure
Each auditory sequence was composed of four words that were presented to the subjects, and it had a congruous (semantically related words) or an incongruous (semantically unrelated words) final word based on its semantic content. The semantically unrelated final words were chosen on the basis of belonging to distant semantic categories and a lack of association according to the association norms (peach, apple, pear, table); or congruous word sequence: (peach, apple, pear, grapes).20,21 The task was performed in a quiet room (the hospital bedroom for patients, a laboratory room for controls) in which the participants were tested one at a time. The sequences (30 congruous and 30 incongruous sequences) were presented orally. Two loudspeakers were placed behind the participant, to the right and left at a distance of 30 cm. To make the cognitive task more simple, the entire battery (60 trials) was subdivided into three sub-sequences of 20 trials (10 congruous and 10 incongruous sequences) each. The order of the sequences was counterbalanced across participants.
EEG Data Reduction
The EEG was recorded with a 64-channel DC amplifier (SYNAMPS system) and acquisition software (NEUROSCAN 4.2). An ElectroCap with Ag/AgCl electrodes was used to record the EEG from active scalp sites that were referred to the earlobes (10/20 system of electrode placement).22 The data were recorded with a sampling rate of 500 Hz, with a frequency band of 0.01 to 50 Hz. An off-line common average reference was successively computed to limit the signal-to-noise ratio. Also, two EOG electrodes were placed on the outer side of the eyes. The impedance of the recording electrodes was monitored for each subject before data collection, and the impedance remained below 5 kΩ. After the EOG correction and visual inspection, only the artifact-free trials were examined (rejected epochs: 6.62%). A successive regression-based eye-movement correction was used. The computerized artifact rejection criterion excluded the peak-to-peak amplitude when it exceeded 50 μV. The peak amplitude measurement was quantified relative to the 100-msec prestimulus, and it considered the most negative peak value within the temporal window of the 300–500 msec poststimulus. The time onset was coincident with the appearance of the final word.
Data Analysis
To exclude possible age, DOC duration, and etiology effect on the dependent measures of ERP, some preliminary correlational analyses were applied to the data. As shown by Pearson correlation coefficients between age and peak amplitude (r=0.123; NS) and latency (r=0.134; NS) no significant correlation was found between the two variables. Also, for DOC duration, no significant effect was found for peak amplitude (r=0.103; NS) and latency (r=0.128; NS). Finally, we can exclude a direct effect of etiology on ERP measure, as shown by correlational index (rpb) for peak amplitude (rpb=0.104; NS) and latency (rpb=0.129; NS).
Two sets of successive analysis were conducted: the first was finalized to compare the general patient category with the control group. The second was aimed at comparing the VS with the MCS subcategory. Two dependent measures were used, respectively, the peak amplitude and the peak latency. The ERPs were entered into a four-way, repeated-measure ANOVA. Independent, repeated factors were group (2), congruence (2), lateralization (2, left and right), and localization (3, fronto-central, temporo-parietal, and occipital). Indeed, to obtain specific regions of interest, the data were averaged for the fronto-central (Fz, F3, F4, Cz, C3, C4), temporo-parietal (T7, T8, Pz, P3, P4), and occipital (O1, Oz, O2) electrode location and for the left (F3, C3, P3, T7, O1), center (Fz, Cz, Oz, Pz), and right (F4, C4, P4, T8, O2) regions.
Results
Patients Versus Controls Comparison
With respect to the peak amplitude, significant main effects were found for congruence (F[1,36]=10.77; p=0.001; η2=0.39) and congruence × localization (F[2,36]=9.08; p=0.001; η2=0.36). No other effect was significant. The patient group was found to have a statistically significant increased amplitude in response to incongruous stimuli (Table 1 [A] and [B]); (Figure 1).
| Fronto-Central | Temporo-Parietal | Occipital | ||||
|---|---|---|---|---|---|---|
| Left | Right | Left | Right | Left | Right | |
| Congruous | 3.60 (1.04) | 3.77 (1.45) | 3.90 (1.43) | 3.87 (1.02) | 3.66 (1.23) | 3.62 (1.09) |
| Incongruous | 4.32 (1.22) | 4.39 (1.16) | 4.05 (1.16) | 3.99 (1.11) | 3.80 (1.12) | 3.81 (1.22) |
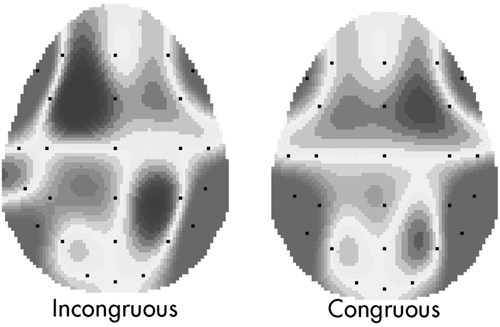
An increased peak amplitude was reported at the fronto-central areas in response to the incongruous condition.
Second, as shown by the post hoc analysis (contrast analysis, Bonferroni corrections for multiple comparisons): for the incongruence condition, a higher fronto-central deflection was found in comparison with temporo-parietal (F[1,14]=12.26; p=0.001; η2=0.41) and occipital (F[1,14]=6.45; p=0.001; η2=0.21) localization (Figure 1). In contrast, the congruous condition did not reveal significant differences between the cortical sites.
Regarding the peak latency variable, significant main effects were found for group × congruence (F[1,36]=7.90; p=0.001; η2=0.35) and group × congruence × localization (F[2,36]=11.09; p=0.001; η2=0.41). No other effect was significant. As revealed by the post-hoc analysis, patients showed a delayed peak in response to the incongruous condition more than did controls (F[1,36]=7.9050; p=0.001; η2=0.34), whereas the congruous condition did not show significant differences between-groups (F[1,36]=1.09; NS; η2=0.15; Table 2). Second, patients showed a more delayed fronto-central deflection in the incongruous condition than did the control group (F[1,36]=7.72; p=0.001; η2=0.34). Moreover, patients showed a greater peak latency in the incongruous than in the congruous condition within the fronto-central site (F[1,36]=7.90; p=0.001; η2=0.35). Other cortical sites did not reveal significant differences.
| Fronto-Central | Temporo-Parietal | Occipital | ||||
|---|---|---|---|---|---|---|
| Patients | Controls | Patients | Controls | Patients | Controls | |
| Congruous | 398 (1.23) | 401 (1.30) | 398 (1.40) | 407 (1.16) | 410 (1.34) | 396 (1.07) |
| Incongruous | 442 (1.54) | 402 (1.16) | 405 (1.18) | 412 (1.13) | 403 (1.23) | 401 (1.04) |
VS Versus MCS Comparison
Peak amplitude and latency measure were entered into two four-way, repeated-measure ANOVAs (Group: 2; VS versus MCS); congruence: 2; lateralization: 2; and localization: 3.
Significant main and interaction effects were found for congruence (F[1,17]=8.76; p=0.001; η2=0.36) and congruence × localization (F[2,17]=11.11; p=0.001; η2=0.42 (Table 3). The incongruous condition showed significant higher peak amplitude than the congruous condition. Moreover, in the incongruous condition, the fronto-central site revealed a higher peak than the temporo-parietal (F[1,17]=9.65; p=0.001; η2=0.39) and occipital sites (F[1,17]=11.02; p=0.001; η2=0.40). No main or interaction effect including the group variable was significant in the analysis (Figure 2).
| Fronto-Central | Temporo-Parietal | Occipital | ||||
|---|---|---|---|---|---|---|
| VS | MC | VS | MC | VS | MC | |
| N400 Peak Amplitude | ||||||
| Congruous | 3.80 (1.15) | 3.78 (1.33) | 3.99 (1.40) | 3.89 | 3.77 (1.21) | 3.90 (1.05) |
| 1.02 | ||||||
| Incongruous | 4.54 (1.28) | 4.49 (1.20) | 3.98 (1.54) | 3.91 | 3.82 (1.14) | 3.80 (1.02) |
| 1.17 | ||||||
| N400 Peak Latency | ||||||
| Congruous | 398 (1.36) | 392 (1.16) | 403 (1.09) | 410 (1.28) | 403 (1.38) | 409 (1.54) |
| Incongruous | 450 (1.09) | 442 (1.22) | 401 (1.14) | 406 (1.33) | 410 (1.01) | 407 (1.30) |
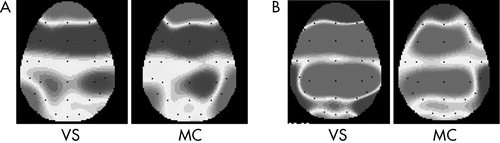
An increased peak amplitude was reported at the fronto-central areas in response to the incongruous condition.
The second analysis, applied to the latency-dependent measure, showed a significant interaction effect of condition × localization (F[1,17]=7.08; p=0.001; η2=0.35). A delayed peak was revealed within the fronto-central site for the incongruous versus congruous condition (F[1,17]=9.13; p=0.001; η2=0.39).
Discussion
Three main results were found in the present research. A first main result was that a morphologically similar N400, peaking at about 410 msec. post-stimulus, more frontally distributed and higher for the incongruous condition, was found for patients and controls. However, also, the comparison between patients’ and controls’ performance revealed a significant difference for N400 latency. In fact, we found a delayed peak within the frontal sites for patients in the incongruous condition as compared with control subjects. Third, MCS and mainly VS diagnosis was not accompanied by the abolition or reduction of the ERP N400 component.
Thus, first, we may suppose that nonconscious patients preserved the ability to execute some modular linguistic functions.23 In a previous study, Burkhardt and Roehm24 found that the amplitude of the N400 is modulated by the degree of plausibility of a conceptual dependency and the strength of the underlying associations: indirect dependencies or conceptual independencies result in an increased negativity. More generally, the strongest relations between two entities revealed the most reduced N400.25
However, some important differences between the present results and previous studies should be underlined. Whereas previous research also found significant differences between congruous and incongruous conditions, with an increased N400 peak amplitude in cases of semantic anomalies, for DOC patients,15 these results were not consistent for both MCS and VS categories. In fact, VS patients were found to produce significantly higher N400 negative deflections in response to incongruous stimuli only in some cases. However, because of the different tasks provided (visual verbal stimuli; complex verbal sentences) in previous studies, the results’ differences may be based on these heterogeneous experimental conditions. The auditory stimulation and the simple associative task between semantic categories we provided in the present experiment may have induced a more direct screening of the preserved abilities in DOC patients.
Nevertheless, in this regard, it is relevant to add some important considerations about the delayed N400 effect we found for patients in response to incongruous associations. This temporal gap could be explained by taking into account the increased difficulty for DOC patients in restoring the appropriate semantic context due to the unattended semantic link (incompatible associations). In other words, in case of DOC, inferences can be made in a less rapid way, in order to support the processing of semantically unrelated information. This may reflect the implication of higher cognitive resources that involve semantic processes and language comprehension, which are only partially preserved in the VS/MCS.
Third, the absence of significant differences between the ERP profile for VS and MCS patients underlies the indistinguishable and similar performance related to the semantic associative task for these two patient categories. We have to underline the fact that VS may preserve some important high-level cognitive functions, such as some semantic associative processes. Patients previously classified as VS patients maintain a consistent ability to respond to semantic meaning, by showing a significant preserved comprehension of incongruous associations. This consideration may contribute to elucidating the question about whether VS patients suffer only from performance problems or whether they have lost their inner knowledge and processing capabilities in the various mental and cognitive domains. Since VS patients were able to process language, this is strong evidence for preserved higher cognitive functions and an argument against the assumption that VS patients only respond at a vegetative level.26
Moreover, the application of multiple screening scales to definite the MCS and the VS categories, in addition to the clinical observation, may have supported a more fine-graded categorization to distinguish the two patients’ categories. Also, the direct comparison between controls’ and patients’ performances, which was absent in some previous studies,15 may guarantee a more systematic analysis of peak profile (its morphological features, the latency window of peak appearance, etc.) and the direct comparability of the normal and pathological group.
However, because of the sample size limitation and the reduced number of patients included in the MCS and VS categories, future research should integrate the present data by comparing larger DOC categories. These “quantitative” criteria of sample inclusion should be integrated with a deeper “qualitative” analysis of classification. In fact, a more systematic comparison between previous diagnostic criteria used in other research to classify the DOC patients (only observational criteria; the use of only one classification criterion or an exclusive behavioral analysis) may support a more complete discussion on the possible contrasting results. Thus, the current diagnostic criteria for DOC, mainly based only on observing patient behavior, may have prevented deeper comprehension of the real cognitive potential of this patient group and correct classification of different consciousness profiles. In some cases, misdiagnosis occurs for patients who are considered to be in VS, and who instead could be more correctly classified as minimally conscious, by adopting ERP measures (and specifically the N400 index). This marker highlights the ability to evaluate intent, semantic associations, and meaning, which are critical components of awareness.
Moreover, the systematic analysis of the etiology of DOC may clarify and justify some significant within-category (MCS, VS, or C) differences. Thus, a stronger comparison related to the clinical profiles of patients may contribute to integration of the present overview.
1 : Behavioral assessment in patients with disorders of consciousness: gold standard or fool’s gold? Prog Brain Res 2009; 177:33–48Crossref, Medline, Google Scholar
2 Balconi M (ed): Psicologia degli stati di coscienza: dalla coscienza percettiva alla consapevolezza di sè [Psychology of conscious states: from perceptive consciousness to self consciousness]. Milano, Led, 2006Google Scholar
3 : Improving the clinical assessment of consciousness with advances in electrophysiological and neuroimaging techniques. BMC Neurol 2010; 10:11Crossref, Medline, Google Scholar
4 : Disability rating scale for severe head trauma: coma to community. Arch Phys Med Rehabil 1982; 63:118–123Medline, Google Scholar
5 : The Disability Rating and Coma/Near-Coma scales in evaluating severe head injury. Neuropsychol Rehabil 2005; 15:442–453Crossref, Medline, Google Scholar
6 : Assessment of consciousness with electrophysiological and neurological imaging techniques. Curr Opin Crit Care 2011; 17:146–151Crossref, Medline, Google Scholar
7 : Coma and consciousness: paradigms (re)framed by neuroimaging. Neuroimage 2012; 61:478–491Crossref, Medline, Google Scholar
8 : fMRI reveals large-scale network activation in minimally-conscious patients. Neurology 2005; 64:514–523Crossref, Medline, Google Scholar
9 : Brain oscillations and BIS/BAS (behavioral inhibition/activation system) effects on processing masked emotional cues. ERS/ERD and coherence measures of alpha band. Int J Psychophysiol 2009; 74:158–165Crossref, Medline, Google Scholar
10 : State of consciousness and ERP (event-related potential) measure: diagnostic and prognostic value of electrophysiology for disorders of consciousness. Neuropsychol Trends 2011; 10:43–54Google Scholar
11 : Event-related potential measures of consciousness: two equations with three unknowns. Prog Brain Res 2005; 150:427–444Crossref, Medline, Google Scholar
12 : Mismatch negativity to the patient’s own name in chronic disorders of consciousness. Neurosci Lett 2008; 448:24–28Crossref, Medline, Google Scholar
13 : Probing consciousness with event-related potentials in the vegetative state. Neurology 2011; 77:264–268Crossref, Medline, Google Scholar
14 : Event-related potentials (MMN and novelty P3) in permanent vegetative or minimally-conscious states. Clin Neurophysiol 2010; 121:1032–1042Crossref, Medline, Google Scholar
15 : How vegetative is the vegetative state? preserved semantic processing in VS patients: evidence from N 400 event-related potentials. NeuroRehabilitation 2004; 19:329–334Medline, Google Scholar
16 : ERPs (event-related potentials), semantic attribution, and facial expression of emotions. Consciousness and Emotion 2003; 4:63–80Crossref, Google Scholar
17 : Comprehending semantic and grammatical violations in Italian. N400 and P600 comparison with visual and auditory stimuli. J Psycholinguist Res 2005; 34:71–98Crossref, Medline, Google Scholar
18 : Semantic processing in comatose patients with intact temporal lobes as reflected by the N400 event-related potential. Neurosci Lett 2010; 474:88–92Crossref, Medline, Google Scholar
19 : Cognitive event-related potentials in comatose and post-comatose states. Neurocrit Care 2008; 8:262–270Crossref, Medline, Google Scholar
20 De Mauro TMancini FVedovelli M: (ed): Lessico di frequenza dell’italiano parlato-LIP. Milano, EtasLibri, 1993Google Scholar
21 : Le associazioni verbali PD-DPSS: norme per 294 parole. G Ital Psicol 2002; 29:153–170Google Scholar
22 : The ten-twenty electrode system of international federation EEG. Electroencephalogr Clin Neurophysiol 1958; 10:371–375Google Scholar
23 : Novelty P3 elicited by the subject’s own name in comatose patients. Clin Neurophysiol 2008; 119:2224–2230Crossref, Medline, Google Scholar
24 : Differential effects of saliency: an event-related brain potential study. Neurosci Lett 2007; 413:115–120Crossref, Medline, Google Scholar
25 : Electrophysiology reveals semantic memory use in language comprehension. Trends Cogn Sci 2000; 4:463–470Crossref, Medline, Google Scholar
26 : The role of early electroclinical assessment in improving the evaluation of patients with disorders of consciousness. Funct Neurol 2011; 26:7–14Medline, Google Scholar



